Abstract
The aim of this experimental study was to evaluate the influence of anaerobic digestion and storage on indicator microorganisms in swine and dairy excreta. Samples were collected every 90 days for 15 months at eight farms, four pig, and four dairy farms, four of them having a biogas plant. Moreover, to evaluate storage effects on samples, 20 l of manure and slurry taken at each farm (digested manure only in farms with a biogas plant) were stored in a controlled climatic chamber at 18 °C, for 6 months. The bacterial load and the chemical-physical characteristics of excreta were evaluated at each sampling time, stored slurry, and manure were sampled and analyzed every 2 months. A high variability of the concentration of bacteria in the different excreta types was observed during the experiment, mainly depending on the type and time of treatment. No sample revealed either the presence of Escherichia coli O157:H7 or of Salmonella, usually linked to the temporary rearing of infected animals in facilities. Anaerobic digestion and storage affected in a significant way the reduction of indicator bacteria like lactobacilli, coliforms, and streptococci. Anaerobic digestion lowered coliforms in pig slurry (− 2.80 log, P < 0.05), streptococci in dairy manure (− 2.44 log, P < 0.001) and in pig slurry (− 1.43 log, P < 0.05), and lactobacilli in pig slurry (− 3.03 log, P < 0.05). Storage lowered coliforms and the other indicators counts, in particular in fresh wastes, while clostridia did not show a reduction in concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the Lombardy region in Northern Italy, livestock farming represents a significant portion of the local economy. In 2010, about 1.5 million cows and 4.8 million pigs (representing, respectively, 27 and 50% of the national total amount), distributed on an agricultural area of about one million hectares, were surveyed (ISTAT 2010). This high concentration of animals poses serious concerns regarding the production of slurries and manure, their impact on groundwater, ammonia and greenhouse gas emissions, and food security resulting from the potential presence of zoonotic pathogens.
In recent years, there has been an increasing interest in the spreading of zoonotic pathogens, their persistence in soils, and the correlation between the presence of pathogens and the safety of agricultural products (Hutchison et al. 2005; Pachepsky et al. 2006; Ziemer et al. 2010; Rogers et al. 2011; Toth et al. 2013). This concern is even more present in Europe and North America, where the availability of “pathogen free” products is a sensitive topic for public opinion (Bicudo and Goyal 2003; Cummings et al. 2009; Newell et al. 2010; Krause and Hendrick 2011). The recycling of these kinds of wastes to agricultural land creates the risk of pathogens, contaminating the environment, entering the food chain, or infecting livestock (Martinez and Burton 2003). Pandey et al. (2014) highlighted the great risk coming from pathogens and related to wastewater effluents for public health.
A clear example is the Escherichia coli O104:H4 outbreak occurred in Germany during the spring of 2011 (3816 cases, including 54 deaths), in which the consumption of bean sprouts was identified as the most likely vehicle of infection (Frank et al. 2011). Other verotoxin-producing strains of Escherichia coli, such as strain O157:H7, able to survive under adverse conditions (Pell 1997), whose reservoir is identified in dairy farms (Wells et al. 1991; Hancock et al. 1994; Zhao et al. 1995), can induce serious symptoms as hemorrhagic colitis, hemolytic uremic syndrome, and thrombocytopenic purpura. Many of the available publications about health risks linked to animal waste disposal are addressed to study Escherichia coli O157:H7 and Salmonella (Huston et al. 2002; Murinda et al. 2002; Blau et al. 2005; Cho et al. 2006; Semenov et al. 2011), while several other pathogens have also been investigated, including Campylobacter jejuni, Listeria monocytogenes, Cryptosporidium parvum, Giardia lamblia, Enterococcus faecalis, and Clostridium spp. (Hutchison et al. 2004; Watcharasukarn et al. 2010).
There is a higher risk of pathogen transfer into the food chain when fresh manure is applied to the land than when stored manure is applied, because in the former case, there is no storage or treatment period to decrease pathogen numbers (Watanabe et al. 1997). As a consequence, the minimization of the sanitary impact of slurries and manure in the environment has to be considered as a primary objective in livestock farming.
Storage is a traditional practice that consists in storing animal excreta for long periods in order to reduce the organic and bacterial loads. Prolonged isolated storage for 3–6 months before land spreading is still the most common practice in Italy. This approach allows the number of pathogens in manure to decrease but not to totally disappear.
Anaerobic digestion performed in biogas plants is a recent alternative way to handle animal wastes for the production of energy and of fertilizers to be spread on cultivated land, limiting the risk for human health and reducing greenhouse gas emission. The usefulness of treatments like digestion, and, traditionally storage, to destroy, or limit, infectious microorganisms in animal waste for land application is well known.
In a recent study, Biswas et al. (2016) evaluated the performance of limited aerobic and anaerobic storage conditions in decay of pathogens in dairy manure at four temperatures under minimal mixing. Results showed that the effects of both limited aerobic and anaerobic storage conditions on pathogen reductions were almost similar in the minimal mixing condition potentially due to poor aeration of dairy manure. Escherichia coli survival was longer than Salmonella and Listeria monocytogenes in all temperature conditions. Salmonella and Listeria monocytogenes levels were reduced to non-detectable level in both limited aerobic and anaerobic storage conditions within 3 days of incubation.
The temperature and hydraulic retention time are crucial factors for pathogenic bacteria survival during anaerobic digestion (Dumontet et al. 1999). Anaerobic digestion can be performed either at 30–38 °C (mesophilic) or thermophilic at 50–55 °C and bacterial inactivation due to temperature is strictly related to time (Olsen and Larsen 1987). Gibbs et al. (1995) and Larsen et al. (1989) found that the time required for a 90% reduction of viable counts of a population of microorganisms (T90) for many bacteria can be counted in hours in thermophilic digestion and in days in mesophilic digestion, compared to weeks and months in conventional treatment (storage). Gibbs et al. 1995, reported at least a T90 of 2 weeks for Escherichia coli and Salmonella typhimurium, of 2.7 weeks for enterococci in storage at 18 °C. Enterococci showed a T90 of 21.4 weeks at a storage temperature of 6–15 °C.
However, pathogens represent a rather limited fraction of the bacteria in the feces of animals, with the exclusion of the acute phases of enteric diseases. Pathogen bacteria are released into the environment on a non-continuous basis, in relation to the health and the immune status of the subjects, and they are not ideal indicators for monitoring the different maturation processes of sewage. The evaluation of more common bacteria, ubiquitous in manure, could be used as “indicators” of the pathogenic potential of the different categories of bacteria that might be present in the feces, because of their similar biochemical and respiratory needs (Bicudo and Goyal 2003). The use of indicator organisms (e.g., fecal coliforms, Escherichia coli) for evaluating pathogen levels has been widely discussed; however, the use of indicator organisms is likely to continue for assessing pathogen levels in water resources potentially for the lack of an alternative reliable solution (Pandey et al. 2014). The use of indicator microorganisms as surrogate for pathogenic fecal organisms in both fate and transport was performed in past studies performed by Wang et al. (2004), Ogden et al. (2001), and Mubiru et al. (2000). In the last decades, the goodness of indicator organism evaluation for assessing pathogen levels in ambient water bodies on the basis of the similar decay is confirmed by many studies (Malakoff 2002; Pandey and Soupir 2012; Pandey et al. 2012; Pandey and Soupir 2013). Smith et al. (1973) found that Salmonella decay in stream water was similar to that of fecal coliforms. In Denmark, the fecal streptococci (FS) method is used for quality assurance of digested residues for common pathogens (Salmonella, Listeria, Campylobacter, and Yersinia; Espensen 1996). This method, however, present the limitation when the temperature in the treatment process exceeds 55 °C, because fecal streptococci are quickly reduced and are impossible to quantify above this temperature (Bendixen 1999). De Luca et al. (1998) found fecal streptococci to be the only indicator bacteria with a statistically significant correlation to Listeria monocytogenes.
In general, the decrease of the counts of microbial indicators also corresponds to a lower concentration of pathogens; this happens in the case of Coliforms for Salmonella spp. and, also, for verotoxigenic Escherichia coli, which is metabolically similar (Vanotti et al. 2005).
For the abovementioned reasons, the present study was aimed at evaluating the effect of anaerobic treatment (at least six complete digestion cycles during the trial) and of storage time on bacteria concentration reductions and on the physical characteristics of livestock wastes.
The effects of storage time (0, 2, 4, and 6 months) on the bacteria concentrations of the eight manure samples (four cattle manures and four pig slurries, two samples for each categories were digestates) were stored in tanks at 18 °C in a climatic cell to avoid undesired environmental additional effects.
Material and methods
Four cattle farms and four pig farms were considered in this study as representatives of Italian intensive cattle and pig husbandry. Four of them, two cattle and two pig farms, had a mesophilic biogas plant. Manure and slurry were spread on land for corn and alfalfa productions.
Animals and farms
Pig farms
Four pig farms were involved in the study. The first farm is a full cycle piggery (from birth to slaughtering), with 12,000 pigs in total (650 sows); the manure is collected under the pit for vacuum system removal and moved to the biogas plant, a mesophilic plant working at 43 °C with a hydraulic retention time (HRT) of 56 days. The plant consists in a primary, a secondary digestion plant, and an ultimate “cold” tank to recover the residual biogas from digested manure.
The second pig farm is a full cycle with 8000 pigs reared from weaning to slaughter (from 35 to 160 kg of live weight). The farm has a slatted floor with vacuum system for manure removal. Manure is collected and moved to a primary tank and then to the mesophilic digestion tank, with a temperature of 37 °C for 40 days of HRT.
The third farm is a full cycle farm with 400 sows; the manure is separated and moved to the tank for 180 days of storage.
The fourth farm is a full cycle farm with 250 sows; the manure is collected into the deep pit and then sent to the tank for 180 days of storage.
Dairy cattle farms
The first farm is a dairy cattle farm with 300 Friesian Holstein dairy heifers; the manure is removed through scrapers and under the pit; then, it is moved to the mesophilic digestion plant (set up in a primary and a secondary digestion plant) working at a temperature of 48 °C, HRT of 90 days.
The second dairy cattle farm reared 600 Friesian Holstein dairy cows; the manure is removed through scrapers and under the pit; then, it is moved to the mesophilic digestion plant(set up in a primary and a secondary digestion plant) working at a temperature of 48 °C, HRT of 90 days. The plant in this farm is identical to the plant adopted by the first dairy farm.
The third dairy cattle farm reared 150 Friesian Holstein dairy cows; the manure falls in to a pre-tank placed under the perforated floor and moved to tank for 120 days of storage.
The fourth dairy cattle farm reared 400 Friesian Holstein dairy cows; the manure is removed through scrapers and under the pit; then, it separated into solid/liquid fractions and stored for 120 days.
Sampling in real conditions
The manure samples were taken in the farms for 15 months every 90 days (six times in the study) to evaluate their physical, chemical, and microbiological characteristics. In the farms with storage pits, the manure was taken directly from the pits, or under the slatted floors. In the farms with anaerobic plants, the samples were taken before and after the digestion process, at the end of the HRT period. The manure was mixed in the lagoons and in the pits; then, five tanks of 10 l were collected from various zones (At middle height of the tank, one sample was taken in the central zone and four in the lateral zones.). Then the collected manure samples were mixed together, and three samples of 100 g for each manure type were collected and taken to the laboratory for microbiological (50 g) and chemical (50 g) analyses.
Sampling of stored manures in controlled climatic conditions
In each farm, 20 l of excreta (fresh manure/slurry for farms with storage tank and digestate product for farms with anaerobic plant) were stored six 6 months at 18 °C to study the effect of storage on bacterial load in manure kept at constant temperature.
At the beginning of the 2 cycles, the manure was collected in every farm, as follows: manure was mixed in the lagoons and in the pits; then, five tanks of 10 l were collected from various zones (At middle height of the tank, one sample was taken in the central zone and four in the lateral zones.). Then, the manure collected in the tanks was mixed together and 20 l were taken to the climatic cell for storage. For the analysis at 0, 2, 4, and 6 months of storage, three samples of manure for each 20-L tank (slurry and/or manure type) were withdrawn at the bottom, in the middle, and in the high part of the tank. The samples (100 g each) were taken to the laboratory for bacterial counts (50 g) and chemical (50 g) analyses within 2 h from sampling.
The climatic control was achieved through a conditioning system, and the temperature was monitored every minute with a datalogger system (HOBO UX100, ELCAM SpA). Microbial concentrations were measured every 2 months, for 6 months, at time 0 = first sampling day, time 1 = second month, time 2 = fourth month, and time 3 at the sixth month. This trial was performed twice in the experimental period.
Microbiological analysis
The presence of the selected “indicator-bacteria” coliforms (Gram-negative, aerobic/facultative anaerobes), enterococci (Gram-positive, facultative anaerobes), lactobacilli (Gram-positive, facultative anaerobes), and clostridia (Gram-positive, sulfite-reducing anaerobes) was evaluated. These microorganisms are indicators of the survival of potentially dangerous pathogens of the same genus. In addition, qualitative bacteriology was also performed to verify the presence and the possible survival of some pathogen bacteria (Escherichia coli O157:H7 just for dairy samples and Salmonella species) in the tested conditions.
Quantitative bacteriology
One gram of each sample was mixed in 9 ml of sterile distilled water and thoroughly homogenized. A series of tenfold dilutions (from 10−1 to 10−7) were then prepared. 0.1 ml of each dilution was used to inoculate three plates for each dilution of four agar selective media using the spread technique. MacConkey agar was used for the enumeration of Coliform species, Slanetz-Bartley agar for Enterococcus species, Rogosa agar for Lactobacillus species, and Iron Sulphite agar for Clostridia species. The water content was determined in 1 g of each sample, testing it by an infrared moisture meter (PSE-484B. Chino Corporation, Kumano, Tokyo, Japan) before and after drying in a vacuum oven at 105 °C. The plates for coliforms were incubated aerobically at 37 °C, 24 h, plates for Enterococcus spp. fat 37 °C, for 72 h. Plates for sulfite-reducing anaerobes were incubated in anaerobiosis at 37 °C for 24 h, and those for Lactobacillus spp. were incubated for 48 h at 45 °C. After incubation, the presence of bacterial colonies on the plates was examined. Only plates with a number of colonies between 15 and 150 were counted, and the results were expressed as colony forming units (CFU) per gram of wet feces.
Qualitative bacteriology
Qualitative assays were performed on the manure samples, before and after treatment, and at different sampling times, to determine the presence of two enteropathogenic bacteria: Salmonella spp. in samples from pigs and cattle and Escherichia coli O157:H7 in samples from cattle.
The sensitivity of the method used for the detection of Salmonella spp. (derived from ISO 6579:2005) has been estimated at 87% of the pathological material from the pig (Mainar-Jaime et al. 2013). For Escherichia coli O157:H7, validation studies of the method ISO 16654-2001 indicate a sensitivity of 96.4% of the plant materials (Tozzoli and Morabito 2014).
For Escherichia coli O157:H7, 10 g of each fecal sample were mixed with 90 ml of buffered peptone water (BPW) and incubated overnight at 37 °C. The colonies in 1 ml of this culture medium were concentrated using immunomagnetic specific anti-O157 beads in an automated system, according to the manufacturer’s recommendations (Dynal, Oslo, Norway). Briefly, the retrieved beads were inoculated on sorbitol MacConkey agar containing cefixime and tellurite (SMACct), then incubated overnight at 37 °C. From each plate, five sorbitol-negative colonies were isolated and identified with biochemical systems and by direct latex agglutination directly with a commercial kit (Oxoid).
For the selective bacteriology of Salmonella spp., 1 g of each fecal sample was inoculated in culture pre-enrichment in buffered peptone water and incubated overnight at 37 °C. One milliliter of this culture was transferred to a 10-ml tube of selective broth Muller-Kauffmann Tetrathionate-Novobiocin (MKTTn), then incubated at 37 °C for 24 h. Finally, this culture was inoculated on XLT4 agar and incubated for 24 h at 37 °C.
Chemical analyses
All samples were dried for 24 h at 40 °C and then for another 24 h at 105 °C (APHA et al. 2005), shredded in a blender, and passed through a 1-mm mesh. Ammonia (NH3–N) and total nitrogen (TKN) were detected on fresh samples. Fresh matter (FM), total solids (TS), and volatile solids (VS) were determined following standard procedures (APHA et al. 2005). Total P and K contents were determined by inductively coupled plasma mass spectrometry (Varian, Fort Collins, USA). Standard samples (National Institute of Standards and Technology, Gaithersburg, MD, USA) and blanks were run with all samples to ensure precision in the analyses. P and K detection was preceded by acid digestion (EPA 1998) of the biomass samples. Total alkalinity or buffer capacity (TAC) and total volatile fatty acids (FOS) concentrations were determined in the bulk samples by a five-times-diluted solution of 2.5 g of wet sample, filtered to 0.45 μm, according to the acid titration method (Lahav et al. 2002).
Statistical analysis
Before the statistical analysis, all the microbiological counts were transformed into log10; data are expressed as log10 CFU per gram. The bacterial counts of samples collected every 3 months in livestock farms were submitted to variance analysis (PROC GLM of the SAS statistical package 9.2, 2013) in order to evaluate the effect of the collecting season on physical characteristics of slurry and on the microbial concentrations.
Microbiological data related to samples before and after anaerobic digestion were processed through variance analysis (PROC GLM of the SAS statistical package 9.2, 2013) to test the effect of type of waste (dairy vs. swine) and of the anaerobic treatment on bacteria concentration reductions; the interaction type for treatment was considered in the model.
A third variance analysis was performed (PROC GLM of the SAS statistical package 9.2, 2013) on samples stored in the climatic cell (four cattle manures and four pig slurries, two samples for each categories were digestates). The variance analysis evaluated the effect of type of waste (dairy vs. swine), treatment (raw manure vs. digestate), and storage time (0, 2, 4, and 6 months) on bacteria concentrations. The interactions types, treatment, and storage time were included in the model.
In the variance analysis, the significance level was considered at least for P < 0.05.
A Pearson correlation procedure (PROC CORR of SAS statistical package, 9.2, 2013) was performed among all the variables to highlight potential correspondences between physical-chemical characteristics and bacterial counts.
Results
Pathogens investigated in the trial (Salmonella and Escherichia coli O157:H7) were not ever detected at any sampling time, indicating that no clinical or subclinical dissemination of these pathogens had occurred during the research period.
No effect of collecting season was found on the samples for all the studied bacteria.
Evaluation of the effect of anaerobic digestion
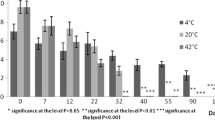
Figure 1 shows the mean values of the microbial load of dairy manure and pigs slurry (clostridia, coliforms, streptococci, and lactobacilli), expressed in log10 CFU per gram, sampled before and after the anaerobic digestion treatment during the experimental study in real conditions.
Streptococci and lactobacilli concentrations were significantly lower (P < 0.05) in dairy raw manure and digestate in comparison to pig wastes.
The anaerobic digestion treatment had a significant overall effect on the decrease of coliforms (P > 0.01), streptococci (P < 0.001), and lactobacilli (P < 0.05). This microbial abatement was evident during the whole sampling campaign.
Clostridia concentration decreased slightly according to the anaerobic treatment in cattle manure from 4.95 to 4.70 log10 CFU/g. The anaerobic digestion induced an increase in the Clostridia population in pig slurry (5.28 vs. 6.02 log10 CFU/g), although not in a significant way.
The coliform count significantly decreased in pig slurry from 5.61 to 2.81 log10 CFU/g (P < 0.05) after the anaerobic treatment. The variation of coliforms in dairy digestate was measured in − 2.19 log in comparison with the fresh manure.
Streptococci counts differed significantly in relation to the manure type (dairy vs. swine, P < 0.001) and after the anaerobic digestion in comparison with the fresh manure (P < 0.001).
In cattle manure, streptococci count was reduced from 4.67 to 2.23 log10 CFU/g (P < 0.001) after the treatment, in pig slurry from 5.43 to 4.00 log10 CFU/g, P < 0.05.
Lactobacilli concentrations showed overall effects of manure type (dairy vs. swine, P < 0.01) and by the digestion treatment (P < 0.05). Pig slurry showed a significant decrease of this concentration in digestate (7.92 vs. 4.89 log10 CFU/g, − 38%; P < 0.05).
Evaluation of storage
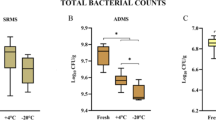
Figure 2 shows the mean values of the microbial load of clostridia, coliforms, streptococci, and lactobacilli in digested and fresh dairy manure at month 0, 2, 4, and 6 of storage in controlled climatic conditions (18 °C).
Clostridia concentrations did not show an overall effect of time of storage in dairy manure, fresh or digested. In pig-digested slurry, clostridia population increased during storage time, with a significant growth from month 0 to month 6. This increase was probably due to the observed reduction of the competitor microorganisms that in normal conditions can inhibit the revitalization of Clostridium spores.
Coliform concentrations in dairy were affected by manure type (fresh vs. digested, P < 0.001) and storage time (P < 0.05); an interaction type for storage time was detected (P < 0.01). Similar counts were measured at the end of storage time for dairy manure and at the beginning of digestate storing time.
This concentration did not vary significantly during the 6 months of storage of the digested manure (2.16 vs. 2.32 log10 CFU/g), while the coliform concentration measured in fresh manure decreased significantly at the end of storage time (5.50 log10 CFU/g at month 0 and 2.01 log10 CFU/g at month 6; P < 0.001). Coliform concentrations was lowered significantly (P < 0.01) by storage in pig raw slurry, from 4.26 log10 CFU/g at month 0 to 1.69 log10 CFU/g at months 4 and 6.
Streptococci concentration in dairy differed significantly in the type of manure (digested vs. fresh manure, P < 0.001) and according to the month of storage (P < 0.05).
Streptococci concentration in digested manure did not vary in a significant way, while they were reduced significantly in fresh manure from month 0 (6.10 log10 CFU/g) to month 2, month 4 (P < 0.01), and at the end of storage (4.31 log10 CFU/g; P < 0.05). In swine slurry, streptococci decreased significantly (5.59 vs. 1.84 log10 CFU/g; P < 0.001), as in digestate samples (4.39 vs. 1.70 log10 CFU/g; P < 0.001).
The statistical analysis revealed an overall significant effect of dairy manure type (fresh vs. digested, P < 0.001) and storage time (P < 0.05) on lactobacilli.
Lactobacilli concentration in fresh manure was measured in 4.81 log10 CFU/g at the month 0 and 2.13 log10 CFU/g at month 6 (P < 0.001), although they showed a non-linear trend. In digestate, this concentration did not vary during all the periods of storage in digested cattle manure.
Pig slurry and digestate concentrations of Lactobacilli were affected by time of storage.
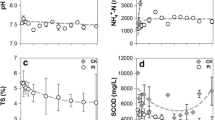
The chemical characteristics of the stored slurries were also monitored. Results (Table 1) showed, as it was expected, a remarkable increase of the total solids due to the physiological dehydration of slurry during the storage. The volatile solids amount was higher in dairy wastes and decreased in time.
The FOS/TAC ratio (FOS are the volatile organic acids, expressed as milligrams per liter of CH3COOH; TAC is the buffer capacity, expressed as milligrams per liter of CaCO3) decreased rapidly, showing the degradation of the volatile acids probably due to a slow biological degradation; pH increased over time.
The Pearson correlation coefficient analysis (Table 2) confirmed that the reduction of coliforms, streptococci, and lactobacilli could be linked to the pH and the FOS/TAC ratio. A diminishing concentration of streptococci resulted inversely proportional to pH (r = − 0.48, P < 0.001), showing that when pH lowered, streptococci concentration increased. On the contrary, clostridia resulted directly proportional to pH (r = 0.33, P < 0.05); their concentration increased with raising pH values.
Discussion
In this study, the results demonstrate an overall significant effect of the anaerobic digestion on the bacterial load of the microbial concentration of indicator microorganisms, except for clostridia.
Anaerobic mesophilic digestion increased clostridia population in pig digested slurry in time (P < 0.01), with a significant increase from the month 0 to the month 6 (P < 0.01). Anaerobic mesophilic digestion did not reduce clostridia levels in cattle digestates, in agreement with Abdelgadir et al. (2014), who found that even thermophilic anaerobic digestion successfully reduced Salmonella spp. and Escherichia coli but not Clostridium perfringens spores.
Their resistance probably depends on their capability of producing endospores, while the observed increase was probably due to the spore re-germination linked to the lowering of the concentration of other bacteria. Similar results were reported by Kearney et al. (1993), Watanabe et al. (1997), and Sahlström (2003).
Due to their spore forming capacity, Clostridium spp. as well as other spore forming bacteria are very resistant. Spores can survive for many years in the environment; many severe diseases are caused by Clostridium spp., such as tetanus (Clostridium tetani), botulism, (Clostridium botulinum), and blackleg (Clostridium chauvoie) (Hirsh and Zee 1999).
The failure in clostridia reduction after anaerobic digestion and storage should be particularly considered, since two bacterial genera, Eubacterium and Clostridium, are most likely the major contributors to odorous volatile fatty acids: it is actually difficult to obtain an effective reduction of clostridia through a simple microbiological process, in agreement with studies performed by Zhu (2000) and Chauret et al. (1999).
Coliforms and the other indicators were considerably reduced by anaerobic digestion treatment, in agreement with Sobsey (1998). In our study, a greater reduction of the investigated bacteria, with the exception of clostridia, was observed in stored wastes in comparison with digested samples, in particular way in pig slurry, considering the initial bacteria concentrations and the final reduction values after the two treatments. These results are in agreement with findings by Pandey et al. (2015) that showed that aerobic processes can be more effective in eliminating pathogens, in comparison with anaerobic digestion. However, in our study, bacteria were reduced but not eliminated. Elimination of bacteria depends on several factors, pH, temperature, availability of nutrients, and also on their initial amount in the waste (Strauch 1991).
The beneficial effects of the anaerobic treatment on the environment should also be taken into account for the reduction of emissions of greenhouse gases, such as methane and nitrous oxide (Møller et al. 2009). In addition, it contributes to reduce global warming, not only from the substitution of fossil fuel by biogas but also from carbon storage in the soil and inorganic fertilizer substitution (Møller et al. 2009).
Storage results highlighted its efficiency to lower the concentration of different microorganisms, especially in fresh manure and slurry, with the exception of Clostridium.
Storage applied after anaerobic digestion lowered lactobacilli and streptococci counts, but only in swine digestates, probably for the already lower counts of these bacteria at the beginning of storage in cattle digestates after the higher temperature of the anaerobic treatment in the cattle farms (Wang et al. 2004).
The substantial reductions of coliform concentration (2.56 log for pig slurry and 3.43 log for dairy manure) are in agreement, although in a less satisfactory way, with a study performed by Coté et al. (2006), who found that a 1-month batch storage of liquid swine manure was sufficient to obtain a 90% reduction of Escherichia coli populations. A storage of 2–4 months can easily reduce fecal indicator microorganism reduction in pig slurries and digestates. Gibbs et al. 1995, reported at least a T90 of 2 weeks for E. coli, of 2.7 weeks for enterococci in storage at 18 °C.
Our results confirmed that prolonged isolated storage for 3–6 months before land spreading, usually performed in Italy, allows the number of pathogens in manure to decrease but not to totally disappear. These limited, although beneficial, results are in agreement with studies of Gibbs et al. (1995) and Martinez et al. (2009).
The correlation coefficient analysis revealed a significant positive relationship between the pH and the bacteria concentrations included in this trial, except for clostridia: coliforms, streptococci, and lactobacilli resulted significantly lowered by pH increase (r = − 033, r = − 0.48, and r = − 0.44, respectively), as it was expected. According to a study performed by Pearson et al. (1987), fecal coliforms in waste ponds reduce more rapidly as the pH increase above 8.50, a particularly large increase in their die-off usually occur when the pH raises from 8.50–8.75 to pH 9.0.
Other researchers showed that extremes in pH are detrimental to organism survival; Parhad and Rao (1974) observed that Escherichia coli counts, in stabilization ponds, declined rapidly at pH above 9.3. More generally, a neutral pH environment seems to favor extended bacterial survival; and acid and alkaline conditions in water can greatly increase fecal coliforms decay rates (McFeters and Stuart 1972). Clostridia concentration seemed to grow with pH raising (r = 0.33). The FOS/TAC ratio was directly correlated with coliforms, streptococci, and lactobacilli concentrations. No references are available with this finding, so further studies are needed to evaluate the relationship of these bacteria levels and FOS/TAC ratios.
Considering the purpose of reusing digested and stored manure and slurry as fertilizers in agriculture, it is important to highlight that the microbiological quality of the samples analyzed in this study did not comply with the microbial parameter thresholds of the Italian law for fertilizers (Escherichia coli < 1000 CFU/g, D.M. 29819/2009).
At this point, an accurate supervision can allow a safe agronomic utilization both of the treated solid and the liquid fractions, limiting the spreading of potentially dangerous materials and improving a sustainable agriculture (Nicholson et al. 2005; Côté et al. 2006).
Conclusions
Anaerobic digestion and storage of dairy and swine manures are confirmed to be effective techniques to limit the presence of coliforms, streptococci, and lactobacilli, with exception of clostridia. Storage was particularly effective on bacteria reduction in fresh manure, also affecting several chemical-physical parameters. Correlations were identified between these parameters and microorganism levels. Further studies are needed to examine in depth the possibility of modeling the fate of indicators and pathogens as a function of the physical-chemical parameters, such as pH and FOS/TAC ratio.
References
Abdelgadir A, Chen X, Liu J, Zhang J, Zhang K, Wang H, Liu N (2014) Characteristics, process parameters, and inner components of anaerobic bioreactors. Biomed Res Int 2014:841573
APHA, AWWA, WEF (2005) Standard methods for the examination of water and wastewater, 22th edn. Water Environment Federation publishing, Alexandria
Bendixen HJ (1999) Hygienic safety––results of scientific investigations in Denmark Sanitation requirements in Danish BGPs. In: Bohm, R., Wellinger, A. (Eds.), Hygienic and environmental aspects of anaerobic digestion: legislation and experiences in Europe, Stuttgart-Hohenheim, pp. 27–47
Bicudo JR, Goyal SM (2003) Pathogens and manure management systems: a review. Environ Technol 24(1):115–130
Biswas S, Pandey PK, Farver TB (2016) Assessing the impacts of temperature and storage on Escherichia coli, Salmonella, and L. monocytogenes decay in dairy manure. Bioprocess Biosyst Eng 39:901–913
Blau DM, McCluskey BJ, Ladely SR, Dargatz DA, Fedorka-Cray PJ, Ferris KE, Headrick ML (2005) Salmonella in dairy operations in the United States: prevalence and anti-microbial drug susceptibility. J Food Prot 68:696–702
Chauret C, Springthorpe S, Sattar S (1999) Fate of Cryptosporidium oocysts, Giardia cysts, and microbial indicators during wastewater treatment and anaerobic sludge digestion. Can J Microbiol 45:257–262
Cho S, Bender JB, Diez-Gonzalez F, Fossler CP, Hedberg CW, Kaneene JB, Ruegg PL, Warnick LD, Wells SJ (2006) Prevalence and characterization of Escherichia coli O157:H7 isolates from Minnesota dairy farms and county fairs. J Food Prot 69:252–259
Côté C, Massé DI, Quessy S (2006) Reduction of indicator and pathogenic microorganisms by psychrophilic anaerobic digestion in swine slurries. Bioresour Technol 97:686–691
Coté C, Villeneuve A, Lessard L, Quessy S (2006) Fate of pathogenic and nonpathogenic microorganisms during storage of liquid hog manure in Quebec. Livest Sci 102:204–210
Cummings KJ, Warnick LD, Alexander KA, Cripps CJ, Gröhn YT, McDonough PL, Nydam DV, Reed KE (2009) The incidence of salmonellosis among dairy herds in the north-eastern United States. J Dairy Sci 92:3766–3774
D.M 18 dicembre 2009. Aggiornamento del decreto ministeriale 22 gennaio 2009, n. 1601, recante: Aggiornamento degli allegati del decreto legislativo 29 aprile 2006, n. 217, concernente la revisione della disciplina in materia di fertilizzanti. (Decreto n. 29818)
De Luca G, Zanetti F, Fateh-Moghadm P, Stampi S (1998) Occurrence of Listeria monocytogenes in sewage sludge. Zentr bl Hyg Umweltmed 201:269–277
Dumontet S, Dinel H, Baloda SB (1999) Pathogen reduction in sewage sludge by composting and other biological treatments: a review. Biol Agric Hortic 16:409–430
EPA (1998) Method EPA 3051: microwave assisted acid digestion of sediments, sludges, soils and oils. EPA, Washington, DC
Espensen B (1996) Praktiske forsog med smitstofreducerende bahandling af husholdningsaffald. DanskVet Tidsskr 79(14):615–622
Frank C, Werber D, Cramer JP, Askar M, Faber M et al (2011) Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 365:1771–1780
Gibbs RA, Hu CJ, Ho GE, Phillips PA, Unkovich I (1995) Pathogen die-off in stored wastewater sludge. Water Sci Technol 31(5–6):91–95
Hancock DD, Besser TE, Kinsel ML, Tarr PI, Rice DH, Paros MG (1994) The prevalence of Escherichia coli O157 H7 in dairy and beef cattle in Washington State. Epidemiol Infect 113:199–207
Hirsh DC, Zee YC (1999) Veterinary microbiology. Blackwell Science, Inc, Oxford
Huston CL, Wittum TE, Love BC, Keen JE (2002) Prevalence of fecal shedding of Salmonella spp. in dairy herds. J Am Vet Med Assoc 220:645–649
Hutchison ML, Walters LD, Avery SM, Synge BA, Moore A (2004) Levels of zoonotic agents in British livestock manure. Lett Appl Microbiol 39:207–214
Hutchison ML, Walters LD, Avery SM, Munro F, Moore A (2005) Analyses of livestock production, waste storage, and pathogen levels and prevalences in farm manures. Appl Environ Microbiol 71(3):1231–1236
ISTAT (2010) 6° Censimento Generale dell’Agricoltura (in Italian). ISTAT, Rome Retrieved on: http://censimentoagricoltura.istat.it/
Kearney TE, Larkin MJ, Frost JP, Levett PN (1993) Survival of pathogenic bacteria during mesophilic anaerobic digestion of animal waste. J Appl Bacteriol 75:215–219
Krause DO, Hendrick S (2011) Zoonotic pathogens in the food chain. CABI Publishing, Wallingford
Lahav O, Morgan B, Loewenthal RE (2002) Rapid, simple, and accurate method for measurement of VFA and carbonate alkalinity in anaerobic reactors. Environ Sci Technol 6:2736–2741
Larsen HE, Munch B, Olsen JE, Nansen P (1989) Om smitsofdrab og smitterisici ved drådning af husdyrgödning i biogasanlaeg. Dansk Vet Tidskrift 72(24):1411–1418
Mainar-Jaime RC, Andrés S, Vico JP, San RB, Garrido V, Grilló MJ (2013) Sensitivity of the ISO 6579:2002/Amd 1: 2007 standard method for detection of Salmonella spp. on mesenteric lymph nodes from slaughter pigs. J Clin Microbiol 51(1):89–94
Malakoff D (2002) Water quality: microbiologists on the trail of polluting bacteria. Science 295(5564):2352–2353
Martinez J, Burton C (2003) Manure management and treatment: an overview of the European situation. In: Saltijeral J (ed) Proceedings of the XI international congress in animal hygiene ISAH 2003. University of Autonoma Metropolitana, Mexico, p 119–133
Martinez J, Dabert P, Barrington S, Burton C (2009) Livestock waste treatment systems for environmental quality, food safety, and sustainability. Bioresour Technol 100:5527–5536
McFeters GA, Stuart DG (1972) Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol 24(5):805–811
Møller J, Boldrin A, Christensen TH (2009) Anaerobic digestion and digestate use: accounting of greenhouses gases and global warming contribution. Waste Manag Res 27:813–824
Mubiru DN, Coyne MS, Grove JH (2000) Mortality of Escherichia coli O157:H7 in two soils with different physical and chemical properties. J Environ Qual 29(6):1821–1825
Murinda SE, Nguyen LT, Ivey SJ, Gillespie BE, Almeida RA, Draughon FA, Oliver SP (2002) Prevalence and molecular characterization of Escherichia coli O157:H7 in bulk tank milk and fecal samples from cull cows: a 12-month survey of dairy farms in east Tennessee. J Food Prot 65:752–759
Newell DG, Koopmans M, Verhoef L, Duizer E, Aidara-Kane A, Sprong H, Opsteegh M, Langelaar M, Threfall J, Scheutz F, van der Giessen J, Kruse H (2010) Food-borne disease—the challenge of 20 years ago still persists while new ones continue to emerge. Int J Food Microbiol 130:S3–S15
Nicholson FA, Groves SJ, Chambers BJ (2005) Pathogen survival during livestock manure storage and following land application. Bioresour Technol 96:135–143
Ogden ID, Fenlon DR, Vinten AJA, Lewis D (2001) The fate of Escherichia coli O157:H7 in soil and its potential to contaminate drinking water. Int J Food Microbiol 66(1/2):111–117
Olsen JE, Larsen HE (1987) Bacterial decimation times in anaerobic digestions of animal slurries. Biol Wastes 21:153–168
Pachepsky YA, Sadeghi AM, Bradford SA, Shelton DR, Guber AK, Dao T (2006) Transport and fate of manure-borne pathogens: modeling perspective. Agric Water Manage 86:81–92
Pandey PK, Soupir ML (2012) Non-point source pollution. Berkshire encyclopedia of sustainability: ecosystem management and sustainability. Berkshire Publishing Group, LLC, Great Barrington
Pandey PK, Soupir ML (2013) Assessing the impacts of E. coli laden streambed sediment on E. coli loads over a range of flows and sediment characteristics. J Am Water Resour Assoc 49(6):1261–1269
Pandey PK, Soupir ML, Rehmann CR (2012) A model for predicting resuspension of Escherichia coli from streambed sediments. Water Res 46:115–126
Pandey PK, Kass PH, Soupir ML, Biswas S, Singh VP (2014) Contamination of water resources by pathogenic bacteria. AMB Express 4:51 http://www.amb-express.com/content/4/1/51
Pandey PK, Biswas S, Vaddella VK, Soupir ML (2015) Escherichia coli persistence kinetics in dairy manure at moderate, mesophilic, and thermophilic temperatures under aerobic and anaerobic environments. Bioprocess Biosyst Eng 38:457–467
Parhad NM, Rao UN (1974) Effect of pH on survival of Escherichia coli. J Water Pollut Control Fed 46:980–986
Pearson HW, Mara DD, Mills SW, Smallman DJ (1987) Physico-chemical parameters influencing faecal bacterial survival in waste stabilization ponds. Water Sci Tech 19(12):145–152
Pell A (1997) Manure and microbes: public and animal health problem? J Dairy Sci 80:2673–2681
Rogers SW, Donnelly M, Peed L, Kelty CA, Mondal S, Zhong Z, Shanks OC (2011) Decay of bacterial pathogens, fecal indicators, and real-time quantitative PCR genetic markers in manure-amended soils. Appl Environ Microbiol 77(4):4839–4848
Sahlström L (2003) A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresour Technol 87(2):161–166
Semenov AV, van Overbeek L, Termorhuizen AJ, van Bruggen AHC (2011) Influence of aerobic and anaerobic conditions on survival of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in Luria-Bertani broth, farm-yard manure and slurry. J Environ Manag 92:780–787
Smith RJ, Twedt RM, Flanigan LK (1973) Relationships of indicator and pathogenic bacteria in stream waters. J Water Pollut Control Fed 45(8):1736–1745
Sobsey MD (1998) Pathogens, fecal indicators and animal waste. In Proc. Of the Annual NC Water Resources Research Conference; April 1, College of Agriculture and Life Sciences; North Carolina State University, Raleigh, NC, 11–12
Strauch D (1991) Survival of pathogenic microorganisms and parasites in excreta, manure and sewage sludge. Rev Sci Tech Off Int Epiz 10(3):813–846
Toth JD, Aceto HW, Rankin SC, Dou Z (2013) Survey of animal-borne pathogens in the farm environment of 13 dairy operations. J Dairy Sci 96(9):1–6
Tozzoli R, Morabito S (2014) The collaborative study for the validation of the ISO 16654:2001. Istituto Superiore di Sanità - Dip. Sanità Pubblica Veterinaria e Sicurezza Alimentare - EU Reference Laboratory for E. coli. Reported at the 21st meeting of CEN/TC 275/WG 6 25–27 June 2014 Washington – USA
Vanotti MB, Patricia D, Millner PD, Hunt PG, Ellison AQ (2005) Removal of pathogen and indicator microorganisms from liquid swine manure in multi-step biological and chemical treatment. Bioresour Technol 96:209–214
Wang L, Mankin KR, Marchin GL (2004) Survival of fecal bacteria in dairy cow manure. Trans ASAE 47(4):1239–1246
Watanabe H, Kitamura T, Ochi S, Ozaki M (1997) Inactivation of pathogenic bacteria under mesophilic and thermophilic conditions. Water Sci Technol 36(6–7):25–32
Watcharasukarn M, Kaparaju P, Steyer JP, Krogfelt KA, Angelidaki I (2010) Screening Escherichia coli, Enterococcus faecalis, and Clostridium perfringens as indicator organisms in evaluating pathogen-reducing capacity in biogas plants. Microb Ecol 58:221–230
Wells JG, Shipman LD, Greene KD, Sowers EG, Green JH, Cameron DN, Downes FP, Martin ML, Griffin PM, Ostroff SM, Potter ME, Tauxe RV, Wachsmuth IK (1991) Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J Clin Microbiol 29:985–989
Zhao T, Doyle MP, Shere J, Garber L (1995) Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl Environ Microbiol 61:1290–1293
Zhu J (2000) A review of microbiology in swine manure odor control. Agric Ecosyst Environ 78:93–106
Ziemer CJ, Bonner JM, Cole D, Vinjé J, Constantini V, Goyal S, Gramer M, Mackie R, Meng XJ, Myers G, Saif LJ (2010) Fate and transport of zoonotic, bacterial, viral and parasitic pathogens during swine manure treatment, storage, and land application. J Anim Sci 88:84–94
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Diane Purchase
Capsule abstract
The present study is aimed to evaluate the effect of anaerobic treatment and storage time on bacteria concentration reductions in swine and dairy manure.
Rights and permissions
About this article
Cite this article
Costa, A., Gusmara, C., Gardoni, D. et al. The effect of anaerobic digestion and storage on indicator microorganisms in swine and dairy manure. Environ Sci Pollut Res 24, 24135–24146 (2017). https://doi.org/10.1007/s11356-017-0011-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0011-5