Abstract
Risk assessing newly synthesized chemicals prior to their applications is extremely important, if we want to ensure substitution of risky chemicals with more benign ones. During the past two decades, many analogs of bisphenol A (BPA) have been manufactured, while their toxicity remains less studied. The aim of this study was to compare the acute toxicity of a synthesized lignin-derived BPA (LD-BP) with that of BPA in representative aquatic organisms including two algal species (Chlorella pyrenoidosa and Scenedesmus obliquus), a cladoceran species (Daphnia magna), and the Japanese medaka (Oryzias latipes). The results revealed that the two algal species showed different responses to the two chemicals. For C. pyrenoidosa, both BPA and LD-BP stimulated growth within 48 h of exposure, except for the 50 mg L−1 of LD-BP treatment. After 96 and 144 h of exposures, BPA stimulated the growth of C. pyrenoidosa at low-exposure concentrations but inhibited its growth at high concentrations, while LD-BP caused a concentration-dependent response in C. pyrenoidosa. S. obliquus exhibited a monotonic concentration-response curve for both BPA and LD-BP exposures. For both D. magna and O. latipes, concentration-responses were monotonic with 96 h–LC50 of BPA and LD-BP of 11.7 and 5.0 mg L−1 and 9.4 and 4.1 mg L−1, respectively. Our results demonstrate that LD-BP is more toxic than BPA in the representative aquatic organisms, and it can pose higher ecological risk to the aquatic ecosystem than BPA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial activities have released a large number of pollutants into the aquatic ecosystems over the past several decades. Among them, bisphenol A (BPA, 2,2-bis-(4-hydroxyphenyl) propane) has been receiving increasing attention due to its adverse effects on aquatic organisms and potential deleterious effects on human beings (Im and Loffler 2016; Rosenmai et al. 2014). Bisphenol A is widely used in the synthesis of hard plastic and epoxy resins as the coating on food cans, water bottles, water pipes, thermal paper receipts, digital media (CDs and DVDs), and electronic equipment lines (Im and Loffler 2016). Its wide industrial applications have driven a continuously rising demand for BPA. The global consumption of BPA is estimated to reach approximately 7.7 billion kg in 2015, and it is predicted that 10.6 billion kg will be manufactured in 2022 (Research and Markets 2016). Bisphenol A from different sources including manufacturers, effluents from wastewater treatment plants (WWTPs), landfill leachates, and improperly disposed BPA-containing products will inevitably enter into the aquatic ecosystems. In fact, BPA has been detected in various waters including the effluents of WWTPs (0.097–370.0 μg L−1), soil leachates (128.0–200.5 μg L−1), surface water (ND–3.9 μg L−1), and drinking water (ND–882.5 μg L−1) around the world (Huang et al. 2012; Makinwa and Uadia 2015; Yamamoto et al. 2001).
Over the past two decades, more than 20 new structural analogs of BPA have been manufactured for different purposes (Kitamura et al. 2005; Chen et al. 2015). Some BPA analogs have been widely used for synthesis of specialized epoxy and polycarbonate resins (Suzuki et al. 2010; Vandenberg et al. 2014). For example, bisphenol B (BPB, 2,2-bis(4-hydroxyphenyl)butane) and bisphenol P (BPP, 2,2-bis(4-hydroxy3-methyl) phenyl propane) have one extra methyl group on the methylene bridge and phenyl rings. They can be used as substitutes for BPA in the production of phenolic resin, rubber additives, and raw materials for pesticides and dyes (Chen et al. 2002; Liao et al. 2012). Bisphenol F (BPF, 4,4′-methylenediphenol) has been used mainly in lacquers, varnishes, adhesives plastics, water pipes, dental sealants, and coatings for food packaging (Cabaton et al. 2009). Bisphenol S (BPS, 4-hydroxyphenyl sulfone) is mainly used in can coatings and thermal receipt papers (Naderi et al. 2014). Bisphenol AF (BPAF, 4,4′-hexafluoroisopropylidene)diphenol), a cross-linker in fluoroelastomers, is mainly used in specialty polymer applications (Matsushima et al. 2010). Increasing efforts are expected to be put forth for the synthesis of more BPA analogs to serve new and special needs from industry.
It is well established that BPA can cause a variety of adverse effects in aquatic organisms including endocrine disruption, cytotoxicity, genotoxicity, reproductive toxicity, and neurotoxicity (Bonefeld-Jorgensen et al. 2007; Crain et al. 2007; vom Saal et al. 1998). A few studies have reported the adverse effects of BPA analogs and compared their toxicities with those of BPA in aquatic organisms (Chen et al. 2002; Im and Loffler 2016; Li et al. 2016). For example, it is reported that the EC50-48 h values of BPB, BPP, and bis(4-hydroxyphenyl)sulfide (TDP) in Daphnia magna were 5.5, 1.6, and 3.5 mg L−1, respectively, which were lower than the 10 mg L−1 reported for BPA (Chen et al. 2002). In terms of the endocrine disruption potential of BPA and BPA analogs, one study finds that the levels of vitellogenin (VTG) in the male zebrafish were elevated after 75 days of exposure to 100 μg L−1 of BPS (Naderi et al. 2014). However, the levels of VTG in the male zebrafish were not altered after 90 days of exposure to BPA at concentrations less than 400 μg L−1 (Keiter et al. 2012). Furthermore, the estrogenicity of bisphenol B was 10 times higher than that of BPA by virtue of the VTG levels in the liver of male Japanese medaka (Yamaguchi et al. 2015). A recent study showed that the exposure to 1.6 μg L−1 of BPA and BPS for 24 h in the zebrafish embryos caused 80 and 140% increase in the newly born neurons in the hypothalamus of the embryos, respectively (Kinch et al. 2015). The above mentioned studies indicate that many BPA analogs might be more toxic than BPA to aquatic organisms. The differences in the toxicity among BPA analogs can be partially attributed to their chemical structures, such as the number of methyl groups on the phenyl rings and the number of methyl groups on the methylene bridge, which can change their hydrophobicity and octanol/water partition coefficient (log K ow ) and therefore alter their uptake into aquatic organisms (Kitamura et al. 2005).
Unlike most analogs of BPA which were synthesized from petrol-based chemicals, lignin-derived bisphenol (LD-BP, 5,5′-methylenebis(2-methoxy-4-methylphenol) is synthesized with a biorenewable and recyclable catalyzer from feedstock (Chen et al. 2015). The synthesized product had high purity (> 99%) as evidenced from the analyses of the 1H nuclear magnetic resonance (NMR) and 13C NMR spectra (Chen et al. 2015). The differences in chemical structures between BPA and LD-BP are seen in Supplementary File 1. In our previous study, it is shown that the biomarkers for effects on the nerve, digestive, antioxidant, and reproductive systems were altered in Japanese medaka exposed to 1.5 mg L−1 of BPA and LD-BP for 60 days. In addition, it seemed that LD-BP was more toxic than BPA in medaka (Li et al. 2016). In this study, the acute effects of BPA and LD-BP in some representative aquatic organisms were evaluated and compared. These organisms include two freshwater algal species, i.e., Chlorella pyrenoidosa and Scenedesmus obliquus, a cladoceran species (D. magna) and the Japanese medaka (Oryzias latipes). The results of this study will provide basic toxicity data for LD-BP in aquatic organisms and help the ecological risk assessment of LD-BP.
Materials and methods
Chemicals and glassware
Bisphenol A (> 97%) (CAS no. 80-05-7; C15H16O2) was obtained from Aladdin Industrial Corporation (Shanghai, China), and lignin-derived bisphenol (C17H20O4) was synthesized (> 99%) (Chen et al. 2015). Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were commercially available and of analytical grade. All glassware and other containers were acid washed, rinsed with deionized water, air-dried, and autoclaved before use.
Test species
The freshwater algae C. pyrenoidosa and S. obliquus strains were purchased from the Institute of Hydrobiology, Chinese Academy of Sciences. The two algal strains have been cultured in a reconstituted medium according to the OECD guideline (no. 201) in the lab for years (OECD 2011). The culture medium was sterilized under pressure of 1.05 kg cm−2 at 121 °C for 30 min prior to inoculation of the algae. The culture of two algal species was maintained in the lab by inoculating new flasks with freshly prepared culturing medium at 1:20 (v/v) once a week. The algae were propagated steadily in the 1-L flasks under illumination of 1500 lx (16 /8 h, light/dark) at 24 ± 1 °C in an incubator. To avoid contamination of the algal species in the lab, the purity of the algae was frequently examined under a microscope with software aiding in algal species counting and identification (Shineso Algacount-Sx, Hangzhou, China).
Daphnids (D. magna) and Japanese medaka (O. latipes) have been maintained in the laboratory for many generations. They were cultured in moderately hard reconstituted (MHR) water (pH 7.0–7.6; total hardness 69.8 mg L−1 as CaCO3) with 16/8 h (light/dark) at 25 ± 1 °C. Daphnids were fed C. pyrenoidosa once a day, and fish were fed newly hatched Artemia nauplii and fish flakes.
Growth inhibition of BPA or LD-BP in two algal species
The growth inhibition assay was performed according to the OECD guidelines for the testing chemicals—testing no. 201 (OECD 2011) with algae. Algal cells were inoculated in 250 mL flasks with 100 mL medium at 25 ± 1 °C with a light intensity of 28 μmol m−2 s−1 (16/8 h, light/dark). Six nominal concentrations of BPA or LD-BP were used in the study for each algal species: 1.6, 3.1, 6.3, 12.5, 25.0, and 50.0 mg L−1. Algae exposed to the same volume of DMSO (carrier solvent for BPA or LD-BP) as the abovementioned six concentrations were treated as the control. Each treatment had four replicates. The algae were acclimated to the exposure conditions for 3 days prior to exposure. One hundred microliters of stock solutions of BPA or LD-BP (dissolved in DMSO and 1000 times of their respective nominal exposure concentrations) was added to the flasks containing 100 mL culture medium to achieve the nominal exposure concentrations. Then, 1 mL of C. pyrenoidosa or S. obliquus from the algal stock was transferred to the flasks with culture medium containing BPA or LD-BP. The solvent control contained DMSO at 0.1% (v/v). After exposure for 48, 96, and 144 h, the absorbance of algal culture was monitored at 750 nm (OD750) on a Multiskan FC (Thermo Scientific, China) spectrophotometer. The growth inhibition rate (GIR) was calculated using the equation: GIR = (A C – A T)/A C × 100%, where A C is the absorbance of the algal culture of the control; A T is the absorbance of the algal culture of the treatments. EC50 values at each assay time were estimated using the nonlinear regression to a log-logistic model (Hill equation) using GraphPad Prism.
Acute toxicity of BPA or LD-BP in D. magna
Acute exposure of BPA and LD-BP in D. magna was conducted according to the modified guidelines of OECD no. 202 for testing chemicals with D. magna (OECD 2004). Approximately 300 adults were divided into 8 containers (each with 1000 mL capacity) to produce neonates for the experiment. The nominal concentrations for BPA or LD-BP were 6.0, 8.0, 10.7, 14.2, 18.9, and 25.3 mg L−1. The solvent control contained 80 μL of DMSO only in 80 mL exposure medium (0.1%, v/v). Each concentration was replicated 4 times. The nominal concentrations for BPA or LD-BP were prepared similarly as above by adding 80 μL of the stock solution (1000× of the nominal concentration) to a 100 mL beaker with 80 mL of MHR water. Then, ten neonates (< 24 h) were transferred into a beaker with the newly prepared exposure medium. The exposure was conducted using a static regime without renewal of the exposure medium. The concentrations of BPA were not quantified. A previous study has demonstrated that after 48 h of exposure, the measured concentrations of BPA were 93.6–95.7% of their nominal concentrations (range from 0.47–7.5 mg/L) in an exposure medium (total hardness 84 mg/L as CaCO3) (Mihaich et al. 2009), which was similar to the exposure medium (MHR water) using in this study. It is therefore reasonable to believe that BPA concentrations will remain over 90% of the nominal concentrations for the exposure in this study. In another study on the effects of BPA in Japanese medaka, it has shown that measured BPA levels in the exposure chambers using a semi-static system were almost identical to their nominal exposure concentrations after 24 h (Yokota et al. 2000). Therefore, in the present study, nominal concentrations of BPA and LD-BP were used. During the exposure for 4 days, food was withheld for the daphnia. Mortality was checked every 12 h, and dead animals were removed from the container at each checking interval.
Acute toxicity of BPA or LD-BP in Japanese medaka
Juvenile medaka (n = 600, approximately 45 days old; weight 67.3 ± 2.2 mg; standard length 1.01 ± 0.13 cm, n = 20) were randomly selected from more than 1500 individuals. The acute exposure was conducted according to the modified guidelines of OECD no. 203. for testing chemicals with fish (OECD 1992). The fish were exposed to 8.5, 9.0, 9.5, 10.0, 12.0, and 16.0 mg L−1 of BPA or 2.0, 4.0, 4.5, 5.0, 5.5, and 6.0 mg L−1 of LD-BP, respectively. The concentrations for the two chemicals were based on results of a preliminary experiment on their acute toxicity to Japanese medaka. Each concentration had four replicates. For the exposure, ten juveniles (45 days) were transferred into a glass beaker (250 mL) with an exposure media of 200 mL MHR water and 200 μL of freshly-made BPA or LD-BP stocks (1000×). The solvent control contained 0.1% DMSO (v/v). Exposure media were not renewed during the exposure according to a previous study on the acute toxicity of BPA in Japanese medaka, in which both larvae and adult medaka were used (Ishibashi et al. 2005). Mortality was recorded every 12 h for 4 days, and dead animals were removed from the beakers at each checking interval.
Statistical analysis
All data were expressed as mean ± standard error (S.E.M). Analysis of variance (ANOVA) was used to detect the difference in the growth inhibition rates of algae, mortality of daphnids, and mortality of Japanese medaka. Tukey’s test was used for the multiple comparisons among treatments. Data were analyzed using the GraphPad Prism 5.0 and OriginPro 7.5 (OriginLab, Northampton, MA, USA).
Results
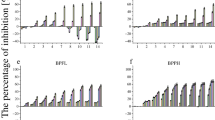
The inhibitory effects of BPA and LD-BP on the growth of C. pyrenoidosa and S. obliquus
For C. pyrenoidosa, after 48 h of exposure, BPA stimulated its growth in a reversed concentration-dependent manner, i.e., the lowest concentration of BPA had the highest stimulation (Fig. 1a). After 96 and 144 h of exposures, the inhibitory effect on growth at concentrations above 10.0 mg L−1 was noticed in C. pyrenoidosa, while a stimulator effects were observed at concentrations lower than 10.0 mg L−1 (Fig. 1a). For LD-BP, after 48 h of exposure, the growth of C. pyrenoidosa was stimulated less relative to BPA, while the inhibition of the growth was observed at the highest concentration (i.e., 50.0 mg L−1) (Fig. 1b). After 96 and 144 h of exposures, C. pyrenoidosa showed a concentration-dependent response to LD-BP. The EC50-96 h of LD-BP was significantly lower than (p < 0.001) that of BPA (Table 1).
For S. obliquus, a clear concentration-dependent response to BPA and LD-BP after 48, 96, and 144 h of exposures was shown (Fig. 2a, b). Its growth was inhibited by approximately 80% compared to the control at the highest test concentrations (50.0 mg L−1) after 96 h. The EC50-96 h of LD-BP was 59% lower than that of BPA (p < 0.01) (Table 1).
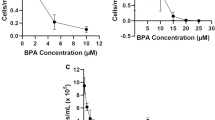
The acute toxicity of BPA and LD-BP in D. magna
For the control, mortality rate was lower than 2.5% after 4 days of exposure. The mortality of D. magna exposed to BPA or LD-BP showed a time- and concentration-dependant pattern (Fig. 3a, b). This coincides with the trends of LC50 values (24–96 h) of BPA and LD-BP for D. magna (Table 1). In general, mortality started earlier in the LD-BP exposure than in the BPA exposure. After 24 h of exposure, mortality started to occur at concentrations higher than 6.0 mg L−1 for LD-BP and 10.7 mg L−1 for BPA. The LC50 values of BPA in the daphnids were approximately 24, 50, 75, and 134% (p < 0.01 in all cases) higher than that of LD-BP after 24, 48, 72, and 96 h of exposure, respectively.
Lethal effect of BPA (a) and LD-BP (b) (each chemical with seven concentrations) in Daphnia magna at 24, 48, 72, and 96 h. The exposure concentrations were from 0 to 25.3 mg L−1. Data were expressed as the means ± standard errors. No error bar on the pillars means that the standard errors equal zero, because all of the organisms were dead (n = 10)
The acute toxicity of BPA and LD-BP in the Japanese medaka
The Japanese medaka showed a concentration-dependent response in mortality to the exposure to both BPA and LD-BP, respectively. Mortality started to show at LD-BP concentrations higher than 4.5 mg L−1 and BPA concentrations above 9.5 mg L−1 after 24 and 48 h of exposures which had a similar response curves for either BPA or LD-BP (Fig. 4). The LC50 values of BPA and LD-BP at 24, 48, 72, and 96 h were estimated to be 10.2, 9.9, 9.8, 9.4 mg L−1 and 5.4, 5.3, 4.9, 4.1 mg L−1, respectively (Table 1). The differences in LC50 values between BPA and LD-BP in medaka after 24, 48, 72, and 96 h of exposures varied from 89 to 129% (p < 0.01 in all cases).
Lethal effect of BPA (a) and LD-BP (b) (each chemical with seven concentrations) in Japanese medaka (O. latipes) at 24, 48, 72, and 96 h. The exposure concentrations of BPA and LD-BP were from 0 to 16.0 mg L−1 and from 0 to 6.0 mg L−1, respectively. The control with no dead is not shown in this figure. Data were expressed as the means ± standard errors. No error bar on the pillars means that the standard errors equal zero, because all of the organisms were dead (n = 10)
Discussion
The comparisons of the effects of BPA and LD-BP
In this study, overall, the toxic effects of LD-BP to the representative aquatic organisms were larger than those of BPA. The EC50-96 h or LC50-96 h values of LD-BP in the two freshwater algae, daphnids and medaka, were 37–80% lower than that of BPA. In our early study in which Japanese medaka were exposed to 1.5 mg L−1 of BPA and LD-BP for 60 days, it was found that, relative to the BPA-exposed medaka, the levels of malondialdehyde (a biomarker for lipid peroxidation) were significantly higher in all the tissues (liver, gill, intestine, brain, gonads) of LD-BP exposed medaka (Li et al. 2016). In addition, LD-BP-exposed male medaka had approximately 55% higher concentrations of vitellogenin (a biomarker for exposure to endocrine disruption chemicals in fish) than BPA-exposed male fish (Li et al. 2016). Bisphenol A and LD-BP differ in their chemical structures in that LD-BP has hydroxyl groups at the three positions of the phenyl rings and extra methyl and methoxyl groups at the phenyl rings of BPA, but no methyl and methoxyl groups between the two phenyl rings (Supplementary File 1). It is hypothesized that the toxicity of BPA analogs is probably positively related to the number of methyl/methoxyl group in the phenyl ring and the methylene bridge between two benzene rings, especially in the former case (Chen et al. 2002). The results from our early study and this study support this hypothesis. In addition, the extra methyl and methoxyl groups in LD-BP might alter the hydrophobicity and octanol/water partition coefficient (log K ow ) (with an extra ·CH3O and ·CH3 group in each phenyl ring of BPA). The estimated log K ow by the software (EPI suite v4.11) of LD-BP (3.80) was slightly higher than that of BPA (3.64) (US EPA 2012).
The inhibitory effects of BPA and LD-BP in C. pyrenoidosa and S. obliquus
The toxic effects of pollutants in algae are generally evaluated by phytotoxicity tests based on the growth inhibition. In this study, the EC50-96 h values of BPA on C. pyrenoidosa and S. obliquus were 44.9 ± 4.8 and 33.9 ± 1.9 mg L−1, respectively, which were comparable to 63.5 and 26.7 mg L−1 for the two same species reported in an early study (Zhang et al. 2012). In addition, the two freshwater algal species showed different responses to the exposures to BPA or LD-BP. The growth of S. obliquus was inhibited at all the concentrations of these two chemicals, while for C. pyrenoidosa, during the first 48 h of exposure, stimulation of growth was observed for both chemicals. The differences in the responses between the two algal species might be due to the differences in the compositions of their cell walls. C. pyrenoidosa is a unicellular algal species, and its cell wall is composed primarily of α-cellulose and glycoprotein (Northcote et al. 1958). S. obliquus is frequently found in the form of four- or eight-celled coenobia, and its cell wall is mainly constituted of mannose (Takeda 1996). It is known that the cell wall of algae plays a critical role in the transport of materials including contaminants in and out of the cell (Latala et al. 2005). Therefore, differences in the composition of the cell walls were likely to affect the uptake of BPA and LD-BP and therefore resulted in the observed difference in their growth inhibition. Furthermore, the differences in growth inhibition by BPA and LD-BP between the two species might be related to their ability to degrade BPA. One study showed that C. fusca can degrade BPA to monohydroxybisphenol A, a low toxic intermediate (Hirooka et al. 2005). Though we have no direct evidence, it is possible that C. pyrenoidosa (a close relative of C. fusca) could also degrade BPA to low toxic intermediates and thus was less sensitive to BPA and LD-BP than S. obliquus.
Furthermore, BPA and LD-BP affected C. pyrenoidosa differently. After 48 h of exposure, LD-BP showed less stimulatory effects in this alga than BPA. After 96 and 144 h of exposure, the stimulatory effects were still obvious for BPA at low concentrations. On the contrary, LD-BP inhibited the growth of C. pyrenoidosa at all the tested concentrations. The exact mechanisms underlying the stimulatory and inhibitory effects of BPA remain unknown and deserve future research.
The acute toxicity of BPA and LD-BP in D. magna
In this study, the LC50-48 h of BPA in D. magna was estimated to be 14.7 mg L−1, which was comparable and within the range of the LC50-48 h values for the same species reported in earlier studies (3.9–20.0 mg L−1) (Alexander et al. 1988; Hendriks et al. 1994; Mu et al. 2005; Stephenson 1983). Again, the higher toxicity of LD-BP in this species than that of BPA might be explained by the differences in their chemical structures. In the evaluation of the toxicity of several BPA analogs in D. magna, it was found that their toxicity was positively related to the number of methyl groups (Chen et al. 2002). Therefore, it is likely that substituting methyl groups in the phenyl ring might increase its toxicity to D. magna.
The acute toxicity of BPA and LD-BP in Japanese medaka
In this study, the LC50-72 h and LC50-96 h value of BPA in medaka was 9.8 and 9.4 mg L−1, respectively. These values were comparable to the results from earlier studies on the acute toxicity of BPA in Japanese medaka. For example, the LC50-96 h value of BPA in Japanese medaka was 13.9 mg L−1 (Ishibashi et al. 2005). The LC50-72 h value of BPA in Japanese medaka was determined to be 6.8 mg L−1 for male fish and 8.3 mg L−1 for female fish (Kashiwada et al. 2002).
The LC50-96 h of LD-BP was significantly lower than that of BPA in Japanese medaka, indicating that LD-BP was more toxic than BPA in acute toxicity testing. In our previous study, it was shown that LD-BP was more estrogenic than BPA and LD-BP. In addition, LD-BP caused higher level of lipid peroxidation than that of BPA when the juvenile Japanese medaka were exposed to the same concentration of these two chemicals for 2 months (Li et al. 2016). Similarly, early studies on the toxicities of BPA analogs have demonstrated that many BPA analogs are more toxic than BPA in fish at various aspects. For instance, the LC50-96 h of BPB was estimated to be 6.1 mg L−1 in larval medaka, which was significantly lower than that of BPA (13.9 mg L−1) (Yokota et al. 2008). In a study on the evaluation of the estrogenicity of BPA and BPB, the lowest observed effect concentration (LOEC) of BPB and BPA to stimulate Vtg gene production in the male medaka was 5 and 50 μmol L−1 (Yokota et al. 2008; Yamaguchi et al. 2015). Again, the increase in the toxicity of BPA analogs relative to BPA might be closely related to the substitutes at the phenyl rings and at the methylene bridge (Chen et al. 2002).
In summary, this study revealed the acute toxic effects of BPA and LD-BP in C. pyrenoidosa, S. obliquus, D. magna, and O. latipes. The two algal species showed different responses to the exposures to both chemicals. The two chemicals affected C. pyrenoidosa differently in that the stimulatory effect was more pronounced in BPA exposure than LD-BP exposure. The acute toxicity of BPA and LD-BP to these three species representative of three trophic levels (primary producer, primary consumer and secondary consumer) in the aquatic food webs was in order of O. latipes > D. magna > C. pyrenoidosa and S. obliquus. In addition, the results of this study and those from our early study (Li et al. 2016) have demonstrated that LD-BP is more toxic than BPA. The results of our studies and those of others on the toxicities of BPA analogs in aquatic organisms suggest that more data on the toxicity of LD-BP and probably other BPA analogs in aquatic organisms are needed prior to their large scale industrial applications.
References
Alexander HC, Dill DC, Smith LW, Guiney PD, Dorn P (1988) Bisphenol A: acute aquatic toxicity. Environ Toxicol Chem 7:19–26
Bonefeld-Jorgensen EC, Long M, Hofmeister MV, Vinggaard AM (2007) Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect 115(Suppl 1):69–76
Cabaton N, Dumont C, Severin I, Perdu E, Zalko D, Cherkaoui-Malki M, Chagnon MC (2009) Genotoxic and endocrine activities of bis(hydroxyphenyl)methane (bisphenol F) and its derivatives in the HepG2 cell line. Toxicology 255:15–24
Chen MY, Ike M, Fujita M (2002) Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol 17:80–86
Chen Q, Huang W, Chen P, Peng C, Xie H, Zhao ZK, Sohail M, Bao M (2015) Synthesis of lignin-derived bisphenols catalyzed by lignosulfonic acid in water for polycarbonate synthesis. ChemCatChem 7:1083–1089
Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette LJ Jr (2007) An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol 24:225–239
Hendriks AJ, Maas-Diepeveen JL, Noordsij A, Gaag MAVD (1994) Monitoring response of XAD-concentrated water in the Rhine delta: a major part of the toxic compounds remains unidentified. Water Res 28:581–598
Hirooka T, Nagase H, Uchida K, Hiroshige Y, Ehara Y, Nishikawa J-I, Nishihara T, Miyamoto K, Hirata Z (2005) Biodegradation of bisphenol A and disappearance of its estrogenic activity by the green alga Chlorella fusca var. vacuolata. Environ Toxicol Chem 24:1896–1901
Huang YQ, Wong CK, Zheng JS, Bouwman H, Barra R, Wahlstrom B, Neretin L, Wong MH (2012) Bisphenol a (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42:91–99
Im J, Loffler FE (2016) Fate of bisphenol A in terrestrial and aquatic environments. Environ Sci Technol 50:8403–8416
Ishibashi H, Watanabe N, Matsumura N, Hirano M, Nagao Y, Shiratsuchi H, Kohra S, Yoshihara S, Arizono K (2005) Toxicity to early life stages and an estrogenic effect of a bisphenol A metabolite, 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene on the medaka (Oryzias latipes). Life Sci 77:2643–2655
Kashiwada S, Ishikawa H, Miyamoto N, Ohnishi Y, Magara Y (2002) Fish test for endocrine-disruption and estimation of water quality of Japanese rivers. Water Res 36:2161–2166
Keiter S, Baumann L, Farber H, Holbech H, Skutlarek D, Engwall M, Braunbeck T (2012) Long-term effects of a binary mixture of perfluorooctane sulfonate (PFOS) and bisphenol A (BPA) in zebrafish (Danio rerio). Aquat Toxicol 118-119:116–129
Kinch CD, Ibhazehiebo K, Jeong JH, Habibi HR, Kurrasch DM (2015) Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci U S A 112:1475–1480
Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S (2005) Comparative study of the endocrine-disrupting activity of bisphenol a and 19 related compounds. Toxicol Sci 84:249–259
Latala A, Stepnowski P, Nedzi M, Mrozik W (2005) Marine toxicity assessment of imidazolium ionic liquids: acute effects on the Baltic algae Oocystis submarina and Cyclotella meneghiniana. Aquat Toxicol 73:91–98
Li D, Chen Q, Cao J, Chen H, Li L, Cedergreen N, Xie H, Xie L (2016) The chronic effects of lignin-derived bisphenol and bisphenol A in Japanese medaka Oryzias latipes. Aquat Toxicol 170:199–207
Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, Kannan K (2012) Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 46:9138–9145
Makinwa TT, Uadia PO (2015) A survey of the level of bisphenol A (BPA) in effluents, soil leachates, food samples, drinking water and consumer products in south-western Nigeria. World Environ 5:135–139
Matsushima A, Liu X, Okada H, Shimohigashi M, Shimohigashi Y (2010) Bisphenol AF is a full agonist for the estrogen receptor ERalpha but a highly specific antagonist for ERbeta. Environ Health Perspect 118:1267–1272
Mihaich EM, Friederich U, Caspers N, Hall AT, Klecka GM, Dimond SS, Staples CA, Ortego LS, Hentges SG (2009) Acute and chronic toxicity testing of bisphenol A with aquatic invertebrates and plants. Ecotox Environ Safe 72:1392–1399
Mu X, Rider CV, Hwang GS, Hoy H, LeBlanc GA (2005) Covert signal disruption: anti-ecdysteroidal activity of bisphenol a involves cross talk between signaling pathways. Environ Toxicol Chem 24:146–152
Naderi M, Wong MY, Gholami F (2014) Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol 148:195–203
Northcote DH, Goulding KJ, Horne RW (1958) The chemical composition and structure of the cell wall of Chlorella pyrenoidosa. Biochem J 70:391–397
OECD (1992) OECD guidelines for the testing of chemicals no. 203. Fish, acute toxicity test
OECD (2004) OECD guidelines for the testing of chemicals no. 202. Daphnia sp., acute immobilisation test and reproduction test
OECD (2011) OECD guidelines for the testing of chemicals no. 201. Freshwater alga, growth inhibition test
Research and Markets (2016) Bisphenol-A—global market overview—key vendors are Hexion Inc., LG Chem Ltd & Mitsubishi Chemical Corporation
Rosenmai AK, Dybdahl M, Pedersen M, Alice van Vugt-Lussenburg BM, Wedebye EB, Taxvig C, Vinggaard AM (2014) Are structural analogues to bisphenol a safe alternatives? Toxicol Sci 139:35–47
Stephenson R (1983) Diphenylol propane: acute toxicity to Daphnia magna and Selenastrum capricornutum. Group research report, shell research Ltd
Suzuki M, Sugiyama T, Musashi E, Kobiyama Y, Kashiwada A, Matsuda K, Yamada K (2010) Use of chitosan for removal of bisphenol A and bisphenol derivatives through tyrosinase-catalyzed quinone oxidation. J Appl Polym Sci 118:721–732
Takeda H (1996) Cell wall sugars of some Scenedesmus species. Phytochemistry 42:673–675
US EPA (2012) Estimation Programs Interface Suite™ for Microsoft® Windows, v4.11. United States Environmental Protection Agency, Washington, DC
Vandenberg LN, Gerona RR, Kannan K, Taylor JA, Breemen RBV, Dickenson CA, Liao C, Yuan Y, Newbold RR, Padmanabhan V, Saal FSV, Woodruff TJ (2014) A round robin approach to the analysis of bisphenol a (BPA) in human blood samples. Environ Health 13:1
vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, Parmigiani S, Welshons WV (1998) A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health 14:239–260
Yamaguchi A, Ishibashi H, Arizono K, Tominaga N (2015) In vivo and in silico analyses of estrogenic potential of bisphenol analogs in medaka (Oryzias latipes) and common carp (Cyprinus carpio). Ecotoxicol Environ Saf 120:198–205
Yamamoto T, Yasuhara A, Shiraishi H, Nakasugi O (2001) Bisphenol A in hazardous waste landfill leachates. Chemosphere 42:415–418
Yokota H, Tsuruda Y, Maeda M, Oshima Y, Tadokoro H, Nakazono A, Honjo T, Kobayashi K (2000) Effect of bisphenol A on the early life stage in Japanese medaka (Oryzias latipes). Environ Toxicol Chem 19(7):1925–1930
Yokota K, Kato C, Hirano M, Ishibashi H, Shiratsuchi H, Tachibana K, Arizono K (2008) Toxicity to early life stages on medaka (Oryzias latipes) and in vitro estrogen intensity of bisphenol compounds. J Environ Toxicol Japan 11:133–142
Zhang W, Xiong B, Sun WF, An S, Lin KF, Guo MJ, Cui XH (2012) Acute and chronic toxic effects of bisphenol A on Chlorella pyrenoidosa and Scenedesmus obliquus. Environ Toxicol 29:714–722
Funding
This study was supported by National Natural Science Foundation of China (NSFC, 41401582; NSFC, 31270549; NSFC, 31270637), China Agriculture Research System (CARS-48) to Yongju Luo and the 100 Talent Program of the Chinese Academy of Sciences to Lingtian Xie.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Cinta Porte
Electronic supplementary material
Supplementary file 1
(DOCX 25 kb).
Rights and permissions
About this article
Cite this article
Li, D., Bi, R., Chen, H. et al. The acute toxicity of bisphenol A and lignin-derived bisphenol in algae, daphnids, and Japanese medaka. Environ Sci Pollut Res 24, 23872–23879 (2017). https://doi.org/10.1007/s11356-017-0018-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0018-y