Abstract
With the boom in industrialization, there is an increase in the level of heavy metals in the soil which drastically affect the growth and development of plants. Nickel is an essential micronutrient for plant growth and development, but elevated level of Ni causes stunted growth, chlorosis, nutrient imbalance, and alterations in the defense mechanism of plants in terms of accumulation of osmolytes or change in enzyme activities like guiacol peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD). Ni-induced toxic response was studied in seedlings of finger millet, pearl millet, and oats in terms of seedling growth, lipid peroxidation, total chlorophyll, proline content, and enzymatic activities. On the basis of germination and growth parameters of the seedling, finger millet was found to be the most tolerant. Nickel accumulation was markedly lower in the shoots as compared to the roots, which was the highest in finger millet and the lowest in shoots of oats. Plants treated with a high concentration of Ni showed significant reduction in chlorophyll and increase in proline content. Considerable difference in level of malondialdehyde (MDA) content and activity of antioxidative enzymes indicates generation of redox imbalance in plants due to Ni-induced stress. Elevated activities of POD and SOD were observed with high concentrations of Ni while CAT activity was found to be reduced. It was observed that finger millet has higher capability to maintain homeostasis by keeping the balance between accumulation and ROS scavenging system than pearl millet and oats. The data provide insight into the physiological and biochemical changes in plants adapted to survive in Ni-rich environment. This study will help in selecting the more suitable crop species to be grown on Ni-rich soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are naturally present in the soil, but their concentration has increased to toxic levels in several places due to industrialization, increased use of fertilizers and pesticides in agriculture, extensive mining, smelting of metals, burning of fossil fuels, production of batteries and other metal products in industries, sewage sludge, and municipal waste. As a result, the natural biogeochemical cycles have been disturbed (Raskin et al. 1994; Shen et al. 2002). Natural resources such as air, water, and soil have been polluted. The accumulation of heavy metals (HMs) is a problem for ecological, nutritional, and environmental balance (Nagajyoti et al. 2010). Heavy metal contamination of soil may pose risks and hazards to humans and the ecosystem through the food chain, drinking of contaminated groundwater, reduction in food quality (safety and marketability) via phytotoxicity, and reduction in land usability for agricultural production causing food insecurity (McLaughlin et al. 2000a, b; Ling et al. 2007). Heavy metals are non-biodegradable, persistent inorganic constituents having cytotoxic, genotoxic, and mutagenic effects on humans, animals, and plants (Flora et al. 2008). Plants need metals for their growth and development; these metals are categorized into essential (Fe, Cu, Zn, Mn, Mg, Mo, Se, Cr, Co, and Ni) and non-essential elements (Cd, Sb, Pb, Ag, As, and Hg) (Afshan et al. 2015). Heavy metals infested in the soil become a nuisance in agriculture as they are found to reduce crop productivity and enter into the food chain (Adrees et al. 2015; Arshad et al. 2016). The European Food Safety Authority (EFSA) has also raised its concern about the presence of Ni in food and feed and recommended systematic investigation on dietary exposure of Ni in food and feed (EFSA 2015). Reduced germination and retardation in the growth of oat seeds were observed in untreated wastewater (Fendri et al. 2013). Clarkson and Luttge (1989) reported that Cu and Zn as well as Ni and Cd compete for the same membrane carriers in plants. Nickel is an essential micronutrient which works as a cofactor for urease, hydrogenase, superoxide dismutase, and glyoxalases and plays a role in nitrogen metabolism, seed germination, and fruit setting (Küpper and Kroneck 2007). Nickel is found in natural conditions at minimal concentrations (10–1000 mg/kg) except in ultramafic or serpentinic soils (Echevarria et al. 2006). Due to continuous human disruptive activities like mining, emission of smelters, and coal and oil burning, levels of Ni have reached up to 200–26,000 mg/kg in soil (Gimeno-García et al. 1996; Izosimova 2005; Sreekanth et al. 2013). In the present scenario, it is essential to assess the toxic effects of Ni on living organisms. At low concentration, Ni has a positive effect on seed germination and is found to be helpful in iron uptake by plants (Hänsch and Mendel 2009; Poonkothai and Vijayavathi 2012). But in excess, it adversely affects the seed germination and seedling growth by hindering the activity of the enzymes such as amylase and protease as well as disrupting the hydrolyzation of food storage in germinating seeds (Seregin and Kozhevnikova 2006). Severe physiological alterations like nutrient imbalance, reduced water potential and transpiration, necrosis, and disorder of cell membrane functions have been observed at higher concentrations in different plant species (Rahman et al. 2005; Ahmad and Ashraf 2012). Adverse effects on plants can be attributed to the direct action of the metal on photosynthetic system and limiting CO2 uptake (Gajewska and Skłodowska 2007b; Sreekanth et al. 2013) or indirectly by competing with several cations like Fe, Mg, Cu, and Zn at high concentrations to prevent them from being absorbed by plants. So with the deficiency of Fe or Zn, chlorosis expression occurs in plants (Khan and Khan 2010). At higher concentrations, Ni can move through phloem and xylem vessels and easily translocate from the root to the upper parts of the plant (Ishtiaq and Mahmood 2012). It can pass through the endodermal barrier and accumulate in the pericycle cells (Seregin and Kozhevnikova 2006). After getting access in the food chain, Ni mostly targets the digestive track and the central nervous system of animals and humans (Hanif et al. 2005). However, Ni-induced harmful effects on plant growth and metabolism mainly depend on its concentrations and exposure time along with the developmental stage of the plant and tolerance level (Khellaf and Zerdaoui 2010; Yusuf et al. 2011). For that reason, it is a prerequisite to know the accumulated level of nickel in edible parts of plants for feed and fodder to ensure human health and food safety (Sreekanth et al. 2013). In accordance, the HM-stressed plants employ metal-specific diverse strategies of cellular metal detoxification including constrained cellular uptake of metals, formation of complex compounds in the cytosol, or vacuolar compartmentalization to survive in the hostile environment (Sharma and Dietz 2006; Gill and Tuteja 2010). Plants posses intricate defense system against oxidative damage which consists of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX) representing the enzymatic system while the non-enzymatic system includes ascorbate, proline, mannitol, glutathione, and phenolic compounds (Bhaduri and Fulekar 2012). Ni is known to both induce and inhibit activity of antioxidative enzymes (Baccouch et al. 2001). Increased activity of APX in pea leaves (Gajewska and Skłodowska 2005) and enhanced level of GPX and SOD in Amaranthus paniculatus were observed (Pietrini et al. 2015), but suppressed activity of CAT, APX, and SOD was also reported in Alyssum bertolonii (Boominathan and Doran 2002).
Proline accumulation is one of the physiological and biochemical modifications which allow a plant to act against heavy metal stress (Schat et al. 1997). Proline might act as an enzyme protector by forming a complex with free radicals (Kaul et al. 2008). Enhanced accumulation of proline in response to heavy metals was reported in several plants such as cabbage (Pandey and Sharma 2002) and Brassica juncea (Thakur and Sharma 2016).
Cereals including wheat, maize, rice, barley, sorghum, and millets are the major food sources of the world (Vigouroux et al. 2011). Human diet majorly depends on wheat and rice, but with population explosion and due to higher nutritional value and improved stress resistance, millets and oats are now getting wider response from food scientists, technologists, and nutritionists in terms of food security and other health benefits (Gupta et al. 2012; Amadou et al. 2013). Oat is also a highly nutritious crop having economic values. Oat seeds have β-amylase protein like wheat, barley, and rye (Ben Halima et al. 2016). Amylases extracted from oat seeds are used in the improvement of the sensory quality and the textural properties of fresh and stored bread (Ben Halima et al. 2015a). Along with this, the oil and nutriment compounds have potential use in industrial applications (Ben Halima et al. 2015b). India is the largest producer of millets (FAO, 2014) with annual production of 11.42 million t where it is used for human beings as well as diverse livestock populations (Saleh et al. 2013).
The objectives of the present study were to provide comparative data on the effect of excessive nickel concentrations on germination, growth, and other physiological and biochemical responses in pearl millet, finger millet, and oats and to get insight into crop-specific response to find out the most tolerant plant which can grow on Ni-contaminated soil.
Material and method
Plant material
Ten varieties of pearl millet (RIB 494, RIB 57, RIB 3135, RIB 192, RIB 20K86, RHB 90, RHB 121, RHB 58, RHB 173, and RHB 177), five varieties of finger millet (PR 202, PES 110, GPU 28, GPU 20, and GPU 66), and three varieties of oat (Quandel jai, H-Javi 08, and NDO 1) were procured from the Agriculture Research Station, Durgapura; the University Agriculture Sciences, GKVK, Bengaluru; and the National Seeds Corporation Ltd., Suratgarh, respectively. The seeds were thoroughly washed with 20% (v/v) Extran® (Merck, India) for 3 min followed by washing with distilled water for three times. Surface sterilization was done with a 0.1% HgCl2 (Merck, India) solution (w/v) for 3 min and then rinsed three times with sterile distilled water. Sterilized seeds were placed on Murashige and Skoog basal medium (HiMedia) having 3% (w/v) sucrose and 0.8% agar (Merck, India). Cultures were grown in controlled environmental condition at 26 ± 1 °C under a 16-h photoperiod with 25 μmol/m2/s photon flux. Various concentrations (0, 10, 15, 20, 25, 30, and 40 ppm) of Ni were added into the medium. For each assay after excision of the plant, the root and shoot were separated. Each experiment was performed in triplicate.
Growth parameters
The percentage of seed germination was calculated to choose the best responding variety for each crop, and then the selected varieties were used for further experiments. Healthy, non-contaminated plants were excised after 15 days of seeding. Root and shoot lengths were measured for each sample. The fresh (FW) and dry weights (DW) of each heavy metal concentration plants were also measured.
Determination of accumulated heavy metal
For the calibration purpose, a stock solution of 1000 ppm was prepared, and by diluting it, a range of 10, 20, 30, 40, 50, and 60 ppm was made. Double-deionized water (Milli-Q Millipore Direct-Q) was used as a blank and for dilution preparation. All the plastics and glassware were soaked in diluted HNO3 (Rankem) and were rinsed with distilled water before use.
A Thermo Fisher Scientific double beam iCE™ 3300 flame atomic absorption spectrophotometer with deuterium background corrector and 50MM Universal finned titanium burner was used in this study. Air acetylene flame with more than 9-psi pressure was applied for measurement.
After excision of the plantlets, they were washed and transferred into a hot-air oven (WTC binder) and were allowed to completely dry at 65 °C. One gram of dried sample was crushed with the help of a mortar and pestle. Subsequently, 10 ml of HNO3 was added per gram of dried sample. This acidic mixture was heated (Tarson Digital Spinot) at 120 °C for 10 min and afterwards at 140 °C until 1-ml volume was left after complete digestion. The final volume of sample was brought to 25 ml with deionized water. All the samples were filtered through a 0.22-μm non-pyrogenic sterile filter (Millex®-GS, Millipore) to obtain a clear and transparent solution. A blank digest was prepared in the same way.
Determination of proline content
Proline concentration was estimated using the method of Bates et al. (1973). Excised seedling tissue (0.5 g) was crushed with a mortar and pestle in 5 ml of 3% sulfosalicylic acid, and the homogenate was centrifuged at 13,000g for 10 min at 4 °C. Two milliliters of freshly prepared ninhydrin reagent and 2 ml of glacial acetic acid were added to 2.0 ml of supernatant. This reaction mixture was boiled at 100 °C for 1 h. On completion of the reaction, the tubes were placed in ice, 4 ml toluene was added, and this was vigorously shaken to extract the chromophore. Absorbance was taken at 520 nm in a UV-visible spectrophotometer (UV-1800, Shimadzu) using toluene as a blank. Proline concentration was determined using a standard curve prepared with known concentrations of proline and their respective absorbance.
Lipid peroxidation
The level of lipid peroxidation was predicted by measuring the accumulated malondialdehyde (MDA) after reaction with thiobarbituric acid (TBA) (Heath and Packer 1968). Fresh 200-mg sample was homogenized in 2 ml of 5% trichloroacetic acid (TCA) and centrifuged for 15 min at 10,000 rpm. In 1 ml of supernatant, 1 ml of 0.5% TBA in 20% TCA was added and heated in boiling water bath for 30 min. After that, the mixture was immediately placed on ice to stop the reaction and the absorbance at 532 and 600 nm was recorded. MDA contents were calculated using the extinction coefficient of 155/mM/cm.
Antioxidant enzyme assays
These enzymes are involved in the detoxification process by converting H2O2 into H2O and molecular oxygen which are produced during photorespiration-β-oxidation of fatty acids and other stress conditions (Lin and Kao 2000). To determine the activities of CAT, GPX, and SOD, 14-day-old plant samples (1 g) were taken and homogenized with a prechilled mortar and pestle in 0.1 M potassium phosphate buffer (pH 7.0). This mixture was centrifuged at 13,000g for 15 min at 4 °C, and the collected supernatant was used to check enzyme activity. All the measurements were taken in triplicates.
Catalase
CAT (EC 1.11.1.6) is a unanimously present, heme-containing oxidoreductase that is localized in peroxisomes. Catalase activity was assayed according to Teranishi (1974) by monitoring the level of H2O2 disappearance (extinction coefficient 39.4 mM/cm). The reaction mixture comprised 50 mM potassium phosphate buffer (pH 7.0) and enzyme extract in a 3-ml volume. To initiate the reaction, 10 mM H2O2 was added and the reduction in absorbance was noted at 410 nm. The CAT activity is defined as one unit of enzyme required to decompose 1 mg of H2O2 in 5 min per gram of fresh weight at 25 °C.
Peroxidase
Guaiacol peroxidase (GPX, EC 1.11.1.7) is a glycoprotein which is present in the cytosol, vacuole, cell wall, and extracellular space (Asada 1992). Its activity was determined according to the method defined by Racusen and Foote (1965). The reaction mixture contained 0.2 ml of enzyme extract prepared in phosphate buffer (pH 7.0), 1 ml of 1% guaiacol, and 0.2 ml of freshly prepared 50 mM H2O2. The assay depends on the conversion of guaiacol to tetraguaiacol in the presence of hydrogen peroxide, and this activity was recorded at 470 nm (extinction coefficient = 26.6/mM/cm).
Superoxide dismutase (SOD; EC 1.15.1.1) activity was assayed using the method given by Beauchamp and Fridovich (1971). To prepare the reaction mixture, the following components were used: 100 mM K-phosphate buffer (pH 7.0), riboflavin, methionine, NBT, and enzyme extract, which were all illuminated for 5 min. One unit of SOD activity is signified as the amount of enzyme required to cause 50% reduction in absorbance.
Estimation of chlorophyll
Photosynthetic pigments Chl a and Chl b and total chlorophyll were estimated using Arnon (1949). In brief, 0.5-g frozen plant sample was homogenized in 80% acetone (v/v) and centrifuged at 10,000 rpm for 10 min. The absorbance of the collected supernatant was taken using 80% acetone as blank.
The amount of different pigments was calculated according to the equation given by Lichtenthaler and Wellburn (1983):
Chla = 12.21A 663 − 2.81A 646
Chlb = 20.13A 646 − 5.03A 663
Total chlorophyll = Chla + Chlb
Results
Effect of Ni on germination and seedling growth
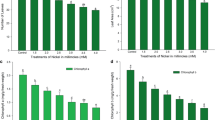
After calculating the germination percentage, varieties RIB 494 of pearl millet, PR 202 of finger millet, and Quandel jai of oats were selected on the basis of their reproducible seed germination rate. These varieties were further used in every experimental setup. Higher Ni concentrations inhibited the germination of seeds of all three plants: finger millet, pearl millet, and oats. After 3 days of incubation on the media containing Ni, the percentage of germination was recorded (Table 1). The maximum inhibition on seed germination was observed in oats at a concentration of 30 ppm which was about 23% whereas no seeds germinated at 40 ppm. At lower concentrations of Ni, particularly at 10 ppm, there was no significant difference in the percentage of seed germination compared to control.
With an increase in Ni concentration, toxic effects were clearly visible on seedling growth. The roots were more affected with suppressed length than shoots. Root length was significantly affected in finger millet and pearl millet. The magnitude of root length reduction due to Ni was the least in the case of pearl millet across all concentrations. In finger millet, there was a slight increase in root length at 10 ppm Ni in the medium. In the case of oats, at 20 ppm Ni, there was negligible root formation observed. The dry weights of the root and shoot were found to be reduced in all three plants with an increase in Ni concentration (Table 2). At higher Ni concentrations, the color of the roots and the stem portion of the shoots turned brownish and shoot length was also markedly suppressed (Fig. 1).
Effect of nickel on the length of a roots and b shoots of seedlings in finger millet, pearl millet, and oats. Data represent arithmetic means ± SE of three experiments. Different letters represent significant differences among plants with the same treatment (Tukey’s test; p < 0.05). Asterisks mean not determined
Accumulation of Ni in root and shoot
The gradual hike of accumulated Ni was observed in all three plants with increased Ni concentrations in the medium (Table 2). The accretion of metal ions was considerably greater in the roots than in the shoots of all three plants. Maximum Ni buildup was found in the roots of finger millet at 40-ppm concentration. The allocation of the amount of Ni from the root to the shoot was greater in finger millet.
Effect of Ni on free proline content
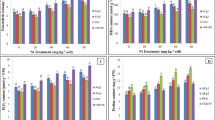
The seedlings exhibited an increase in proline content as the concentration of Ni rises in the medium. At 40 ppm, proline content was 4.3- and 4-fold in the shoot than that of control in finger millet and pearl millet, respectively, while in oats, no seed germination occurred. Similarly, in the root part, at 40 ppm, 5.7- and 5.4-fold proline was measured in finger millet and pearl millet, respectively. Oats showed a rise in proline level up to 2.8-fold in the shoot and 3.5-fold in the root (Fig. 2).
Effect of various concentrations of Ni on free proline content in a roots and b shoots of finger millet, pearl millet, and oats. Data represent arithmetic means ± SE. Different letters represent significant differences among plants with the same treatment (Tukey’s test; p < 0.05). Asterisks mean not determined
Effect of Ni on lipid peroxidation
Lipid peroxidation was measured with the formation of MDA content. Lipid peroxidation of the roots and the shoots of finger millet, pearl millet, and oats decreased significantly with an increase of concentration of Ni. The lowest MDA content was measured in oats at 30 ppm (Fig. 3).
MDA content in a roots and b shoots and SOD activity in c roots and d shoots of seedlings in finger millet, pearl millet, and oats in response to different concentrations of Ni. Data represent arithmetic means ± SE of three experiments. Different letters represent significant differences among plants with the same treatment (Tukey’s test; p < 0.05). Asterisks mean not determined
Effect of Ni on antioxidative enzymes
SOD activity in the roots and shoots of finger millet, pearl millet, and oats exhibited augmentation in relation to rising concentrations of externally supplied Ni as compared to controls (Fig. 3). The roots and shoots of finger millet showed higher activity of SOD (Fig. 3). In contrast, catalase activity of both the roots and shoots of finger millet, pearl millet, and oats was reduced significantly with an increase in metal concentration (Fig. 4). The peroxidase activity of the roots and shoots of finger millet, pearl millet, and oats was boosted up initially and then declined (Fig. 4). In the shoot of finger millet, a noteworthy increase in GPX activity was measured up to 20 ppm Ni concentration; after that, there was no significant rise measured. Instead, a slight reduction was observed. Likewise, in the roots, peroxidase activity increased up to 20-ppm concentration, then it declined. In the case of pearl millet, the peroxidase activity surged significantly in the shoots up to 15-ppm concentration. After that, a considerable reduction was measured with increasing concentration of Ni; although, in the roots, GPX activity increased up to 15-ppm concentration, then no significant reduction was seen. Similarly, in the shoots of oats, peroxidase activity upregulated significantly up to 25 ppm. After that, the activity diminished and in the roots, the peroxidase activity rose initially and became constant at 25-ppm concentration.
Effect of nickel on catalase activity in a roots and b shoots and peroxidase activity in c roots and d shoots of finger millet, pearl millet, and oats. Data represent arithmetic means ± SE of three experiments. Different letters represent significant differences among plants with the same treatment (Tukey’s test; p < 0.05). Asterisks mean not determined
Chlorophyll content
The total chlorophyll content decreased significantly with the increase of Ni concentrations in all three crops (Fig. 5). In pearl millet, maximum chlorophyll reduction was observed, whereas minimal effect was seen in finger millet.
Change in the total chlorophyll content in finger millet, pearl millet, and oat seedlings after treatment with different concentrations of Ni. Data represent arithmetic means ± SE of three experiments. Different letters represent significant differences among plants with the same treatment (Tukey’s test; p < 0.05). Asterisks mean not determined
Discussion
The present study investigated the toxic effect of nickel over a range of concentrations (0, 10, 15, 20, 25, 30, and 40 ppm) on seedling growth parameters and biochemical changes in finger millet, pearl millet, and oats. The data obtained helped in identifying the tolerant crop which could be used for phytoremediation or as feed and fodder in Ni-contaminated areas.
Earlier studies showed that nickel infestation in the soil or medium leads to the inhibition of seed germination (Rao and Sresty 2000; Hossein Khoshgoftarmanesh and Bahmanziari 2012). Notable nickel-induced inhibition of seed germination was recorded in all three crops with the increase in concentration of Ni. Less than 40% of the seeds of finger millet and pearl millet germinated at 40-ppm concentration, and no oat seeds germinated at 40 ppm.
Ni accumulation in the roots and shoots of finger millet, pearl millet, and oats and its adverse impacts on plant physiology were studied. Ishtiaq and Mahmood (2012) reported that at higher concentration, Ni easily moves through phloem and xylem vessels, so it can be translocated smoothly from the root to the upper parts of plants (Bai et al. 2013). It was manifested that plants having more tolerant power show more accumulation of heavy metals in their roots (Rao and Sresty 2000). In our results, finger millet was found to be the most tolerant plant among all tested plants with the highest percentage of germination and accumulation of Ni in the roots. Dry matter accumulation in the roots and shoots of plants was also affected negatively on nickel treatment. Higher concentrations of Ni reduced the root and shoot significantly which is in line with the previous studies (Zurayk et al. 2002; Maheshwari and Dubey 2009).
Proline is likely to be involved in osmoregulation in a water imbalance condition under heavy metal stress in plants; along with this, it is also involved in cellular antioxidative defense mechanism as shown in proline-overproducing transgenic Chlamydomonas reinhardtii (Kaul et al. 2008). Proline plays an important role in protecting the enzymes against toxicity induced by Cd and Zn (Siripornadulsil et al. 2002). Enhanced free proline content in the roots and shoots of all three crops, finger millet, pearl millet, and oats, was observed with the increase in Ni concentration which is aligned with the established HM-dependent proline accumulation in several plant species (Gajewska and Skłodowska 2005; Sharma and Dietz 2006).
Lipid peroxidation measured by MDA content is an effective sign of oxidative damage. Ni-stressed plants exhibit an increased level of lipid peroxidation (Maheshwari and Dubey 2009; Pietrini et al. 2015), but our result showed a reduction in MDA content, which is in sync with other studies (Gajewska and Skłodowska 2007b; Thakur and Sharma 2016). A reduction in lipid peroxidation in the roots and shoots was observed in all three plants tested, and it was found to be the highest in finger millet followed by pearl millet and oats. Constitutively, high antioxidant capacity and high synthesis of antioxidant enzymes may prevent the oxidative damage and make the plant resistant to oxidative stress.
Heavy metal (Cr, Cu, Ni, Cd, Zn, Pb, etc.) toxicity leads to the production of reactive oxygen species in both agronomic and non-agronomic plants which results in physiological and biological disorders that cause significant damage to cellular constituents and enzymatic imbalance (Yan et al. 2008; Siddiqui et al. 2013). To alleviate these reactive oxygen species, plants have specific ROS-scavenging machinery that includes the production of intracellular SOD, catalase, and peroxidase (Mittler 2002; Adrees et al. 2015). Assche and Clijsters (1990) have reported that SOD serves as a key guard to prevent plants from heavy metal-induced oxidative stress. The capacity of plants to overcome oxidative stress partly relies on the induction of SOD activity and consequently on the upregulation of other downstream antioxidant enzymes (Alscher et al. 2002). SOD destroys superoxide, forming H2O2, which in turn may be detoxified by catalase and peroxidase; consequently, the formation of the hydroxyl radical is prevented since it is produced by the interaction of superoxide and H2O2, being catalyzed by transition metal ions (Elstner 1982). Some research group mainly focused on antioxidative response generated in the roots as they sequester more Ni than the shoot parts (Baccouch et al. 2001; Boominathan and Doran 2002). Increased SOD activity was observed in the roots and shoots of all three plants tested with an increase of metal ion concentration which proves its role in ROS scavenging and supports previous studies (Rao and Sresty 2000; Pietrini et al. 2015).
Catalase and peroxidase are important antioxidative enzymes that function in the cells to prevent the buildup of reactive oxygen species (Halliwell and Gutteridge 1999). Edreva et al. (1989) reported that plant peroxidases have been used as genetic markers in genetic physiological and pathological studies and play the role of “stress enzymes” in plants. Peroxidases also play an important role in the defense mechanism against oxidative stress conditions (Gabbrielli et al. 1987). Peroxidase activity increased at lower concentrations of Ni, and then it decreased at higher concentrations in the plantlets of all three crops treated. It has been reported earlier also that the increased peroxidase activity is not only correlated with metal ion concentration (Van Assche et al. 1988) but is also related with biomass production (Rao and Sresty 2000). Previously, Gabbrielli et al. (1987) and Van Assche et al. (1988) reported increased peroxidase activity in the leaves of Silene italica and Phaseolus vulgaris, respectively. Among all three plants tested, finger millet showed the maximum increase in peroxidase activity. Reduced peroxidase activity was also reported in the leaves of Brassica sp. (Nouairi et al. 2009) and B. juncea (Thakur and Sharma 2016) and the roots of A. bertolonii (Boominathan and Doran 2002) while increased peroxidase activity was reported in wheat shoots (Gajewska et al. 2006).
Catalase is also a major player in cellular defense system as it is known to catalyze the decomposition of hydrogen peroxide to water and oxygen, and it is one of the key enzymes involved in the removal of toxic peroxides (Mhamdi et al. 2010). An increase in catalase activity can be explained by an increase in its substrate, i.e., to maintain the level of H2O2 as an adaptive mechanism of the plants (Reddy et al. 2005). Catalase exhibited a substantial decline in activity due to Ni treatment in the plantlets of all three plants tested. It has been reported that heavy metals can inhibit the synthesis of catalase and other oxidase proteins during seed germination (Gajewska and Skłodowska 2007a). Nickel-induced reduction in catalase activity has also been reported in B. juncea (Thakur and Sharma 2016), wheat shoots (Gajewska et al. 2006), and pigeon pea (Rao and Sresty 2000).
Toxic levels of heavy metals adversely affect photosynthetic apparatus and chlorophyll content, which are used to monitor the heavy metal-induced damage in the leaves (Schickler and Caspi 1999; Asopa et al. 2016). Reduced chlorophyll content and interruption of electron transport due to Ni toxicity were also reported in other studies (Gajewska and Skłodowska 2007b; Sreekanth et al. 2013). At higher concentrations of Ni, it was observed in maize that photosynthetic protein complexes and the rate of Hill reaction got affected (Ghasemi et al. 2012). In the present study, chlorophyll content was reduced with an increase in Ni concentration in the medium; maximum decay in chlorophyll content was observed in pearl millet as compared to finger millet and oats.
Conclusions
The heavy metal nickel strongly influences germination parameters of crop plants, and it is worthy to simulate how cereal crops are affected and how tolerant or susceptible they are against this metal. The roots have higher capacity to accumulate Ni than the stem shoot part which suggests a plant’s ability to mitigate the effects of heavy metal toxicity by accumulating it in the roots. Also, plants show defense response such as activation of antioxidative enzymes and proline accumulation to prevent or fix the harmful effects caused by metal stress. Our data proved metal tolerance of three plants in the following order: finger millet > pearl millet > oats. Testing tolerance mechanism towards metal toxicity is crucial to broaden our understanding of the physiological responses that allow plants to survive in polluted soils, which can help to improve traits of plants, which can then be used for phytoremediation and production of food in otherwise non-fertile soils.
References
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Zia-ur-Rehman M, Irshad MK, Bharwana SA (2015) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162
Afshan S, Ali S, Bharwana SA, Rizwan M, Farid M, Abbas F, Ibrahim M, Mehmood MA, Abbasi GH (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ Sci Pollut Res 22:11679–11689
Ahmad MSA, Ashraf M (2012) Essential roles and hazardous effects of nickel in plants. Rev Environ Contam Toxicol 214:pp125–pp167
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress. J Exp Bot 53:1331–1341
Amadou I, Gounga ME, Le G-W (2013) Millets: nutritional composition, some health benefits and processing—a review. Emirates J Food Agric 25:501
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1
Arshad M, Ali S, Noman A, Ali Q, Rizwan M, Farid M, Irshad MK (2016) Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch Agron Soil Sci 62:533–546
Asada K (1992) Ascorbate peroxidase—a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 85:235–241
Asopa PP, Bhatt R, Sihag S, Kothari S, Kachhwaha S (2016) Effect of cadmium on physiological parameters of cereal and millet plants—a comparative study. Int J Phytoremediation, 00-00
Assche FV, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Baccouch S, Chaoui A, El Ferjani E (2001) Nickel toxicity induces oxidative damage in Zea mays roots. J Plant Nutr 24:1085–1097
Bai C, Liu L, Wood BW (2013) Nickel affects xylem Sap RNase A and converts RNase A to a urease. BMC Plant Biol 13:1
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Ben Halima N, Khemakhem B, Fendri I, Ogata H, Baril P, Pichon C, Abdelkafi S (2016) Identification of a new oat β amylase by functional proteomics. Biochim Biophys Acta 1864:52–61
Ben Halima N, Borchani M, Fendri I, Khemakhem B, Gosset D, Baril P, Pichon C, Ayadi MA, Abdelkafi S (2015a) Optimized amylases extraction from oat seeds and its impact on bread properties. Int J Biol Macromol 72:1213–1221
Ben Halima N, Ben Saad R, Khemakhem B, Fendri I, Abdelkafi S (2015b) Oat (Avena sativa L.): oil and nutriment compounds valorization for potential use in industrial applications. J Oleo Sci 64:915932
Bhaduri AM, Fulekar M (2012) Antioxidant enzyme responses of plants to heavy metal stress. Rev Environ Sci Biotechnol 11:55–69
Boominathan R, Doran PM (2002) Ni-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertolonii. New Phytol 156:205–215
Clarkson DT, Luttge U (1989) Mineral nutrition: divalent cations, transport and compartmentation. Prog Bot 51:93–112
Echevarria G, Massoura ST, Sterckeman T, Becquer T, Schwartz C, Morel JL (2006) Assessment and control of the bioavailability of nickel in soils. Environ Toxicol Chem 25:643–651
Edreva AM, Georgieva ID, Cjholakova NI (1989) Pathogenic and non-pathogenic stress effects on peroxidases in leaves of tobacco. Environ Exp Bot 29:365–377
EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) (2015) Scientific opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J 13:202 http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016H1111, http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016H1110
Elstner EF (1982) Oxygen activation and oxygen toxicity. Ann Rev Plant Physiol 33:73–96
FAO (Food and Agriculture Organization) (2014) http://www.fao.org/faostat/en/#data/QC Accessed 10 November 2016
Fendri I, Ben Saad R, Khemakhem B, Ben Halima N, Gdoura R, Abdelkafi S (2013) Effect of treated and untreated domestic wastewater on seed germination, seedling growth, amylase and lipase activities in Avena sativa L. J Sci Food Agric 93:1568–1574
Flora S, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 128:501
Gabbrielli R, Pandolfini T, Vergnano O (1987) Peroxidase involvement in tolerance mechanisms. G Bot Ital 21:200–201
Gajewska E, Skłodowska M (2005) Antioxidative responses and proline level in leaves and roots of pea plants subjected to nickel stress. Acta Physiol Plant 27:329–340
Gajewska E, Skłodowska M, Słaba M (2006) Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol Plant 50:653–659
Gajewska E, Skłodowska M (2007a) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 20:27–36
Gajewska E, Skłodowska M (2007b) Relations between tocopherol, chlorophyll and lipid peroxides contents in shoots of Ni-treated wheat. J Plant Physiol 164:364–366
Ghasemi F, Heidari R, Jameii R, Purakbar L (2012) Effects of Ni2+ toxicity on Hill reaction and membrane functionality in maize. J Stress Physiol Biochem, 8
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gimeno-García E, Andreu V, Boluda R (1996) Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ Pollut 92:19–25
Gupta N, Srivastava A, Pandey V (2012) Biodiversity and nutraceutical quality of some indian millets. Proceed Natl Acad Sci India Section B: Biological Sci 82:265–273
Halliwell B, Gutteridge JMC (1999) Free radicles in biology and medicine, 4th edn. Oxford University Press, New York
Hanif MA, Nadeem R, Rashid U, Zafar MN (2005) Assessing pollution levels in effluents of industries in city zone of Faisalabad, Pakistan. J Appl Sci 5:1713–1717
Hänsch R, Mendel RR (2009) Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opin Plant Biol 12:259–266
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation Archives of biochemistry and biophysics. Arch Biochem Biophys 125:189–198
Hossein Khoshgoftarmanesh A, Bahmanziari H (2012) Stimulating and toxicity effects of nickel on growth, yield, and fruit quality of cucumber supplied with different nitrogen sources. J Plant Nutr Soil Sci 175:474–481
Ishtiaq S, Mahmood S (2012) Phytotoxicity of nickel and its accumulation in tissues of three Vigna species at their early growth stages. J Appl Bot Food Qual 84:223
Izosimova A (2005) Modelling the interaction between calcium and nickel in the soil-plant system. Bundesforschungsanstalt für Landwirtschaft (FAL), German
Kaul S, Sharma S, Mehta I (2008) Free radical scavenging potential of L-proline: evidence from in vitro assays. Amino Acids 34:315–320
Khan MR, Khan MM (2010) Effect of varying concentration of nickel and cobalt on the plant growth and yield of chickpea. Aust J Basic Appl Sci 4:1036–1046
Khellaf N, Zerdaoui M (2010) Growth response of the duckweed Lemna gibba L. to copper and nickel phytoaccumulation. Ecotoxicology 19:1363–1368
Küpper H, Kroneck PM (2007) Nickel in the environment and its role in the metabolism of plants and cyanobacteria. Met Ions Life Sci 2:31–62
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lin CC, Kao CH (2000) Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul 30:151–155
Ling W, Shen Q, Gao Y, Gu X, Yang Z (2007) Use of bentonite to control the release of copper from contaminated soils. Aust J Soil Res 45:618–623
Maheshwari R, Dubey R (2009) Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regul 59:37–49
McLaughlin MJ, Zarcinas BA, Stevens DP, Cook N (2000a) Soil testing for heavy metals. Commun Soil Sci Plant Anal 31:1661–1700
McLaughlin MJ, Hamon RE, McLaren RG, Speir TW, Rogers SL (2000b) Review: a bioavailability-based rationale for controlling metal and metalloid contamination of agricultural land in Australia and New Zealand. Aust J Soil Res 38:1037–1086
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Botany 61(15):4197–4220. doi:10.1093/jxb/erq282
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nagajyoti P, Lee K, Sreekanth T (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Nouairi I, Ammar WB, Youssef NB, Miled DDB, Ghorbal MH, Zarrouk M (2009) Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol Plant 31:237–247
Pandey N, Sharma CP (2002) Effect of heavy metals Co 2+, Ni 2+ and Cd 2+ on growth and metabolism of cabbage. Plant Sci 163:753–758
Pietrini F, Iori V, Cheremisina A, Shevyakova NI, Radyukina N, Kuznetsov VV, Zacchini M (2015) Evaluation of nickel tolerance in Amaranthus paniculatus L. plants by measuring photosynthesis, oxidative status, antioxidative response and metal-binding molecule content. Environ Sci Pollut Res 22:482–494
Poonkothai M, Vijayavathi BS (2012) Nickel as an essential element and a toxicant. Int J Environ Sci 1:285–288
Racusen D, Foote M (1965) Protein synthesis in dark-grown bean leaves. Can J Bot 43:817–824
Rahman H, Sabreen S, Alam S, Kawai S (2005) Effects of nickel on growth and composition of metal micronutrients in barley plants grown in nutrient solution. J Plant Nutr 28:393–404
Rao KM, Sresty T (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Raskin I, Kumar PBAN, Dushenkov S, Salt DE (1994) Bioconcentration of heavy metals by plants. Curr Opin Biotechnol 5:285–290
Reddy AM, Kumar SG, Jyonthsnakumari G, Thimmanaik S, Sudhakar C (2005) Pb induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.) Chemosphere 60:97–104
Saleh AS, Zhang Q, Chen J, Shen Q (2013) Millet grains: nutritional quality, processing, and potential health benefits. Compr Rev Food Sci Food Saf 12:281–295
Schat H, Sharma SS, Vooijs R (1997) Heavy metal-induced accumulation of free proline in a metal-tolerant and a nontolerant ecotype of Silene vulgaris. Physiol Plant 101:477–482
Schickler H, Caspi H (1999) Response of antioxidative enzymes to nickel and cadmium stress in hyperaccumulator plants of the genus Alyssum. Physiol Plant 105:39–44
Seregin I, Kozhevnikova A (2006) Physiological role of nickel and its toxic effects on higher plants. Russ J Plant Physiol 53:257–277
Sharma SS, Dietz K-J (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Shen Z, Li X, Wang C, Chen H, Chua H (2002) Lead phytoextraction from contaminated soil with high biomass plant species. J Environ Qual 31:1893–1900
Siddiqui MH, Al-Whaibi MH, Ali HM, Sakran AM, Basalah MO, AlKhaishany MY (2013) Mitigation of nickel stress by the exogenous application of salicylic acid and nitric oxide in wheat. Aust J Crop Sci 7:1780
Siripornadulsil S, Traina S, Verma DPS, Sayre RT (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 14:2837–2847
Sreekanth T, Nagajyothi P, Lee K, Prasad T (2013) Occurrence, physiological responses and toxicity of nickel in plants. Int J Environ Sci Technol 10:1129–1140
Teranishi Y, Tanaka A, Osumi M, Fukui S (1974) Catalase activities of hydrocarbon-utilizing Candida yeasts. Agric Biol Chem 38:1213–1220
Thakur S, Sharma SS (2016) Characterization of seed germination, seedling growth, and associated metabolic responses of Brassica juncea L. cultivars to elevated nickel concentrations. Protoplasma 253:571–580
Van Assche F, Cardinaels C, Clijsters H (1988) Induction of enzyme capacity in plants as a result of heavy metal toxicity: dose-response relations in Phaseolus vulgaris L., treated with zinc and cadmium. Environ Pollut 52:103–115
Vigouroux Y, Barnaud A, Scarcelli N, Thuillet A-C (2011) Biodiversity, evolution and adaptation of cultivated crops. Comptes rendus biologies 334:450–457
Yan R, Gao S, Yang W, Cao M, Wang S, Chen F (2008) Nickel toxicity induced antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L. cotyledons. Plant Soil Environ 54:294–300
Yusuf M, Fariduddin Q, Hayat S, Ahmad A (2011) Nickel: an overview of uptake, essentiality and toxicity in plants. Bull Environ Contam Toxicol 86:1–17
Zurayk R, Sukkariyah B, Baalbaki R, Ghanem DA (2002) Ni phytoaccumulation in Mentha aquatica L. and Mentha sylvestris L. Water Air Soil Pollut 139:355–364
Acknowledgments
The authors are grateful to the Council of Scientific and Industrial Research (CSIR), the Indian Council of Medical Research (ICMR), and the Rajiv Gandhi National Fellowship (RGNF) for financial support. We also express sincere thanks to DBT-IPLS facility and DRS Phase II, Department of Botany, University of Rajasthan, for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Gupta, V., Jatav, P.K., Verma, R. et al. Nickel accumulation and its effect on growth, physiological and biochemical parameters in millets and oats. Environ Sci Pollut Res 24, 23915–23925 (2017). https://doi.org/10.1007/s11356-017-0057-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0057-4