Abstract
Mercury (Hg) is a highly toxic and widely distributed metal that is bioaccumulated in insectivorous mammals and may cause adverse effects on the reproductive system. Bats are considered excellent Hg bioindicators due to their wide distribution, life span, trophic position, metabolic rate and food intake. However, few studies have analysed Hg residues in bats, and to the best of our knowledge, no studies have been made in the Iberian Peninsula. The main aim of this study was to undertake the first ever assessment of Hg exposure in Schreiber’s bent-winged bats inhabiting a natural cave in the southeast of Spain. The findings suggest that Schreiber’s bent-winged bats in the sampling area are chronically exposed to low levels of Hg. The Hg concentrations found in different tissues (fur, kidney, liver, muscle and brain) were below the threshold levels associated with toxic effects in mammals. Non-gestating females showed Hg concentrations in the brain and muscle that doubled those found in gestating females. This could be due to Hg mobilization from the mother to the foetus in gestating females, although other factors could contribute to explain this result such as variations in hunting areas and the insect-prey consumed and/or different energetic needs and average food consumption during the breeding season. Hg levels were 1.7 times higher, although not significant, in foetus’ brains than in the maternal brains, and Hg concentration in foetus’ brain was significantly correlated with levels in the corresponding mothers’ kidney. These results suggest that there could be an active mother-to-foetus transfer of Hg in bats, which would be of special relevance in a scenario of higher Hg exposure than that found in this study. However, further research is needed to support this view due to the limited number of samples analysed. Given the scarce ecotoxicological data available for bats and their protected status, we encourage further opportunistic studies using carcasses found in the field, the validation of non-destructive samples such as fur and guano for Hg monitoring, and new modelling approaches that will increase the data needed for proper ecological risk assessment in bat populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ecosystems are continuously threatened by contaminants such as metals, which are ubiquitous and occur naturally in the environment, so there are always background concentrations of these elements in the environment. Monitoring chronic exposure of animals and humans to toxic metals and evaluating their effects is a global health concern. During recent decades, the anthropogenic production of mercury has increased and subsequently the presence of this metal in the environment has also increased (Pacyna et al. 2001; Maxson 2005; Scheuhammer et al. 2007; Streets et al. 2009). Although ecosystems are efficient in the filtration and retention of many metals, Hg can be easily transferred through the aquatic and terrestrial systems, and it is readily available in food webs (Morel et al. 1998; Benoit et al., 2003; Ward et al. 2010). In this sense, it is well known that Hg in the form of methylmercury (MeHg) can enter the food web, where it biomagnifies along the food chain (Cristol et al. 2008; Jackson et al. 2011; Henderson et al. 2012; Syaripuddin et al. 2014; Yates et al. 2014). The adverse effects of environmental Hg on the health of wildlife, including mammals, have been widely reported (Wolfe et al. 1998; Scheuhammer et al. 2007). MeHg has been proved to be a potent neurotoxic metal, affecting the reproductive system of mammals, including bats (Wolfe et al. 1998; Scheuhammer et al. 2007; Nam et al. 2012; Syaripuddin et al. 2014; Yates et al. 2014). In addition, it is transferred across the placenta and is able to selectively concentrate in the foetal brain, where it may cause developmental alterations leading to foetal death (Wolfe et al. 1998). Moreover, lactational transfer of MeHg to offspring has also been observed (Nam et al. 2012; Yates et al. 2014; Wolfe et al. 1998).
Large spatial scale monitoring is useful for evaluating the exposure to this metal and its related effects on wildlife and humans (Schmeltz et al. 2011). Different wild animals are considered good bioindicators of contaminant exposure and are widely used in biomonitoring programs across the world (e.g. birds; García-Fernández et al. 2008; Sánchez-Virosta et al. 2015; Espín et al. 2016). Bats are recognized as good bioindicator species for metal pollution (Jones et al. 2009; Zukal et al. 2015), and may be considered excellent Hg bioindicators due to the fact that (1) many bat species are distributed across wide geographic ranges and, while individuals of several species live in habitats that are relatively pristine, other individuals of the same species live near heavily industrialized areas or point sources of Hg emission (Yates et al. 2014); (2) most bat species are relatively long-living (Kunz and Lumsden 2003; Brunet-Rossinni and Austad 2004; Dietz et al. 2009) and so Hg may accumulate with age (Yates et al. 2014); (3) many bats are at high trophic levels making them susceptible to biomagnification, especially insectivorous species (O’Shea and Johnston, 2009; Syaripuddin et al. 2014; Yates et al. 2014); and (4) bats may be exposed to higher Hg loads compared to other animals of similar size due to their high metabolic rate and food intake (Kurta et al. 1989; Streit and Nagel 1993a; Hickey et al. 2001; Wada et al. 2010). However, despite their potential as bioindicator organisms, Hg exposure and its potential detrimental effects are poorly documented in wild bat populations (Zukal et al. 2015; Hernout et al. 2016b).
The main aim of this study was to undertake the first ever assessment of Hg exposure in Schreiber’s bent-winged bats from the Iberian Peninsula. The Schreiber’s bent-winged bat (Miniopterus schreibersii) (Kuhl 1817) is a small-sized bat distributed across Europe, North Africa and Turkey. In the southeast of Spain, this species shows sexual size dimorphism, females being bigger than males (Lisón 2012). It is cave-dwelling and may appear in natural caves, tunnels and mines ranging 350–1000 m.a.s.l. (Lisón et al. 2011), but always near water bodies (Rainho and Palmeirim 2011). Its diet is composed of flying insects which it hunts in open areas and water bodies (Dietz et al. 2009). They form large colonies, especially in the breeding season when up to 30,000 bats may congregate (Lisón 2014). However, in the southeast of Spain, its population has declined by about 70% during the last two decades (Lisón et al. 2011). As suggested by other researchers, exposure to metals may be a potential stressor that affects bat populations that should be evaluated (Mickleburgh et al. 2002; Zukal et al. 2015).
Our specific aims were as follows: (1) to determine the Hg concentrations in different tissues of Schreiber’s bent-winged bats (fur, kidney, liver, muscle and brain), (2) to study the differences in Hg concentrations according to sex and reproductive status (gestating and non-gestating females), and (3) to evaluate the mother-foetus transfer of Hg during pregnancy. For this purpose, it is important to consider that many bat species, including the Schreiber’s bent-winged bat, are rare or threatened and their protection status limits the acquisition of samples, meaning that they are frequently unavailable.
Material and methods
Study area and sample collection
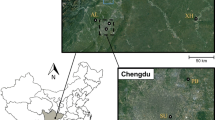
Las Yeseras Cave (1°1′45″ N; 38°4′52″ W) is located in the northeast of Murcia province, southeast of Spain (Fig. 1). It is a natural cave in a karstic zone with a large citric plantation around it, and a large number of ponds and a dam. This cave has a substantial colony of Schreiber’s bent-winged bat composed of at least 3000 individuals, and it is catalogued as Special Area of Conservation being part of the Natura 2000 network (Lisón et al. 2011). The agricultural soils in this semi-arid region have metal concentrations following the sequence Fe > Mn > Zn > Cr > Ni > Cu > Pb > Co > Cd, and concentrations are similar to those found in other areas of Spain, although some plots had high Pb and Cd concentrations (Micó et al. 2006). Although Micó et al. (2006) did not analyse Hg concentrations in soils, Pérez-Sirvent et al. (2009) found a background value of 0.4 mg/kg (values ranged from 0.1 to 2.3 mg/kg) in agricultural soils located in a semi-arid zone in the southeast of Murcia. This background value was similar to those reported in other areas of similar lithological characteristics, although 16% of the samples showed Hg concentrations much above background levels (Pérez-Sirvent et al. 2009). The high salinity of some of the streams in the area (Millán et al. 2011), together with acidification of the water, could facilitate the uptake of these metals into the food chain (Little et al. 2015a).
The bat colonies were regularly checked, and 100 bats were captured during a survey in April 2009 in order to collect data on morphological measurements, sex and reproductive status. Unfortunately, 24 adult bats died accidentally during handling. However, the unfortunate death of these individuals provided an interesting opportunity to increase our knowledge of this species. Ecological risk assessment in bat populations is limited by a lack of data, and there is a great need for this kind of ecotoxicological study to help in the decision making in wildlife conservation (Zukal et al. 2015).
In the present study, we took an advantage of the carcasses to analyse Hg concentrations in different tissues. The sampling was authorized by the local Government of the province of Murcia. The carcasses were preserved in glass jars with pure ethanol and frozen until necropsies and metal analyses were made in October 2011. Few studies have documented the effect of preservative solutions on the interpretation of metal analysis (Simmons 2014). Hernout et al. (2016a) evaluated the leaching of metals into formaldehyde, and their results suggested that leaching may occur, especially in the case of Cu. However, these authors did not evaluate the potential leaching of Hg. Gibbs et al. (1974) found that specimens of fish preserved in formalin, ethyl alcohol and isopropyl alcohol had lower Hg concentrations than unpreserved frozen subsamples. However, Hill et al. (2010) compared Hg concentrations of museum fish to Hg concentrations of unpreserved fish collected from the rivers, and results were not significantly different. These authors concluded that preserved museum fish specimens can be used to evaluate historical changes and predict current levels of Hg contamination (Hill et al. 2010). Poulopoulos (2013) found an increase in mean Hg concentration in fish muscle of 5% after 12 months of formalin/ethanol preservation. Therefore, the Hg concentrations provided in the present study may be slightly under or overestimated due to the preservation of samples in ethanol solution. However, we believe that the leaching would have been minimized taking into account that the whole carcasses preserved in ethanol were conserved frozen instead of at room temperature. We therefore think that our data provide a good approximation of Hg exposure in the individuals studied.
Carcasses were thawed at room temperature, and during the necropsy, body mass (g), forearm lengths (mm) and organ weights (g) were determined for all the bats; sex was also registered (Table 1). A total of 120 samples of kidney, liver, brain, muscle and fur (n = 24 for each tissue) were collected via necropsy from adult bats. In addition, we collected the brain of the foetuses from 7 gestating females. After sample collection, the samples were preserved in Eppendorf tubes and frozen at −20 °C until analysis. A total of 13 adult males, 11 adult females (7 gestating and 4 non-gestating females) and 7 foetuses were necropsied. Gestating females were at the end of the gestation period. The age was determined by the degree of ossification of the growth plaques in the epiphysis of the phalanges on the finger (Dietz et al. 2009).
Mercury analysis
Total mercury (THg) was analysed in 127 samples from 24 adult bats and 7 foetuses in a Milestone DMA-80 direct mercury analyser by atomic absorption spectrophotometry with a detection limit of 0.005 ng following the method described by Espín et al. (2012). Samples (0.2 g wet weight for liver, 0.04 g for kidney, 0.1 g for brain, 0.7 g for muscle and 0.05 g for fur, approximately) were loaded in a nickel boat and analysed. Hg levels in all samples well exceeded the limit of detection. A calibration curve was traced with 11 points (in duplicate) from 0 to 1004 ng of Hg. The method was tested for precision and accuracy using a certified reference material (CRM) (Hg Standard for AAS, Fluka, 1000 mg/l Hg in 12% nitric acid, prepared with high purity Hg metal, HNO3-TraceSELECT® and water TraceSELECT®Ultra). The recovery of total Hg from 5 replicates of CRM diluted to 1 ppm was 98.4 ± 6.6% (mean ± standard deviation). The coefficient of variation for repeatability was 6.7%. Hg concentrations were referred to wet or fresh weight (w.w.). Due to the small size of the matrix samples used, we decided to analyse samples in fresh weight to avoid sample loss during the drying process. Other researchers have analysed Hg in bat samples on a wet and fresh basis (Yates et al. 2014). Dry weight concentrations can be estimated by multiplying the w.w. result by a conversion factor of 3.7 for liver and 3.9 for kidney (Hartmann 2000). Although some authors used a washing process prior to Hg analysis to remove any external contamination in fur (e.g. Flache et al. 2015), other researchers do not describe any washing process for fur samples prior to Hg determination (e.g. Yates et al. 2014). In the present study, fur samples were not washed and a potential overestimation of Hg concentrations in fur due to external contamination cannot be excluded. Yates et al. (2014) found a significant positive correlation between methylmercury (MeHg) and THg concentrations in bat fur, with MeHg accounting for 71–95% (average 86%) of the THg measured. Thus, given the very limited sample size to analyse both MeHg and THg, THg can be used as a surrogate measure for MeHg for the purpose of this investigation.
Statistical analysis
All analyses were carried out using the SPSS v.15.0 statistical package. Reported Hg values represent the mean ± standard deviation and range (min-max). The data were tested for normality using the Kolmogorov-Smirnov test. Since Hg concentrations were not distributed normally, the data were log-transformed. Differences in Hg concentrations among tissues were tested with ANOVA and Tukey’s test. Differences in Hg concentrations between sexes, reproductive status in females and between mother and foetus were tested using t tests. Pearson’s correlation coefficient was used in order to calculate the relationship between variables. The level of significance was set at α = 0.05.
Results
Hg concentrations in tissues of adult Schreiber’s bent-winged bats
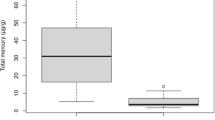
Hg concentrations in tissues of adults Schreiber’s bent-winged bats are detailed in Table 1. The Hg distribution pattern was fur > kidney > liver > muscle > brain (Table 1, Fig. 2). We found significant differences in Hg concentrations among tissues (F = 96.85; d.f. = 4, 115; p < 0.001). The Tukey test shows that Hg levels were significantly higher in fur than in the other tissues (p < 0.001), and in kidney than the rest of tissues (p < 0.001); there were no significant differences between the liver and muscle (p = 0.945; Fig. 2). Hg concentrations in the liver and brain (r = 0.499; p = 0.013) and the muscle and brain (r = 0.799; p < 0.001; Table 2) were positively correlated. We did not find significant correlations between the forearm length or body mass and the Hg concentrations in the different tissues.
Hg concentrations in Schreiber’s bent-winged bats according to sex and reproductive status
No significant differences in Hg concentrations were found between males and females (Table 1). When considering the reproductive status of females, significant differences in Hg concentrations were found for the brain (t = 2.561; d.f. = 9; p = 0.031) and muscle (t = 3.186; d.f. = 9; p = 0.011), non-gestating females having higher Hg levels than gestating females in both tissues (Table 1).
Hg concentrations in gestating females and foetus of Schreiber’s bent-winged bats
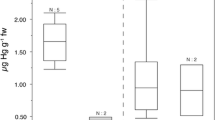
Foetus brains showed higher, although not significant, Hg concentrations than the brain of gestating females (t = 4.860; d.f. = 7; p = 0.055) (Table 1). However, a significant negative correlation was found between Hg concentrations in kidney of gestating females and the brain of the corresponding foetus (r = −0.762; p = 0.047; Table 3 and Fig. 3).
Discussion
Hg concentrations in tissues of adult Schreiber’s bent-winged bats
In the present study, the highest Hg concentrations were found in the fur, followed by the kidney, liver, muscle and brain. Previous studies that involved feeding mice with diets containing Hg found the same distribution pattern, i.e. fur > kidney > liver > muscle > brain (Hussain et al. 1999; Bourdineaud et al. 2008, 2012). Sulphur-keratin proteins of keratinocytes in fur have an affinity for MeHg during the growing phase (Díez 2009; Dorea 2011), and the fact that fur is considered an important route of Hg elimination in bats (Wada et al. 2010) could explain the higher Hg levels found in the fur in the present study. Little is known regarding the suitability of using bat fur as a tool for metal biomonitoring, particularly when analysing Hg and when comparing concentrations in the fur and internal organs (Hernout et al. 2016b). Some studies have found that fur Hg concentrations are higher in bats captured in contaminated sites compared to those from reference sites (Wada et al. 2010; Nam et al. 2012; Yates et al. 2014). The higher Hg concentrations in the fur could be related with the bat capacity to eliminate part of this metal through this matrix during fur growth (Wada et al. 2010; Syaripuddin et al. 2014; Yates et al. 2014; Zukal et al. 2015; Little et al. 2015b). However, although other researchers have found that Hg concentrations in the fur are tightly correlated with endogenous levels in blood (Wada et al. 2010; Yates et al. 2014) and in the liver and brain (Nam et al. 2012; Zukal et al. 2015) in bats, we failed to find significant correlations between Hg concentrations in the fur and internal tissues (Table 2). Hg sequestered in the fur indicates internal Hg levels at the time of fur growth (Yates et al. 2014). Thus, several factors (e.g. changes in diet and fat mobilization during breeding) may produce alterations in internal tissue concentrations that are not reflected in the Hg sequestered in the fur, lowering these correlations. For example, it is known that both males and females of this species lose weight during the dry season (Lisón 2014), and changes in body condition or starvation may alter the liver lipid content and/or liver mass or may produce a remobilization of Hg body burdens, which subsequently leads to increased concentrations in the liver, as shown for other pollutants in birds (Crosse et al. 2013). Usually, the moulting season occurs during the end of summer and beginning of autumn (Fraser et al. 2013) and, as the individuals were sampled in spring, the bats did not manifest the characteristics of the moulting season (Fraser et al. 2013). Therefore, the time elapsing between the last moulting season and the moment of sampling may be an important factor affecting correlations between Hg concentrations in fur and internal tissues. However, due to the limited number of samples in the present study, we cannot draw such a firm conclusion, and this question should be answered in future research. Together with this potential time effect, another methodological bias that could explain the lack of significant correlations is potential external contamination in our fur samples. As the fur samples were not washed before Hg determination, an overestimation of Hg concentrations in fur cannot be excluded. However, as previously suggested by other researchers (Hernout et al. 2016b), any potential external contamination could have been washed off while carcasses were stored in the preservative solution (pure ethanol), which would have the same effect as washing using ethanol or acetone.
Mercury levels in the fur were 3–12 times lower than those found in the fur of different bat species from non-contaminated zones (insectivorous (Eptesicus fuscus) from USA (Wada et al. 2010); insectivorous (Hipposideros, Rhinolophus) and frugivorous (Cynopterus, Megaerops) from Malaysia (Syaripuddin et al. 2014); ten species of bats from USA (Yates et al. 2014); insectivorous (Myotis lucifugus) from Canada (Little et al. 2015a)) and 24–116 times lower than those observed in bats inhabiting contaminated habitats (insectivorous (E. fuscus) from USA (Wada et al. 2010); insectivorous (M. lucifugus) from USA (Nam et al. 2012); ten species of bats from USA (Yates et al. 2014)). When comparing Hg concentrations in the fur with the critical Hg threshold in hair (10 μg/g) associated with neurobehavioural disorders in mammals (Wren 1986; Hickey et al. 2001; Nam et al. 2012), the concentrations in bats in the present study were seen to be far below this critical level (Table 1).
Our results showed that the second tissue with the highest Hg concentration was the kidney, followed by the liver and muscle, while the lowest Hg concentrations were found in the brain (Table 1, Fig. 2). Hg is distributed to different organs, the main accumulation pattern depending on whether exposure is to organic or inorganic forms of the metal (Gupta 2011; Bernhoft 2012). Inorganic Hg mainly accumulates in the kidneys, and significant deposition may also occur in the liver. Although inorganic Hg does not cross the blood-brain barrier efficiently, it can accumulate in brain and foetal tissues (Gupta 2011; Bernhoft 2012). Organic Hg, such as MeHg, accumulates in a higher proportion in the brain and foetus (especially in the foetal brain) compared to inorganic forms, because of its ability to penetrate the blood-brain and placental barriers (Gupta 2011). Some authors suggest that the MeHg demethylation is mainly conducted in the brain (Vahter et al. 1995), and this process could limit the neurotoxicity of Hg (Krey et al. 2012). In spite of this, MeHg can also accumulate in the liver and kidneys (Gupta 2011; Bernhoft 2012). The Hg accumulation does not only depend on the forms of mercury but also on the frequency of the exposure. In cases of chronic exposure, the kidney:liver ratio of Hg has been proposed as a means of distinguishing between exposure to MeHg and inorganic Hg (Scheuhammer 1987). According to this author, during inorganic Hg exposure, only the kidney accumulates high levels of Hg; thus, the kidney:liver ratio will be much higher than unity, whereas if the exposure is to MeHg, the ratio will be much closer to unity (<2, Scheuhammer 1987). In the present study, the kidney:liver ratio was 3.9, which could be considered characteristic of exposure to both inorganic Hg and MeHg. In accordance with this, the significant positive correlations found between Hg concentrations in the liver and brain (r = 0.499, p = 0.013) and the muscle and brain (r = 0.799, p < 0.001) and the lack of correlations between kidney and other organs (Table 2) could be reflecting exposure to and accumulation of both Hg forms simultaneously. Relatively large amount of organic Hg can accumulate in the brain, and while demethylation takes place in this organ, it has been suggested that this process does not occur in the muscle (Gupta 2011). Therefore, chronic exposure to MeHg could result in strong correlations between muscle and brain concentrations and significant but weaker correlations between liver and brain Hg levels, since the liver also accumulates substantial proportions of inorganic Hg (Bernhoft 2012). On the other hand, a simultaneous exposure to inorganic Hg would mainly result in renal accumulation (Gupta 2011; Bernhoft 2012), which could explain the lack of significant correlations between Hg concentrations in the kidney and the other internal tissues. However, additional studies measuring total Hg and MeHg simultaneously are needed to support this hypothesis since other factors such as the limited number of samples in this study could affect the correlations. Moreover, due to the small size of the matrix samples, the samples were analysed in fresh weight to avoid sample loss during the drying process, which could involve small variation in the moisture content in the samples and affect the correlations between tissues.
The toxicity of Hg is in part related to its differential accumulation in the tissues, kidney and brain being the major target organs (Gupta 2011). The maximum renal and hepatic Hg concentrations found in the present study (2.23 μg/g w.w. in the kidney and 0.38 μg/g w.w. in the liver, c.a. 8.7 μg/g d.w. and 1.4 μg/g d.w. in the kidney and liver, respectively, using a conversion factor of 3.9 and 3.7 according to Hartmann (2000)) were well below those associated with adverse effects (ataxia, anorexia, paralysis, nephrotic and neurologic lesions) or death in other mammals such as mink (Mustela vison) or otter (Lutra canadensis) when they were orally exposed during an intermediate duration exposure (20–60 μg/g w.w., Thompson 1996, Wolfe et al. 1998). In general terms, the Hg concentrations found in brain samples in the present study (0.09 μg/g w.w. or c.a. 0.36 μg/g d.w. using a conversion factor of 4, see Zukal et al. (2015) for details) were similar to those found by Nam et al. (2012) in the brain of bats in non-contaminated or reference sites (0.34 μg/g d.w.; Nam et al. 2012), and 20 times lower than levels found for contaminated sites (7.14 μg/g d.w.; Nam et al. 2012). More importantly, mean Hg concentrations in brain were lower than those related with neurological and neurochemical effects in other mammals like minks or otters (Wolfe et al. 1998).
Schreiber’s bent-winged bat has a preference for watercourses, forest and scrubland zones (Lisón et al. 2013). They feed on flying insects, which they hunt in open areas and water bodies (Dietz et al. 2009). In the study area, the species mainly forage in wetlands and rivers (Lisón et al. 2010; Lisón 2014). Several studies have demonstrated that invertebrates can act as biovectors, transferring Hg from lakes to terrestrial predators (e.g. Haro et al. 2013; Hernout et al. 2013, 2015; Tweedy et al. 2013), and most of the THg in larvae seems to be in the form of MeHg in freshwater food webs (Haro et al. 2013). Despite their preference for aquatic-based prey in the study area, the Hg concentrations found in internal tissues and fur suggest that Schreiber’s bent-winged bats in the present study are exposed to low levels of Hg, possibly to a combination of both inorganic Hg and MeHg forms. This could be indicative of relatively low Hg pollution in the area, although due to the small sample size and other methodological limitations, this should be interpreted with caution and more studies are needed for a more accurate assessment of Hg contamination. Moreover, our study area is characterized by a semi-arid climate with c.a. 300 mm of annual rainfall and an average temperature of 17 °C. Rainfall has been found to be an important factor influencing Hg levels in blood of birds in this region, since it contributes to the removal of Hg from the atmosphere, local wet deposition and mobilization (Espín et al. 2014). Therefore, the low annual rainfall in the area could contribute to the lower Hg mobilization compared to other sites with more abundant rainfall. In a different study on guano samples collected in the same cave, Hg was detected even in the lower layers of guano (authors’ unpublished data). Thus, we believe that the colony of Schreiber’s bent-winged bats studied is chronically exposed to low doses of this metal.
Hg concentrations in Schreiber’s bent-winged bats according to sex and reproductive status
There were no significant differences in Hg concentrations between males and females in any of the tissues studied (Table 1). This lack of significant differences between sexes in bats has been observed by other authors (Lueftl et al. 2003; Nam et al. 2012; Syaripuddin et al. 2014). This suggests that both sexes are equally exposed to dietary Hg and that the distribution pattern of Hg in the body is not dependent on sex (Kalisinska et al. 2012; Henderson et al. 2012; Syaripuddin et al. 2014; Yates et al. 2014). However, some studies have described significant differences in Hg concentration between sexes (Allinson et al. 2006; Yates et al. 2014), so the lack of significant differences in the present study may be due to the low number of samples available.
On the other hand, we found significant differences for Hg levels in the brain and muscle between gestating and non-gestating females, non-gestating females having Hg concentrations two times higher in these tissues (Table 1). Numerous studies have provided evidence of the placental transfer of MeHg in humans and other mammals (e.g. Dock et al. 1994; Chen et al. 2014). Therefore, Hg mobilization from mother to foetus and, consequently, lower Hg concentrations in gestating females could explain this difference (Wolfe et al. 1998). In this sense, Greenwood et al. (1978) observed that the half-life of Hg clearance in blood was lower in lactating females than in non-lactating females. This difference may also be related to variations in hunting areas and insect-prey consumed and/or different energetic needs and average food consumption by Schreiber’s bent-winged females during the breeding season, which could affect the Hg intake. In France, it was observed that hunting areas of lactating females were larger than those of gestating females in this species (Vincent et al. 2010). Moreover, the gestating females showed a preference for urban areas, crops and water bodies, while the lactating females preferred woodland areas (Vincent et al. 2010). In addition, differences in food consumption and energy budgets have been observed in other bat species (Mclean and Speakman 1999; Encarnação and Dietz 2006). In this sense, Encarnação and Dietz (2006) found that pregnant females of Daubenton’s bats (Myotis daubentonii) in Germany spent significantly more time foraging during the periods of pregnancy than females during the post-lactation period. These authors calculated that the insect intake of female bats was 8.0 g during pregnancy and 4.9 g per day during post-lactation (providing 5.0 and 3.0 kJ of ingested energy per gramme body mass per day). However, there have been no studies showing the foraging areas, diet and energetic needs of Schreiber’s bent-winged bats in Spain (Lisón 2014), although significant differences in the diet before and after the breeding season have been found in other bat species near the study area (Lisón et al. 2015). Therefore, further studies are needed in order to evaluate the potential differences in the prey composition and the average food consumption by non-gestating and gestating females, and to ascertain whether these differences affect Hg exposure in bats.
Hg concentrations in gestating females and foetus of Schreiber’s bent-winged bats
Different researchers have reported that ready maternal transfer of MeHg across the placenta selectively concentrates Hg in the foetal brain of mammals and may produce embryonic pathological effects and malformations, altering the breeding success (Reuhl et al. 1981; Wolfe et al. 1998). Our results showed that Hg concentrations in the brain of the foetus were higher (1.7 times) than the corresponding levels found in the mothers, although the difference was not significant (p = 0.055; Table 1). In rats fed with MeHg, Yang et al. (1972) found Hg concentrations in the foetal brain that were twice as high as in the maternal brain, and a similar phenomenon was described by Wannag (1976) in the same species. Moreover, we found a significant negative correlation between Hg concentration in foetus brain and the mothers’ kidney (Table 3 and Fig. 3). In this regard, some studies have found a correlation between Hg levels in brain of neonate rats and a lower retention of inorganic Hg in their mothers (Jugo 1976; Yoshida et al. 2002). These results could suggest that the bat females transfer Hg to their foetus across the placenta. This transfer of Hg may also occur later during lactation (Streit and Nagel 1993b). Metallothioneins (MT) probably play an important role in such Hg distribution. Although without significant differences, the mean Hg renal concentration in pregnant females of this study was almost twice as high as levels found in non-pregnant females (Table 1; t = −1.0846; d.f. = 9; p = 0.306). Yoshida et al. (2002) showed that pregnant mice had higher renal MT levels than non-pregnant mice and that these MT levels were greatly increased after 24 h of exposure to Hg vapour. These authors also concluded that a large amount of Hg in the placenta was associated with MT and that MT in maternal tissues protect against Hg transfer from mother to foetus, regulating Hg distribution in the foetus and playing a defensive role against Hg toxicity (Yoshida et al. 2002).
The mean Hg concentrations in foetus brain (0.12 μg/g w.w. or ca. 0.48 μg/g d.w. using a conversion factor of 4, see Zukal et al. (2015) for details) were lower than the levels associated with neurological and neurochemical effects in other mammals (Wolfe et al. 1998). It is important to consider that bats are hibernating animals and in other hibernating mammals, such as polar bears, it has been observed that the Hg transfer is high during spring (Knott et al. 2012). Since the breeding period in bats occurs in spring, the risks of Hg for bat foetuses could be higher than in non-hibernating mammals.
Although the results of the present study could suggest maternal transfer of Hg, the correlation between Hg concentrations in kidney of gestating females and brain of foetuses (p = 0.047) was at the limit of statistical significance, which could be related with the small sample size. Therefore, we recommend further research to improve understanding of the maternal transfer of Hg to offspring in bats. As discussed by Zukal et al. (2015), given the conservation and protection status of bat species in different countries, it is not possible to perform in vivo experiments using bats or even use them in contaminant monitoring programs. Therefore, the acquisition of data in bats is strictly limited (Zukal et al. 2015). Carcasses found in the field present a good opportunity to increase our knowledge, although as shown in this article, opportunistic studies of this nature present limitations such as the number of samples or the availability of pregnant bats. We encourage the use of modelling, which could be an interesting approach, given the scarce ecotoxicological data available in bats. This kind of studies would be a valuable contribution to filling the data gap in this field and would have important implications for ecological risk assessment in bat populations and decision making in wildlife conservation.
Conclusions
This study is the first ever assessment of Hg exposure in a bat species (M. schreibersii) from the Iberian Peninsula. Our findings suggest that Schreiber’s bent-winged bats in Las Yeseras Cave are chronically exposed to low levels of Hg. Bats could be exposed to both inorganic Hg and MeHg forms, but additional studies measuring total Hg and MeHg simultaneously are needed to support this hypothesis. The Hg concentrations found in different tissues are below the threshold levels associated with toxic effects in mammals. Non-gestating females showed Hg concentrations two times higher in the brain and muscle than those found in gestating females. This could be due to Hg mobilization from the mother to the foetus in gestating females, although other factors could explain this, such as variations in hunting areas and the insect-prey consumed and/or different energetic needs and average food consumption during the breeding season that could affect the Hg intake. Further studies evaluating the diet composition and average food consumption in non-gestating and gestating females are needed in order to better understand these differences.
Our findings do not support fur as a good indicator of Hg concentrations in internal tissues in bats. The lack of correlations between Hg concentrations in the fur and internal tissues suggests that additional studies are needed in order to better understand the transfer of Hg into the fur in bat species. Guano deposits form different areas may also be a relevant non-destructive matrix for monitoring trends in metal exposure, and new studies using this type of sample are recommended.
Despite the variation in moisture content in the samples, and the potential underestimation and/or overestimation of Hg concentrations due to methodological bias (effect of preservative solution in Hg content and external contamination for fur samples), we think that the results reported in this study are a good approximation of Hg exposure in the bat colony evaluated.
The present study suggests that there could be an active mother-to-foetus transfer of Hg in bats, which would be especially relevant in scenarios of higher Hg exposure than that reported in this study. However, this result should be interpreted with caution due to the limited number of samples. Given the scarce ecotoxicological data available in bats and the protected status of bats in many countries, we encourage further opportunistic studies using carcasses found in the field, the validation of non-destructive samples such as fur and guano for Hg monitoring, and new modelling approaches in order to fill in this data gap. Such knowledge is needed for proper ecological risk assessment in bat populations and decision making in wildlife conservation.
References
Allinson G, Mispagel C, Kajiwara N et al (2006) Organochlorine and trace metal residues in adult southern bent-wing bat (Miniopterus schreibersii bassanii) in southeastern Australia. Chemosphere 64:1464–1471. doi:10.1016/j.chemosphere.2005.12.067
Benoit JMJ, CCC G, Heyes A et al (2003) Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. ACS Symp 835:1–33
Bernhoft RA (2012) Mercury toxicity and treatment: A review of the literature. J Environ Public Health. doi:10.1155/2012/460508
Bourdineaud J-P, Bellance N, Bénard G et al (2008) Feeding mice with diets containing mercury-contaminated fish flesh from French Guiana: a model for the mercurial intoxication of the Wayana Amerindians. Environ Health 7:1–13. doi:10.1186/1476-069X-7-53
Bourdineaud J-P, Laclau M, Maury-Brachet R et al (2012) Effects of methylmercury contained in a diet mimicking the Wayana Amerindians contamination through fish consumption: mercury accumulation, metallothionein induction, gene expression variations, and role of the chemokine CCL2. Int J Mol Sci 13:7710. doi:10.3390/ijms13067710
Brunet-Rossinni AK, Austad SN (2004) Ageing studies on bats: A review. Biogerontology 5:211–222
Chen Z, Myers R, Wei T et al (2014) Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol 24:537–544. doi:10.1038/jes.2014.26
Cristol DA, Brasso RL, Condon AM et al (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:335. doi:10.1126/science.1154082
Crosse JD, Shore RF, Jones KC, Pereira MG (2013) Key factors affecting liver {PBDE} concentrations in sparrowhawks (Accipiter nisus). Environ Pollut 177:171–176. doi:10.1016/j.envpol.2013.02.006
Dietz C, von Helversen O, Nill D (2009) Bats of Britain, Europe and Northwest Africa. A&C Black Publishers Ltd., London
Díez S (2009) Human health effects of methylmercury exposure. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology. Springer New York, New York, NY, pp 111–132
Dock L, Rissanen RL, Vahter M (1994) Demethylation and placental transfer of methyl mercury in the pregnant hamster. Toxicology 94:131–142. doi:10.1016/0300-483X(94)90033-7
Dorea JG (2011) Environmental contaminants as biomarkers of fish intake: a case for hair mercury concentrations. Eur J Clin Nutr 65:419–420
Encarnação JA, Dietz M (2006) Estimation of food intake and ingested energy in Daubenton’s bats (Myotis daubentonii) during pregnancy and spermatogenesis. Eur J Wildl Res 52:221–227. doi:10.1007/s10344-006-0046-2
Espín S, García-Fernández AJ, Herzke D et al (2016) Tracking pan-continental trends in environmental contamination using sentinel raptors—what types of samples should we use? Ecotoxicology 25:777–801. doi:10.1007/s10646-016-1636-8
Espín S, Martínez-López E, Gómez-Ramírez P et al (2012) Razorbills (Alca torda) as bioindicators of mercury pollution in the southwestern Mediterranean. Mar Pollut Bull 64:2461–2470. doi:10.1016/j.marpolbul.2012.07.045
Espín S, Martínez-López E, León-Ortega M et al (2014) Factors that influence mercury concentrations in nestling eagle owls (Bubo bubo). Sci Total Environ 470–471:1132–1139. doi:10.1016/j.scitotenv.2013.10.063
Flache L, Czarnecki S, Düring R-A et al (2015) Trace metal concentrations in hairs of three bat species from an urbanized area in Germany. J Environ Sci 31:184–193. doi:10.1016/j.jes.2014.12.010
Fraser EE, Longstaffe FJ, Fenton MB (2013) Moulting matters: the importance of understanding moulting cycles in bats when using fur for endogenous marker analysis. Can J Zool Can Zool 91:533–544. doi:10.1139/cjz-2013-0072
García-Fernández AJ, Calvo JF, Martínez-López E, María-Mojica P, Martínez JE (2008) Raptor ecotoxicology in Spain: a review on persistent environmental contaminants. AMBIO J Hum Environ 37(6):432–439
Gibbs RH, Jarosewich E, Windom HL (1974) Heavy metal concentrations in museum fish specimens: effects of preservatives and time. Science 184:475–477. doi:10.1126/science.184.4135.475
Greenwood MR, Clarkson TW, Doherty RA et al (1978) Blood clearance half-times in lactating and nonlactating members of a population exposed to methylmercury. Environ Res 16:48–54. doi:10.1016/0013-9351(78)90140-8
Gupta RC (2011) Mercury. In: Gupta RC (ed) Veterinary toxicology: basic and clinical principles, 2nd edn. Academic Press, Oxford, pp 537–543
Haro RJ, Bailey SW, Northwick RM et al (2013) Burrowing dragonfly larvae as biosentinels of methylmercury in freshwater food webs. Environ Sci Technol 47:8148–8156. doi:10.1021/es401027m
Hartmann R (2000) Deskription der Schwermetallgehalte in Knochen, Organen und Haaren von Fledermäusen (Chiroptera) im Zeitraum 1987 bis 1999. Dissertation, Georg August Universität, Göttingen
Henderson BL, Chumchal MM, Drenner RW et al (2012) Effects of fish on mercury contamination of macroinvertebrate communities of grassland ponds. Environ Toxicol Chem 31:870–876. doi:10.1002/etc.1760
Hernout BV, Bowman SR, Weaver RJ et al (2015) Implications of in vitro bioaccessibility differences for the assessment of risks of metals to bats. Environ Toxicol Chem 34:898–906. doi:10.1002/etc.2871
Hernout BV, Somerwill KE, Arnold KE et al (2013) A spatially-based modeling framework for assessing the risks of soil-associated metals to bats. Environ Pollut 173:110–116. doi:10.1016/j.envpol.2012.08.017
Hernout BV, Arnold KE, McClean CJ et al (2016a) A national level assessment of metal contamination in bats. Environ Pollut 214:847–858. doi:10.1016/j.envpol.2016.04.079
Hernout BV, McClean CJ, Arnold KE et al (2016b) Fur: a non-invasive approach to monitor metal exposure in bats. Chemosphere 147:376–381. doi:10.1016/j.chemosphere.2015.12.104
Hickey MBC, Fenton MB, MacDonald KC, Soulliere C (2001) Trace elements in the fur of bats (Chiroptera: Vespertilionidae) from Ontario and Quebec, Canada. Bull Environ Contam Toxicol 66:699–706
Hill J, Chumchal MM, Drenner RW et al (2010) Use of preserved museum fish to evaluate historical and current mercury contamination in fish from two rivers in Oklahoma, USA. Environ Monit Assess 161:509–516. doi:10.1007/s10661-009-0764-5
Hussain S, Atkinson A, Thompson SJ, Khan AT (1999) Accumulation of mercury and its effect on antioxidant enzymes in brain, liver, and kidneys of mice. J Environ Sci Heal Part B 34:645–660. doi:10.1080/03601239909373219
Jackson AK, Evers DC, Folsom SB et al (2011) Mercury exposure in terrestrial birds far downstream of an historical point source. Environ Pollut 159:3302–3308. doi:10.1016/j.envpol.2011.08.046
Jones G, Jacobs DS, Kunz TH et al (2009) Carpe noctem: the importance of bats as bioindicators. Endanger Species Res 8:93–115. doi:10.3354/esr00182
Jugo S (1976) Retention and distribution of 203HgCl2 in suckling and adult rats. Health Phys 30:240–241
Kalisinska E, Lisowski P, Kosik-Bogacka DI (2012) Red fox Vulpes vulpes (L., 1758) as a bioindicator of mercury contamination in terrestrial ecosystems of North-Western Poland. Biol Trace Elem Res 145:172–180
Knott KK, Boyd D, Ylitalo GM, O’Hara TM (2012) Lactational transfer of mercury and polychlorinated biphenyls in polar bears. Chemosphere 88:395–402. doi:10.1016/j.chemosphere.2012.02.053
Krey A, Kwan M, Chan HM (2012) Mercury speciation in brain tissue of polar bears (Ursus maritimus) from the Canadian Arctic. Environ Res 114:24–30
Kunz TH, Lumsden LF (2003) Ecology of cavity and foliage roosting bats. In: Kunz TH, Fenton MB (eds) Bat ecology. University of Chicago Press, Chicago, U.S., pp 3–89
Kurta A, Bell GP, Nagy KA, Kunz TH (1989) Energetics of pregnancy and lactation in free-ranging little brown bats (Myotis lucifugus). Physiol Zool 62:804–818
Lisón F (2012) Datos biométricos de cinco especies de murciélagos (Mammalia: Chiroptera) de la Región de Murcia (SE España). An Biol 34:35–40
Lisón F (2014) Murciélago de cueva - Miniopterus schreibersii. In: Salvador A, Luque-Larena JJ (eds) Enciclopedia Virtual de los Vertebrados Españoles. Museo Nacional de Ciencias Naturales, Madrid Available: http://www.vertebradosibericos.org/. Accessed 05/05/2015
Lisón F, Aledo E, Calvo JF (2011) Los murciélagos (Mammalia: Chiroptera) de la Región de Murcia (SE España): distribución y estado de conservación. An Biol 33:79–92
Lisón F, López-Espinosa JA, Calvo JF, Jones G (2015) Diet of the meridional serotine Eptesicus Isabellinus in an urban semiarid Mediterranean landscape. Acta Chiropterologica 17:371–378. doi:10.3161/15081109ACC2015.17.2.013
Lisón F, Palazón JA, Calvo JF (2013) Effectiveness of the Natura 2000 Network for the conservation of cave-dwelling bats in a Mediterranean region. Anim Conserv 16:528–537. doi:10.1111/acv.12025
Lisón F, Yelo ND, Haz Á, Calvo JF (2010) Contribución al conocimiento de la distribución de la fauna quiropterológica de la Región de Murcia. Galemys 22:11–28
Little ME, Burgess NM, Broders HG, Campbell LM (2015a) Mercury in little brown bat (Myotis lucifugus) maternity colonies and its correlation with freshwater acidity in Nova Scotia, Canada. Environ Sci Technol 49:2059–2065. doi:10.1021/es5050375
Little ME, Burgess NM, Broders HG, Campbell LM (2015b) Distribution of mercury in archived fur from little brown bats across Atlantic Canada. Environ Pollut 207:52–58. doi:10.1016/j.envpol.2015.07.049
Lueftl S, Freitag B, Deutz A, Tataruch F (2003) Concentration of heavy metals in European bats (Microchiroptera). Fresenius Environ Bull 12:353–358
Maxson P (2005) Mercury flows in Europe and the world: the impact of decommissioned chlor-alkali plants. In: Pirrone E, Mahaffey KR (eds) Dynamics of mercury pollution on regional and global scales, atmospheric processes and human exposures around the world. Springer, New York, U.S., pp 25–50
Mclean JA, Speakman JR (1999) Energy budgets of lactating and non-reproductive brown long-eared bats (Plecotus auritus) suggest females use compensation in lactation. Funct Ecol 13:360–372. doi:10.1046/j.1365-2435.1999.00321.x
Mickleburgh SP, Hutson AM, Racey PA (2002) A review of the global conservation status of bats. Oryx 36:18–34. doi:10.1017/S0030605301000011
Micó C, Peris M, Sánchez J, Recatalá L (2006) Heavy metal content of agricultural soils in a Mediterranean semiarid area: the Segura River Valley (Alicante, Spain). Spanish J Agric Res 4:363–372
Millán A, Velasco J, Gutiérrez-Cánovas C et al (2011) Mediterranean saline streams in Southeast Spain: what do we know? J Arid Environ 75:1352–1359. doi:10.1016/j.jaridenv.2010.12.010
Morel FMM, Kraepiel AML, Amyot M (1998) The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst 29:543–566. doi:10.1146/annurev.ecolsys.29.1.543
Nam DH, Yates D, Ardapple P et al (2012) Elevated mercury exposure and neurochemical alterations in little brown bats (Myotis lucifugus) from a site with historical mercury contamination. Ecotoxicology 21:1094–1101. doi:10.1007/s10646-012-0864-9
O’Shea TJ, Johnston JJ (2009) Environmental contaminants and bats: investigating exposure and effects. In: Kuntz TH, Parsons S (eds) Ecological and behavioral methods for the study of bats. Johns Hopkins University Press, Baltimore, U.S., pp 500–528
Pacyna EG, Pacyna JM, Pirrone N (2001) European emissions of atmospheric mercury from anthropogenic sources in 1995. Atmos Environ 35:2987–2996. doi:10.1016/S1352-2310(01)00102-9
Pérez-Sirvent C, Martínez-Sánchez MJ, García-Lorenzo ML et al (2009) Geochemical background levels of zinc, cadmium and mercury in anthropically influenced soils located in a semi-arid zone (SE, Spain). Geoderma 148:307–317. doi:10.1016/j.geoderma.2008.10.017
Poulopoulos J (2013) Long-term changes to food web structures and mercury biomagnification in three large, inland North American lakes. Dissertation, Queen’s University, Kingston, Ontario, Canada
Rainho A, Palmeirim JM (2011) The importance of distance to resources in the spatial modelling of bat foraging habitat. PLoS One 6(4):e19227. doi:10.1371/journal.pone.0019227
Reuhl KR, Chang LW, Townsend JW (1981) Pathological effects of in utero methylmercury exposure on the cerebellum of the golden hamster. Environ Res 26:281–306. doi:10.1016/0013-9351(81)90205-X
Sánchez-Virosta P, Espín S, García-Fernández AJ, Eeva T (2015) A review on exposure and effects of arsenic in passerine birds. Sci Total Environ 512-513:506–525. doi:10.1016/j.scitotenv.2015.01.069
Scheuhammer AM (1987) The chronic toxicity of aluminium, cadmium, mercury, and lead in birds: a review. Environ Pollut 46:263–295. doi:10.1016/0269-7491(87)90173-4
Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW (2007) Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 36:12–18. doi:10.1579/0044-7447(2007)36[12:EOEMOT]2.0.CO;2
Schmeltz D, Evers DC, Driscoll CT et al (2011) MercNet: a national monitoring network to assess responses to changing mercury emissions in the United States. Ecotoxicology 20:1713–1725. doi:10.1007/s10646-011-0756-4
Simmons JE (2014) Fluid preservation: a comprehensive reference. Rowman & Littlefield, Lanham, Maryland
Streets DG, Zhang Q, Wu Y (2009) Projections of global mercury emissions in 2050. Environ Sci Technol 43:2983–2988. doi:10.1021/es802474j
Streit B, Nagel A (1993a) Element assessment in tissue samples from European bats (Microchiroptera). Fresenius Environ Bull 2:162–167
Streit B, Nagel A (1993b) Heavy metal by lactation in a bat colony. Fresenius Environ Bull 2:168–173
Syaripuddin K, Kumar A, Sing KW et al (2014) Mercury accumulation in bats near hydroelectric reservoirs in peninsular Malaysia. Ecotoxicology 23:1164–1171. doi:10.1007/s10646-014-1258-y
Thompson DR (1996) Mercury in birds and terrestrial mammals. In: Beyer WN, Meador JP (eds) Environmental contaminants in wildlife. Interpreting tissue concentrations. CRC Press, London, pp 341–356
Tweedy BN, Drenner RW, Chumchal MM, Kennedy JH (2013) Effects of fish on emergent insect-mediated flux of methyl mercury across a gradient of contamination. Environ Sci Technol 47:1614–1619. doi:10.1021/es303330m
Vahter ME, Mottet NK, Friberg LT et al (1995) Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol Appl Pharmacol 134:273–284. doi:10.1006/taap.1995.1193
Vincent S, Nemoz M, Aulagnier S (2010) Activity and foraging habitats of Miniopterus schreibersii (Chiroptera: Miniopteridae) in southern France: implications for its conservation. Hystrix, Ital J Mammal 22:57–72
Wada H, Yates DE, Evers DC et al (2010) Tissue mercury concentrations and adrenocortical responses of female big brown bats (Eptesicus fuscus) near a contaminated river. Ecotoxicology 19:1277–1284. doi:10.1007/s10646-010-0513-0
Wannag A (1976) The importance of organ blood mercury when comparing foetal and maternal rat organ distribution of mercury after methyl mercury exposure. Acta Pharmacol Toxicol (Copenh) 38:289–298. doi:10.1111/j.1600-0773.1976.tb03123.x
Ward DM, Nislow KH, Folt CL (2010) Bioaccumulation syndrome: identifying factors that make some stream food webs prone to elevated mercury bioaccumulation. Ann N Y Acad Sci 1195:62–83
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17:146–160. doi:10.1002/etc.5620170203
Wren CD (1986) A review of metal accumulation and toxicity in wild mammals: I. Mercury. Environ Res 40(1):210–244. doi:10.1016/S0013-9351(86)80098-6
Yang MG, Krawford KS, Garcia JD et al (1972) Deposition of mercury in foetal and maternal brain. Exp Biol Med 141:1004–1007. doi:10.3181/00379727-141-36920
Yates DE, Adams EM, Angelo SE et al (2014) Mercury in bats from the northeastern United States. Ecotoxicology 23:45–55. doi:10.1007/s10646-013-1150-1
Yoshida M, Satoh M, Shimada A et al (2002) Maternal-to-fetus transfer of mercury in metallothionein-null pregnant mice after exposure to mercury vapor. Toxicology 175:215–222. doi:10.1016/S0300-483X(02)00084-7
Zukal J, Pikula J, Bandouchova H (2015) Bats as bioindicators of heavy metal pollution: history and prospect. Mamm Biol 80:220–227
Acknowledgements
This work was supported by the Fundación Séneca (CARM) with the MASCA’2014 Project (19481/PI/14). FL was supported by a fellowship (Programa MECE Educación Superior) from the Chilean Ministry of Education and postdoctoral fellowship (Programa de Formación de Investigadores Postdoctorales) from Universidad de La Frontera, Chile. SE was funded by the Academy of Finland (project number 265859 to Dr. Tapio Eeva) and by Fundación Séneca-Agencia de Ciencia y Tecnología de la Región de Murcia (20031/SF/16 to Dr. Silvia Espín). The first version of the manuscript was considerably improved by the comments of the anonymous reviewers and the editor. To the best of our knowledge, no conflict of interest, financial or other, exists.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Céline Guéguen
Rights and permissions
About this article
Cite this article
Lisón, F., Espín, S., Aroca, B. et al. Assessment of mercury exposure and maternal-foetal transfer in Miniopterus schreibersii (Chiroptera: Miniopteridae) from southeastern Iberian Peninsula. Environ Sci Pollut Res 24, 5497–5508 (2017). https://doi.org/10.1007/s11356-016-8271-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8271-z