Abstract
To further treat the reclaimed municipal wastewater and rehabilitate the aquatic ecosystem of polluted urban rivers, an 18.5-km field-scale ecological restoration project was constructed along Jialu River, a polluted urban river which receives only reclaimed municipal wastewater from Zhengzhou City without natural upland water dilution. This study investigated the potential efficiency of water quality improvement, as well as genotoxicity and cytotoxicity reduction along the ecological restoration project of this polluted urban river. Results showed that the chemical oxygen demand (COD) and ammonia nitrogen (NH3-N) of the reclaimed municipal effluent were reduced by more than 45 and 70%, respectively, meeting the Chinese surface water environmental quality standard level IV, while the total phosphorus and metal concentrations had no significant reduction along the restoration project, and Pb concentrations in all river water samples exceeded permissible limit in drinking water set by WHO (2006) and China (GB5749-2006). The in vitro SOS/umu assay showed 4-nitroquinoline-1-oxide equivalent (4-NQO-EQ) values of reclaimed municipal wastewater of 0.69 ± 0.05 μg/L in April and 0.68 ± 0.06 μg/L in December, respectively, indicating the presence of genotoxic compounds. The results of 8-hydroxy-2′-deoxyguanosine (8-OHdG) and hepatic cell apoptosis in zebrafish after a chorionic long-term (21 days) in vivo exposure also demonstrated that the reclaimed municipal wastewater caused significant DNA oxidative damage and cytotoxicity. After the ecological purification of 18.5-km field-scale restoration project, the genotoxicity assessed by in vitro assay was negligible, while the DNA oxidative damage and cytotoxicity in exposed fish were still significantly elevated. The mechanisms of DNA oxidative damage and cytotoxicity caused by the reclaimed municipal wastewater need further study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Along with accelerating and rapidly growing urbanization and industrialization of China, demand and usage of accessible runoff from urban rivers both by municipalities and industries are increasing. In recent years, more than 3 × 107 t of reclaimed wastewater from municipal wastewater treatment plants (MWPs) were discharged into urban rivers per year (Yi et al. 2011). Many urban freshwater bodies are receiving these wastewaters, and downstream aquatic ecosystems have experienced considerable degradation. In order to further improve the water quality of reclaimed municipal wastewater and rehabilitate the aquatic ecosystems of polluted urban rivers, the advanced treatment of reclaimed municipal wastewater using ecological technology has gained attention in China, as the treatment is less costly and requires less space and time compared to other advanced technologies. Sheng et al. (2012) reported that chemical oxygen demand (COD) was reduced from 250 to 50 mg/L, and ammonia nitrogen (NH3-N) was reduced from 27 to 4 mg/L along a 23-km river remediation project in the Shijin River, North China. In another separate field-scale experiment, COD concentrations were reduced by 70% in an ecologically restored urban river in Guangzhou City, South China (Sheng et al. 2012, 2013). Although the COD of municipal wastewater was significantly reduced in the Shijin River, there were still a number of contaminants left that cannot be removed (Sheng et al. 2012, 2013).
Despite the fact that most of the synthetic chemical contaminants present in municipal wastewater are at very low and fluctuating concentrations, reclaimed wastewater from MWPs can still pose genotoxicity and cytotoxicity to organisms (Kaur et al. 2014; Wang et al. 2007, 2013; Wu et al. 2010). While it has been shown that advanced treatment reduces the genotoxicity and cytotoxicity of reclaimed wastewater in an ecologically restored urban river, a basic understanding of this process is still lacking and its extent (Schwarzenbach et al. 2006). A large number of micropollutants that cause genotoxicity are present in the reclaimed municipal wastewater; however, they typically occur at low concentrations or at levels below the detection limit, which makes it difficult to estimate the genotoxicity by chemical analytical detection methods. In recent decades, several bioassays, including the micronucleus test, the SOS/umu test, and the Ames test, have been successfully used to assess the genotoxicity of wastewater (Mathur et al. 2007). The SOS/umu test is frequently used as a short-term, high-throughput in vitro genotoxicity assay for detecting DNA-damaging agents (Xu et al. 2014). Using the SOS/umu test, Magdeburg et al. (2014) found that some pollutants in MWP effluents could induce genotoxicity. Margot et al. (2013) also found that the metabolites of some pollutants in municipal wastewater could lead to genotoxic effects by using SOS/umu test. In recent years, in vivo genotoxicity assays gradually gained attention, as it can provide more information about the actual ecological risk (Roos and Kaina 2006; Valavanidis et al. 2009). In animal tissues and cells, DNA is the main target for environmental geno-toxins, and a predominant and stable form of an oxidative product of DNA, 8-hydroxy-2′-deoxyguanosine (8-OHdG), has been widely used as a biomarker for oxidative stress and DNA mutagenesis risks in animals (Esperanza et al. 2015). Flow cytometry (FCM) is another effective way to investigated necrosis apoptosis and the genotoxicity. Using FCM, Xian et al. (2011) found that cells exposed to 10 mg/L NH3-N for 24 h showed a significant increase in apoptosis and genotoxicity (Xian et al. 2011). Cytotoxicity and genotoxicity of prooxidant herbicides to the microalga Chlamydomonas was also demonstrated by using the FCM (Esperanza et al. 2015). There are many studies that investigated the relationship of DNA damage induced apoptosis and genotoxicity of pure toxic substances such as tributyltin and certain polycyclic aromatic hydrocarbons with in vitro bioassays (CHAKRABARTI 2003; Li et al. 2015).
Jialu River, which is a polluted urban river flowing through Zhenzhou City and represents a tributary of the Huaihe River basin in North China, is estimated to receive about 200 kt of reclaimed wastewater from Zhengzhou City, without any natural upland water dilution. The COD and NH3-N concentrations in Jialu River did not reach the level V value of Chinese surface water environmental quality standard (GB 3838-2002), which may lead to serious ecological risk. To further treat the reclaimed municipal wastewater and rehabilitate the aquatic ecosystem of the Jialu River, an 18.5-km field-scale ecological restoration project was implemented between 2009 and 2011. The river channel was newly reconstructed, and the channel was made wider (120–170 m) than before (30–60 m). Diverse species of aquatic plants, such as Acorus calamus L., Cyperus imbricatus Retz., Ceratophyllum demersum L., and Scirpus tabernaemontani Vahl, were planted along the restoration site in the Jialu River. Solar aerators at the Xiangyunsi section and rubber dams were used to add dissolved oxygen (DO) to river water (Supplementary materials). This study aimed to investigate the potential efficiency of the reclaimed municipal wastewater along the 18.5-km field-scale ecological restoration project to improve water quality, and to reduce genotoxicity and cytotoxicity of conventionally treated wastewater.
Materials and methods
A field-scale ecological restoration project along the Jialu River
The study was conducted in a segment of the Jialu River in northern China (Fig. 1). Specifically, the drainage systems designed to treat wastewater and help restore the habitats were in between site S0 (Shijiahe section, the first rubber dam, 34° 52′ 13.5 N, 113° 34′ 02.1″ E) and site S3 (Xiangyunsi section, 18.5 km to S0, 34° 52′ 09.1″ N, 113° 43′ 30.3″ E) with a total length of 18.5 km. There are two more sites along the channel were selected for this study, which are site S1 (the second rubber dam, 7.7 km to S0, 34° 52′ 12.2″ N, 113° 36′ 04.2″ E) and site S2 (the third rubber dam,11.7 km to S0, 34° 52′ 12.2″ N, 113° 36′ 04.2″ E) (Fig. 1). The average river flow upstream of the sewage treatment works was 216.5∼328.9 L/s, and nearly 100% of river flow was reclaimed municipal wastewater from Zhengzhou City, without dilution by natural runoff.
Water sample collection
Water samples were collected every day at 0.5 m below the water surface between December 10 and 18 (air −2–5 °C, water 2–3 °C) of 2013 and between April 1 and 8 (air 4–9 °C, water 5–6 °C) of 2014 from sites S0 to S3. There was no rainfall on any of the sampling days. The hydraulic retention times (HRTs), average length of time the municipal wastewater remains in the 18.5 km stretch, between December 10 and 18, 2013, were 40.7 h (S0), 42.6 h (S1), 44.8 h (S2), and 40.2 h (S3), respectively. The HRTs between April 1 and 8, 2014, were 40.2 h (S0), 43.5 h (S1), 45.2 h (S2), and 39.6 h (S3), respectively.
Conventional water quality measurement
Water samples were first filtered through a 0.45 μm GFF filter (Xinya Purify Apparatus Company, Shanghai, China) to remove suspended particles. The same samples were also used to measure routine water quality parameters including chemical oxygen demand (COD), NH3-N, total phosphorus (TP), and DO, which were determined using a DR2800 instrument (HACH, USA). Concentrations of metals and other elements, including Na, Ca, Mg, K, Ba, Fe, Al, Cu, Li, Mn, Ni, and Pb, were measured by inductive coupled plasma-atomic emission spectrometry (ICP-AES, J-A1100 Jarrel-Ash, USA). DO was determined using dissolved oxygen analyzer (Eutech DO700, ThermoFisher, USA). Water samples used for use in the in vivo toxicity assay were stored in glass bottles at 4 °C before being used for fish exposure within 48 h.
Genotoxicity by SOS/umu assay
After being filtered, 1 L water samples were transferred to C18 cartridges using a solid-phase extraction apparatus at a flow rate of 10 mL/min (Zhenxiang Commerce and Trade Co., Ltd., China). Ten milliliters of both acetone and dichloromethane were pumped into each cartridge at 2.0 mL/min for elution. Then, eluates were mixed in a scaled centrifuge tube, and air-dried under a slow nitrogen flow. The residues were then dissolved in 1 mL dimethyl sulfoxide (DMSO). The concentrated stock was used to prepare exposure solutions for in vitro genotoxicity assay.
The genotoxicity of water samples was evaluated following the SOS/umu method based on Salmonella typhimurium TA1535/pSK1002 (Yan et al. 2014). Briefly, bacterial cultures grown overnight were diluted ten times with fresh TGA medium (KeyGEN Biotech, Nanjing, China) and incubated at 37 °C for 1.5 h with shaking (150 rpm). The test was performed on a microplate with the mixture consisting of 20 μL of 10 × TGA (KeyGEN Biotech, Nanjing, China), 70 μL of bacterial culture, and 180 μL concentrated stock (180 μL DMSO used as control sample). The microplate was then incubated at 35 °C for 2 h with shaking (150 rpm). Then, the incubated mixture was diluted ten times with fresh TGA medium and was further incubated for 2 h. The absorbance at 595 nm was measured, and then, 20 μL O-nitrophenyl β-D-galactopyranoside (6 mg/mL solution) were added to the mixture and incubated for 20 min. After that, 50 μL Na2CO3 (1 mol/L solution) were added to terminate the reaction. Then, the absorbance was measured at 550 and 415 nm to calculate the genotoxicity. Toxicity tests were performed in triplicate. The activity of all samples was calculated by the following formula (Eq. 1):
4-Nitroquinoline-1-oxide (4-NQO), of which eight concentration gradients from 0.1 to 25 μg/L were set, was used as reference for the dose-response curve. The genotoxicity effect of water sample was then expressed as the equivalent concentration of 4-NQO (4-NQO-EQ, μg/L).
8-OHdG measured in zebrafish
Zebrafish (Danio rerio), which were obtained from the Institute of Hydrobiology of Chinese Academy of Sciences (Wuhan, China), were used for a 21-day exposure chronic toxicity test. Each individual used weighed between 0.25 and 0.3 g. Fish were first acclimatized in recirculating water for 3 weeks (25 ± 0.5 °C, 12: 12-h light/dark cycles). Fish were then exposed to water samples for 4 days to test their acute toxicity, and survival rate was monitored. Bioassays were conducted for 21 days chronic toxicity effects, while the control was exposed to MilliQ water with inorganic salt (CaCl2 = 2 mmol/L, MgSO4 = 0.5 mol/L, NaHCO3 = 0.5 mol/L, KCl = 0.05 mol/L) to maintain the osmotic pressure balance in fish cells (GB/T 21808-2008). A total of 60 zebrafish were used for each treatment of water samples.
After 21-day exposure, each fish was weighed and the liver was collected. Hepatic tissue samples were then homogenized with PBS (0.1 g liver with 1 ml PBS, pH = 7.4) by 2 mL cold liver grinder that was prestored at −20 °C in a freezer. The supernatant was then transferred into clean tubes and was used for in vivo genotoxicity assay. Total protein content was measured by the Bradford assay using serum albumin as standard (Bradford 1976; Sun et al. 2016). The concentration of 8-OHdG in zebrafish, which indicates hepatic oxidative DNA damage, was monitored using a zebrafish 8-OHdG enzyme-linked immunosorbent assay (ELISA) kit (Nanjing Jiancheng Bioeng Inst., China). Competitive ELISA for 8-OHdG was performed according to manufacturer’s protocol. Each experiment was performed in triplicate. The average concentration of 8-OHdG per microgram of DNA for each group was then calculated. The detection limits for 8-OHdG analysis using ELISA was 0.1 ng/L.
Cytotoxicity measured by flow cytometry
After 21-day exposure, ten zebrafish were randomly selected from each treatment group and their livers were aseptically isolated, and resuspended in cold 10 mM PBS buffer (137 mM NaCl, 2 mM KCl, 0.1%NaN3,pH = 7.2), and the blood vessels were carefully removed. Tissues were then digested by 0.25% pancreatin at 37 °C for 5 min, and 40 μL of trypsin-neutralized solution (KeyGen Biotech, Nanjing, China) was used to terminate the reaction. Then, approximately 1 × 105∼5 × 105 cells were collected by centrifugation at 2000 rpm for 5 min and washed by PBS twice. Cells were then stained with by 5 μL Annexin V (Annexin V-FITC Apoptosis Kit, KeyGEN Biotech, Nanjing, China) and 5 μL propidium iodide (PI) in 500 μL binding buffer and incubated for 5 min. Cells stained by both Annexin V and PI are necrotic cells, while those stained only by Annexin V represent apoptotic cells, cells neither stained by Annexin V nor PI are normal. Apoptosis and necrosis counts were then determined using FCM (BD Bioscience, USA).
Statistical analysis
Graphs were generated using GraphPad Prism 5.0 software (San Diego, CA, USA). All data were expressed as mean ± standard deviation (SD). Tests for normality and homogeneity of variances indicated that all the data were normally distributed and had homogeneic variance in SPSS 13.0. Then, the data were analyzed statistically using Dunnett’s multiple t test in SPSS 13.0. Statistical significance was set at a level of p (p ≤ 0.05).
Results and discussion
Conventional water quality improvement along the ecological restoration project
A summary of the conventional water quality determined in water samples collected along 18.5-km ecologically restored urban river is presented in Table 1. Results showed that conventional water quality was improved significantly through the restoration of 18.5-km field-scale ecological restoration project. The COD concentrations at S0 site samples were significantly higher than the level V values of Chinese surface water environmental quality standard (GB3838-2002, 2002). And the COD concentrations were significantly reduced from 81 ± 2 mg/L (in April) and 62 ± 3 mg/L (in December) at site S0 to 54 ± 1 mg/L (in April) and 50 ± 4 mg/L (in December) at site S1, respectively. At site S3, COD levels decreased to 39 ± 3 and 38 ± 1 mg/L in April and in December, respectively, hence meeting the IV level of Chinese surface water environmental quality standard (i.e., 30 ∼ 40 mg/L). NH3-N levels were also significantly reduced along the ecological restoration project, dropping from 4.7 ± 1.1 mg/L (April) and 4.2 ± 2.0 mg/L (December) at site S0 to 1.1 ± 0.1 mg/L (April) and 1.8 ± 0.1 mg/L (December) at site S3, which were better than the IV (1.5 mg/L) and V (2.0 mg/L) threshold levels of Chinese surface water standard (GB3838–2002). It was reported that NH3-N could induce production of reactive oxygen species at concentrations greater than 1 mg/L that could potentially damage DNA (Jiang et al. 2012; Xian et al. 2011). In this study, the NH3-N levels at S3 site were greater than 1 mg/L, which may indicate that the water posed the risk of DNA damaged to aquatic lives. In short, the COD and NH3-N concentrations were reduced by more than 45 and 70%, respectively. However, the TP in river samples collected from all four sampling sites did not change significantly (Table 1).
There are only a few reports on urban river remediation and restoration of effluents of municipal wastewater, and most of which are still based on laboratory work on constructed wetlands (Cao et al. 2012; Wu et al. 2011). An urban river after ecological restoration in Guangzhou (South China) was observed to naturally remove more than 70% COD from MWP effluents. Sheng et al. (2013) also reported that a field-scale experiment in an urban river in northern China removed 80% COD and decreased its levels to 50 mg/L at the end of the river. At the same time, it also reduced NH3-N from nearly 30 to 4 mg/L (Sheng et al. 2012, 2013). These two field-scale experiments demonstrated that COD and NH3-N in polluted urban rivers could be removed effectively by ecological restoration projects. DO is essential for the oxidation of pollutants (Wang et al. 2015), and thus helpful in ecosystem restoration in a river (Ning et al. 2014). In this study, the DO concentrations continuously and significantly increased along the ecological restoration project in Jialu River (Table 1). The cost of the Jialu River restoration was about 0.018 Yuan/t, which was mainly labor costs such as cleaning of the rubber dams, sampling and control, and site inspection. It requires less cost compared to other advanced treatment processes, such as ozonation, active carbon adsorption, and MBR (Rousseau et al. 2008).
Concentrations of most heavy metal elements such as Cu, Fe, Ni, and Pb had partly or slightly reduction through 18.5-km field-scale ecological restoration project, while the Mg, K, Na, Si, and Mn concentrations were not significantly changed (Table 2). Pb, Cr, Ni, Fe, and Cu also known to cause genotoxicity by damaging DNA or inhibiting fidelity of DNA synthesis (Kaur et al. 2014; Esperanza et al. 2015). The Pb concentrations in Jialu River exceeded permissible limit in drinking water set by WHO (2006) and China (GB5749-2006, 2006) at 0.01 mg/L. The permissible limit in drinking water set by WHO (2006) and China (GB5749-2006, 2006) of Ni, Fe, and Cu were 0.02, 0.3, and 2 mg/L, respectively (Kaur et al. 2014). The concentration of Ni at S3 was 0.011 ± 0.001 mg/L in April and 0.010 ± 0.001 mg/L in December; the concentration of Fe at S3 was 0.07 ± 0.01 mg/L in April and 0.08 ± 0.01 mg/L in December; the concentration of Cu at S3 was 0.011 ± 0.001 mg/L in April and 0.007 ± 0.001 mg/L in December. Although some metals were partly removed, the residual heavy metals, such as Pb, may still cause genotoxicity.
Genotoxicity by SOS/umu assay in vitro
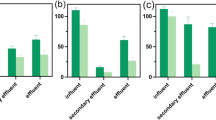
4-NQO-EQ values of site S0 samples were 0.69 ± 0.05 μg/L in April and 0.68 ± 0.06 μg/L in December, and slightly decreased to 0.50 ± 0.03 and 0.49 ± 0.04 μg/L at site S1, and further decreased to 0.27 ± 0.02 and 0.28 ± 0.01 μg/L at site S3, respectively (Fig. 2). The predicted no-effect concentration of 4-NQO was 0.64 μg/L 4-NQO, which was based on 12 no observed effect concentration data points of genotoxicity (carcinogenesis) obtained from a number of studies with fish, mice and cell lines following exposure to 4-NQO (Xu et al. 2014). In cases where 4-NQO equivalents exceeded 0.64 μg/L, as the case at site S0, the water samples were likely to pose genotoxic risk to aquatic life. The 4-NQO-EQs of site S3 samples in both April and in December ranged from 0.25 to 0.30 μg/L and were less than the PNEC value, which indicated that the genotoxicities of site S3 samples in Jialu River were reduced to be negligible after the purification of 18.5-km field-scale ecological restoration project.
DNA damage in zebrafish after 21-day exposure
8-OHdG levels in zebrafish exposed to site S0 water were 134.6 ± 8.0 ng/L in April and 132.2 ± 11.4 ng/L in December after 21 days of exposure, which were significantly greater than those of the control group. This revealed that exposure to the S0 water samples resulted in significant oxidative damage to DNA in zebrafish, indicating that the river water was genotoxic. These results were congruent with the results of the SOS/umu assay. Along the ecological restoration project stretch of the Jialu River, 8-OHdG levels in zebrafish were significantly decreased for water samples from sites S1 and S2, and that of site S3 further decreased to 63.6 ± 2.8 ng/L in April and 68.4 ± 3.4 ng/L in December (Fig. 3). Compared to the control group, 8-OHdG levels at site S3 showed a significantly greater genotoxicity than the control sample during both sampling seasons, which suggests that long-term exposure to effluent-dominated river water, even after treatment through the ecological restoration project, may still cause DNA damage in zebrafish. These results were different from those of the in vitro (SOS/mum) assay. These differences could be due to the following possibilities. Firstly, in SOS/mum in vitro assay experiments, some volatile organic pollutants could be lost during the nitrogen-drying process; in addition, NH3-N and metal ions were not captured by the enriching process using C18 cartridges (Magdeburg et al. 2014). It is then possible that the in vitro test undertaken in this study might not have been able to evaluate the toxic effects of NH3-N or heavy metals in water, while many of these pollutants can cause genotoxicity to zebrafish (Kaur et al. 2014; Xian et al. 2011). Secondly, S. typhimurium and zebrafish have very different metabolic capacity, as well as are characterized by different structural properties that can affect the uptake of chemicals. For example, the bacterial cell wall can prevent some micropollutants entering into the cells, while fish cells have no cell wall (Petit et al. 1995). Meanwhile, some chemicals could be metabolically activated in zebrafish, but not in bacteria. For example, many polycyclic and heterocyclic aromatic hydrocarbons, such as 3,4-diol-DB[a,j]A, had represented more genotoxicity in eukaryote cells rather than in S. typhimurium, as these chemical may be metabolic activated by cytochrome P450 enzymes (CYPs) which were only expressed in eukaryote cells (Xu et al. 2005). Lastly, zebrafish may bioaccumulate many pollutants in river water during 21-day exposure, which would result in greater exposure than in the short-term SOS/mum assay.
Cytotoxicity assay using FCM
Chakrabarti (2003) and Li et al. (2015) reported that DNA damage may lead to cytotoxicity, which can be induced by toxic pollutants such as tributyltin and polycyclic aromatic hydrocarbons (PAHs) (CHAKRABARTI 2003; Li et al. 2015). Excessive mitochondrial injury and oxidized DNA products such as 8-OHdG may directly cause apoptosis and necrosis in cells (Kultz 2005). Two cellular strategies have evolved for coping with DNA damage. First, DNA damage is repaired (Kultz 2005). Second, cells that contained DNA damage due to exposure to toxic substance are removed from the tissue mainly by apoptosis or necrosis. Nonrepaired DNA damage often has harmful consequences that manifest as gene mutations (or called genotoxicity) (Kultz 2005).
FCM has been shown to provide an objective, reproducible, and sensitive characterization of the cytotoxicity level in a large number of cells. However, very few studies have investigated the link between the percentage of necrosis and apoptosis and the genotoxicity level in natural water body with zebrafish bioassay. Zebrafish exposed for 21 days, were observed to have significantly upregulated concentrations of 8-OHdG (Fig. 3), which may indicate genotoxic effects of contaminants in the water samples tested. To better confirm the genotoxic effects in zebrafish, the FCM-based detection of necrosis and apoptosis were analyzed in hepatic cells. Results showed that the apoptosis percentages of hepatic cells of fish exposed to S0 water were 20.9 ± 1.1% in April and 18.9 ± 1.1% in December, and the necrosis percentages were 7.9 ± 0.3% in April and 6.7 ± 0.5% in December, which all were significantly greater than that of control group (Table 3). This indicated that the reclaimed municipal wastewater poses obvious cytotoxicity to zebrafish after 21 days of exposure. Although apoptosis and necrosis percentages of hepatic cells of fish exposed to site S1 and S2 water were significantly decreased compared to S0 water, they were still significantly greater than that of the control group, indicating the river water still posed cytotoxic risks to zebrafish. This is in agreement with the results of 8-OHdG in zebrafish. The apoptosis and necrosis percentages of fish exposed to S3 water had further decreased to 4.2 ± 0.6 and 2.4 ± 0.1% in April and 4.7 ± 0.5 and 2.2 ± 0.2% in December, respectively. The percentages of apoptosis at S3 during both sampling seasons were also significantly greater than those of the control, which indicates that the water still had genotoxic properties and caused DNA damage and DNA damage-induced apoptosis. Considering this, we hypothesized that the river water still posed cytotoxic and genotoxic risks to zebrafish.
For NH3-N could interfere with energy metabolism through impairment of the tricarboxylic acid cycle in the mitochondria and cause oxidative damage to cells, it may lead to multiple deleterious effects on aquatic lives (Chew and Ip 2014). Notably, many fishes may suffer from oxidative stress exposed to 1 mg/L NH3-N within 2 weeks, and this concentration of ammonia was not regarded as high environmental ammonia (Chew and Ip 2014; Jiang et al. 2012; Xian et al. 2011). In this study, the NH3-N concentrations in all water samples were more than 1 mg/L, indicating that all water samples might pose oxidative stress to zebrafish. As one of the most toxic heavy metals, prolonged Pb exposure could increase the toxicity effects in fish because Pb could be accumulated in fish from contaminated water (Alves and Wood 2006). Alsop et al. (2016) had examined the possible toxicity effects of Pb in juvenile rainbow trout and found that the 0∼97 μg/L Pb could be accumulated and would cause damage to the gill, liver, and kidney (Alsop et al. 2016). Jurczuk et al. (2006) had also found that Pb could enhance the concentration of malondialdehyde and reduce the glutathione concentrations, which might be the main mechanisms of the peroxidative action by Pb (Jurczuk et al. 2006). The Pb concentrations in S3 water samples were 57 μg/L in December and 45 μg/L in April, indicating the S3 might pose oxidative stress to zebrafish.
Another possibility of genotixicity in S3 is that the micropollutants causing genotoxicity present in the municipal wastewater may not be totally removed in the ecological restoration project. There is no direct evidence from literature that the ecological restoration project would remove the genotoxic organics, such as polycyclic aromatic hydrocarbons, some pharmaceuticals and so on. However, some literature had reported that the polycyclic aromatic hydrocarbons cannot be effectively removed in wetlands, and the water in wetlands may still pose genotoxicity and carcinogensis risks (Li et al. 2014; Zhang et al. 2011). The genotoxic organics in the ecological restoration project will be studied further.
Conclusions
This study showed that COD and NH3-N in reclaimed municipal wastewater could be effectively removed, and DO could be continuously and significantly increased along 18.5-km field-scale ecological restoration project in Jialu River. The results of SOS/umu, FCM, and 8-OHdG assay demonstrated that the reclaimed municipal wastewater could induce genotoxicity, obvious DNA oxidation, and cytotoxicity, which may correlate with the presence of NH3-N, exceedingly high concentrations of Pb and other pollutants. After the ecological purification of the 18.5-km field-scale restoration project, the genotoxicity assessed by in vitro assay was negligible, while the DNA oxidative damage and cytotoxicity level were still significant in exposed fish. The mechanisms of DNA oxidative damage and cytotoxicity caused by the reclaimed municipal wastewater need further study.
References
Alsop D, Ng TYT, Chowdhury MJ, Wood CM (2016) Interactions of waterborne and dietborne Pb in rainbow trout, Oncorhynchus mykiss: bioaccumulation, physiological responses, and chronic toxicity. Aquat Toxicol 177:343–354
Alves LC, Wood CM (2006) The chronic effects of dietary lead in freshwater juvenile rainbow trout (Oncorhynchus mykiss) fed elevated calcium diets. Aquat Toxicol 78:217–232
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao WP, Zhang HH, Wang YM, Pan JZ (2012) Bioremediation of polluted surface water by using biofilms on filamentous bamboo. Ecol Eng 42:146–149
CHAKRABARTI KKFT (2003) Genotoxic effects of PAH containing sludge extracts in Chinese hamster ovary cell cultures. Biomed Environ Sci 1:008
Chew SF, Ip YK (2014) Excretory nitrogen metabolism and defence against ammonia toxicity in air-breathing fishes. J Fish Biol 84:603–638
Esperanza M, Cid A, Herrero C, Rioboo C (2015) Acute effects of a prooxidant herbicide on the microalga Chlamydomonas reinhardtii: screening cytotoxicity and genotoxicity endpoints. Aquat Toxicol 165:210–221
GB3838-2002 (2002) The surface water environmental quality standard. The State Environmental Protection Administration of China. (in Chinese)
GB5749-2006 (2006) Standards for drinking water quality. The State Health Administration of China. (in Chinese)
Jiang Q, Lv L, Jiang G, Minter E, Wang Q, Huang W, Dong S, Yang J (2012) Acute effects of ammonia on antioxidative response and gill Na+/K+ ATPase activity of juvenile Australian red claw crayfish, Cherax quadricarinatus. Journal of Freshwater Ecology 27, 551–560
Jurczuk M, Moniuszko-Jakoniuk J, Brzóska MM (2006) Involvement of some low-molecular thiols in the peroxidative mechanisms of lead and ethanol action on rat liver and kidney. Toxicology 219:11–21
Kaur J, Chaudhary A, Kaur R, Arora S (2014) Assessment of mutagenic, genotoxic, and cytotoxic potential of water samples of Harike wetland: a Ramsar site in India using different ex vivo biological systems. Ecotoxicology 23:967–977
Kultz D (2005) Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257
Li G, Lang Y, Yang W, Peng P, Wang X (2014) Source contributions of PAHs and toxicity in reed wetland soils of Liaohe estuary using a CMB–TEQ method. Sci Total Environ 490:199–204
Li B, Sun L, Cai J, Wang C, Wang M, Qiu H, Zuo Z (2015) Modulation of the DNA repair system and ATR-p53 mediated apoptosis is relevant for tributyltin-induced genotoxic effects in human hepatoma G2 cells. J Environ Sci 27:108–114
Magdeburg A, Stalter D, Schliusener M, Ternes T, Oehlmann J (2014) Evaluating the efficiency of advanced wastewater treatment: target analysis of organic contaminants and (geno-)toxicity assessment tell a different story. Water Res 50:35–47
Margot J, Kienle C, Magnet A, Weil M, Rossi L, de Alencastro LF, Abegglen C, Thonney D, Chevre N, Scharer M, Barry DA (2013) Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? ScienceTotal Environ 461, 480-498
Mathur N, Bhatnagar P, Mohan K, Bakre P, Nagar P, Bijarnia M (2007) Mutagenicity evaluation of industrial sludge from common effluent treatment plant. Chemosphere 67:1229–1235
Petit F, Valotaire Y, Pakdel F (1995) Differential functional activities of rainbow-trout and human estrogen-receptors expressed in the yeast Saccharomyces-cerevisiae. Eur J Biochem 233:584–592
Roos WP, Kaina B (2006) DNA damage-induced cell death by apoptosis. Trends Mol Med 12:440–450
Rousseau DPL, Lesage E, Story A, Vanrolleghem PA, De Pauw N (2008) Constructed wetlands for water reclamation. Desalination 218, 181-189
Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, von Gunten U, Wehrli B (2006) The challenge of micropollutants in aquatic systems. Science 313:1072–1077
Sheng YQ, Chen FZ, Sheng GY, Fu JM (2012) Water quality remediation in a heavily polluted tidal river in Guangzhou, South China. Aquat Ecosyst Health 15:219–226
Sheng YQ, Qu YX, Ding CF, Sun QY, Mortimer RJG (2013) A combined application of different engineering and biological techniques to remediate a heavily polluted river. Ecol Eng 57:1–7
Sun J, Ji XW, Zhang R, Huang Y, Liang Y, Du JH, Xie XC (2016) Endocrine disrupting compounds reduction and water quality improvement in reclaimed municipal wastewater: A field-scale study along Jialu River in North China. Chemosphere: 157, 232-240
Valavanidis A, Vlachogianni T, Fiotakis C (2009) 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27:120–139
Wang LS, Hu HY, Wang C (2007) Effect of ammonia nitrogen and dissolved organic matter fractions on the genotoxicity of wastewater effluent during chlorine disinfection. Environ Sci Technol 41:160–165
Wang CC, Niu ZG, Zhang Y (2013) Health risk assessment of inhalation exposure of irrigation workers and the public to trihalomethanes from reclaimed water in landscape irrigation in Tianjin, North China. J Hazard Mater 262:179–188
Wang Q, Xie H, Zhang J, Liang S, Ngo HH, Guo W, Liu C, Zhao C, Li H (2015) Effect of plant harvesting on the performance of constructed wetlands during winter: radial oxygen loss and microbial characteristics. Environ Sci Pollut Res Int 22:7476–7484
WHO (2006) Guidelines for drinking-water quality, third edition,Word Health Organization
Wu QY, Hu HY, Zhao X, Li Y (2010) Effects of chlorination on the properties of dissolved organic matter and its genotoxicity in secondary sewage effluent under two different ammonium concentrations. Chemosphere 80:941–946
Wu HM, Zhang JA, Li PZ, Zhang JY, Xie HJ, Zhang B (2011) Nutrient removal in constructed microcosm wetlands for treating polluted river water in northern China. Ecol Eng 37:560–568
Xian JA, Wang AL, Chen XD, Gou NN, Miao YT, Liao SA, Ye CX (2011) Cytotoxicity of nitrite on haemocytes of the tiger shrimp, Penaeus monodon, using flow cytometric analysis. Aquaculture 317:240–244
Xu WL, Warshawsky D (2005): Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicology and Applied Pharmacology 206, 73-93
Xu JY, Zhao CT, Wei DB, Du YG (2014) A toxicity-based method for evaluating safety of reclaimed water for environmental reuses. J Environ Sci-China 26:1961–1969
Yan Y, Jiang WW, Li N, Ma M, Wang DH, Wang ZJ, Rao KF (2014) Assessing of genotoxicity of 16 centralized source-waters in China by means of the SOS/umu assay and the micronucleus test: initial identification of the potential genotoxicants by use of a GC/MS method and the QSAR Toolbox 3.0. Mutat Res-Gen Tox En 763:36–43
Yi LL, Jiao WT, Chen XN, Chen WP (2011) An overview of reclaimed water reuse in China. J Environ Sci-China 23:1585–1593
Zhang Z, Rengel Z, Meney K, Pantelic L, Tomanovic R (2011) Polynuclear aromatic hydrocarbons (PAHs) mediate cadmium toxicity to an emergent wetland species. J Hazard Mater 189:119–126
Acknowledgements
We gratefully acknowledge generous support provided by NSFC (41203062 and 51438008), Jiangsu Nature Science Fund (BK20151378), China’s National Key Project of Science and Technology (2012ZX07204-001; 2015ZX07204-007), and the Fundamental Research Funds for the Central Universities (090514380001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Markus Hecker
Electronic supplementary material
ESM 1
(DOCX 608 kb)
Rights and permissions
About this article
Cite this article
Sun, J., Zhang, R., Qin, L. et al. Genotoxicity and cytotoxicity reduction of the polluted urban river after ecological restoration: a field-scale study of Jialu River in northern China. Environ Sci Pollut Res 24, 6715–6723 (2017). https://doi.org/10.1007/s11356-016-8352-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8352-z