Abstract
Seasonal variations of trace metal concentrations (Hg, Cd, Pb, Cr, Zn, and Cu) were investigated in the hepatopancreas and soft tissues (remaining parts of bivalves such as gill, mantle, foot, and muscle) of Tapes decussatus and Mytilus galloprovincialis from the Homa Lagoon. The highest metal concentrations were recorded frequently in February and July in M. galloprovincialis and T. decussatus, respectively. Trace metal levels (except Zn) in the hepatopancreas were higher than those in soft tissue for both bivalves. The results showed that the mean concentrations of Hg, Cd, and Zn for M. galloprovincialis were higher compared to T. decussatus in both tissues. The metal concentrations in both bivalves from the Homa Lagoon were generally below the values of provisional tolerable weekly intake (PTWI) estimates, in terms of human health risk. According to hazard quotient (HQ), Cr values for both species and Cd values for only M. galloprovincialis were found greater than one. Also, total hazard index (THI) values were greater than one in both bivalves, having a potential risk for consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Metal pollution is known to be widespread, and metal pollutants have long-term effects on marine environments, such as coastal lagoons. High metal concentrations in the marine environments are derived from natural and anthropogenic sources. Industrial, agricultural, and urban sources contribute to the lagoon environment either directly or by means of watercourses discharging their contents into the lagoon (Irato et al. 2003).
Lagoons are characterized by being relatively isolated from the open sea and connected to the coastal waters through channels, which makes them highly vulnerable to impacts, particularly near the shoreline and in bodies of water with restricted circulation. Therefore, lagoons can demonstrate a certain buffer capacity to external impact within stable thresholds. On the other hand, high levels of external pressures over prolonged periods can lead to the irreversible deterioration of these ecosystems (Viaroli et al. 2007; María-Cervantes et al. 2009). Lagoons are ecologically vital ecosystems which are involved in fisheries and productivity, serving as migration routes and feeding and nursery grounds of many organisms (Chapman and Wang 2001; Uluturhan et al. 2011). Besides, lagoons, which provide economic benefits, have importance for the protection of coastal fish populations as stated in the Water Framework Directive (WFD) and the protection of the water status within transitional waters. Also, lagoons are widely studied for the assessment of the reserve effects on marine protected areas throughout the world (EU 2000; Pérez-Ruzafa et al. 2011).
The availability of metals can be a threat to the exposed organisms in aquatic ecosystems. Marine organisms can be able to tolerate or excrete excessive amounts of trace metals after exposure (Fernandes et al. 2008). Some aquatic organisms are utility indicators for the availability of metal when exposed at sublethal levels (Válega et al. 2008). For this reason, the monitoring of aquatic organisms can be very useful to understand not only the sources of metal pollution but also the risks to the trophic chain even to humans as top consumers (Caçador et al. 2012). Bivalves are useful biological indicators for identifying biologically available metals due to inadequate movement capability, filter-feeding dietary type, long-term expose ability, having high tolerance to the environmental conditions, and wide distribution in the geographical area (Szefer 1986; Irato et al. 2003; El-Sikaily et al. 2004; Roméo et al. 2005). Also, bivalves are extensively used as sentinel organisms in metal biomonitoring research (Rainbow and Philips 1993; Cravo et al. 2004) The digestive gland usually plays a major role for identifying metal detoxification and accumulation in bivalves. Due to being a target organ, bioaccumulation in digestive gland was commonly reported in biomonitoring of metal pollution during the past decades (Regoli and Orlando 1994; Pereira et al. 2009).
In this study, trace metal levels were investigated in the hepatopancreas and soft tissues of Mytilus galloprovincialis and Tapes decussatus from the Homa Lagoon for assessment of metal pollution. Over the past two decades, some studies have been focused on metal pollution both in biota and sediment in the Homa Lagoon (Mordoğan et al. 1990; Sunlu and Egemen 1998; Atılgan and Egemen 2001; Dora et al. 2007; Uluturhan et al. 2011). In this area, there are no recent studies about biomonitoring of trace metal pollution in bivalves as bioindicators. The main goals of this study are (i) to determine seasonal metal concentrations in the hepatopancreas and soft tissues of T. decussatus and M. galloprovincialis, (ii) to observe variances of metal concentrations between species, tissues, and seasons, and (iii) to evaluate trace metal levels according to provisional tolerable weekly intake (PTWI) and hazard quotient (HQ) estimates based on WHO/FAO and ATSDR values for human health risk assessment, due to the socioeconomic importance of the high fishing activity within the Homa Lagoon.
Material and methods
Study area and selected bivalve species
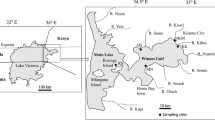
The Homa Lagoon is an ecologically and economically important and only active lagoon in the Izmir Bay, eastern Aegean Sea. The Homa Lagoon is located in the Gediz Delta (at the mouth of Gediz River) and covers an area of approximately 18 km2 (Fig. 1). It is quite shallow with depths varying between 0.5 and 1.0 m. It is adjacent to the most important salt pan of Turkey and the Izmir Bird Paradise in the bay. The Gediz Delta and the Homa Lagoon have been described as protected wetlands according to Ramsar Convention since 1998 (Parlak et al. 2006). The Homa Lagoon is affected by the Gediz River which is heavily polluted due to agricultural drainage water, industrial wastewater, and domestic wastewater from the entire area (Uluturhan et al. 2011).
The clam (T. decussatus L.) is a commercially valuable bivalve, and it is naturally distributed from the south and west coasts of the British Isles to the Mediterranean Sea and also along the Atlantic coasts from Norway to Senegal (Breber 1985). This species is distributed along the coastlines of the Aegean and Marmara Seas. Also, it has been recently reported from the Izmir Bay (Serdar et al. 2007). It lives in muddy-sand sediments of tidal flats or shallow coastal areas. The mussel (M. galloprovincialis L.) is another economically important bivalve and widely distributed from the Mediterranean to Black Sea in Turkish coastlines. It is found on temperate, sheltered, and rocky shores (Branch and Steffani 2004). These species are caught, intensively consumed as food by local people, and also exported to other countries every year. This study was focused on these bivalves due to their good bioindicator capacity and wide distribution in the Homa Lagoon.
Sampling and trace metal analysis
The physicochemical parameters such as salinity, pH, and temperature of seawater were measured in situ by a multi-parameter portable meter (WTW) during the bivalve sampling. Dissolved oxygen (DO) was determined according to Winkler titration method at sampling points from the Homa Lagoon (Table 1).
The samples of T. decussatus and M. galloprovincialis were collected seasonally (Spring: May 2013; Summer: July 2013, Autumn: October 2013; and Winter: February 2014) by hands from sampling points and transported to the laboratory in plastic boxes. Epiphytes were removed from the shells of bivalves. All samples were divided into 2–4 groups according to their shell lengths (pooled 10–20 samples per group) (Table 1). Hepatopancreas tissues were separated from other soft tissues of the samples. Soft tissues contain all other remaining parts of bivalves (such as gonad, gill, heart, mantle, foot, and muscle) in each group.
The pooled tissue samples were freeze-dried (Labconco Freeze Dryer System) and immediately homogenized. Triplicate samples were weighted about 1 g and digested in acid mixture (5:1 HNO3:HClO4) with a microwave oven (Milestone 1200) (UNEP 1982, 1984). Metal analyses were determined by flame (for Cu and Zn), graphite furnace (for Cd, Pb, and Cr), and cold vapor (for Hg) techniques using atomic absorption spectrometers (Varian 280FS and 280Z). The detection limits for trace metals were Hg 0.05 μg kg−1, Cd 0.10 μg kg−1, Pb 0.10 μg kg−1, Cr 0.10 μg kg−1, Cu 0.03 μg g−1, and Zn 0.01 μg g−1. Standard reference materials (NIST SRM 2976) were used for accuracy and validity of analytical measurements. The values obtained from the analysis of SRMs were as follows: Hg, 0.061 ± 0.004:0.070 ± 0.005; Cd, 0.82 ± 0.16:0.76 ± 0.18; Pb, 1.19 ± 0.18:1.33 ± 0.09; Cr, 0.50 ± 0.16:0.61 ± 0.03; Cu, 4.02 ± 0.33:4.15 ± 0.10; and Zn, 137 ± 13:146 ± 3.0 (certified/reference values:observed values in μg g−1 dry weight ± standard deviation).
Human health risk assessment
The health risk assessment for humans was conducted by using provisional tolerable weekly intake (PTWI) and reference dose factor (RfD) estimates (FAO/WHO 2004). The exposure levels of metals were determined using the estimated daily intake. The estimated daily intake (EDI) (μg/kg/day) was calculated by the following equation (Eq. 1):
where C bivalve mean trace metal concentration in bivalve (μg g−1 wet weight), dcbivalve daily bivalve consumption (1.15 g/capita/day) per person of Turkey in (FAO 2011), and bw the average body weight (70 kg) of target population in this study.
EDI was calculated using the mean concentrations (converted from dry weight to wet weight of samples. The mean conversion ratios were found as 0.180 in whole body of both bivalves). The non-carcinogenic health risk to local consumers through the consumption of metal-contaminated bivalves was characterized by a hazard quotient (HQ). The hazard quotient was calculated by the following equation (Eq. 2):
For estimating the consumers risk of several metals, total hazard index (THI) has been developed (USEPA 2010). THI was calculated by the sum of HQ values for all metals (Guerra et al. 2010, Eq. 3). If the HQ or THI was less than one, there would be no obvious risk of excessive intake of metal from bivalve consumption (Sridhara Chary et al. 2008; USEPA 2013).
Statistical analyses
Statistical analyses were performed by Statistica 8.0 (2007) for Windows (StatSoft, Inc., Tulsa, USA). Data were log-transformed where necessary for meeting the normal distribution assumptions of ANOVA and Pearson’s product-moment correlation tests. Pearson’s product-moment correlation test was used to check for significant relationships between metal concentrations and sample lengths. A two-way ANOVA test was used for investigating statistical differences between seasons and tissue types for each species (p < 0.05). Also, a three-way ANOVA test was performed for significant differences among seasons, species, and tissue types (p < 0.05).
Results
Seasonal distribution of trace metal concentrations in bivalves
The metal concentrations (mean ± SD) and their ranges (Hg, Cd, Pb, Cr, Zn, and Cu) in M. galloprovincialis and T. decussatus from the Homa Lagoon were given in Figs. 2 and 3 and Table 2, respectively. In soft tissues of M. galloprovincialis, the lowest concentrations of Hg (0.095 μg g−1), Cd (0.22 μg g−1), and Zn (79.8 μg g−1) were detected in May. The lowest Pb and Cu levels were found in July as 0.81 and 2.09 μg g−1, respectively. Minimum Cr concentration was observed (0.10 μg g−1) in October. On the other hand, the maximum levels of Cd (0.51 μg g−1) and Pb (2.47 μg g−1) were found in October. The Cr level (6.21 μg g−1) increased to its maximum in May. The highest concentrations of Hg (0.154 μg g−1), Cu (5.63 μg g−1), and Zn (232 μg g−1) were observed in February (Table 2).
For the hepatopancreas of M. galloprovincialis, Cd (0.29 μg g−1) and Zn (61.0 μg g−1) were at their lowest levels in May. The minimum concentrations of Hg (0.139 μg g−1), Pb (0.99 μg g−1), Cr (2.44 μg g−1), and Cu (4.21 μg g−1) were determined in July. Otherwise, Cr concentration reached its highest value as 12.4 μg g−1 in May. The highest levels of Cd (0.48 μg g−1) and Zn (147 μg g−1) were obtained in October. The highest values of Hg (0.251 μg g−1), Pb (2.77 μg g−1), and Cu (11.9 μg g−1) were found in February (Table 2).
For M. galloprovincialis, mean concentrations of Hg, Cd, Pb, Cu, and Zn levels demonstrated considerable increases in both tissues from May to February. Only Cr levels followed irregular profiles depended on seasons (Fig. 2).
In soft tissues of T. decussatus, the concentrations of Hg (0.073 μg g−1) and Cd (0.10 μg g−1) reached their minimum levels in February. The lowest values of Pb and Cu were observed in May and February. Cr and Zn were found at their minimum levels as 0.50 and 52.9 μg g−1 respectively, in October. The maximum levels of Hg (0.132 μg g−1), Cd (0.33 μg g−1), Pb (1.35 μg g−1), Cr (13.4 μg g−1), Cu (9.57 μg g−1), and Zn (82.2 μg g−1) were determined in July (Table 2).
For the hepatopancreas of T. decussatus, the results showed that the minimum concentrations of Pb (0.88 μg g−1) and Cr (2.35 μg g−1) were found in May. Minimum levels of Cd, Cu, and Zn were observed as 0.12, 13.7, and 80.4 μg g−1, respectively, in July. The Hg was at its lowest level (0.146 μg g−1) in October. The maximum concentrations of Hg (0.281 μg g−1) and Zn (96.1 μg g−1) were found in May. Pb (3.95 μg g−1) and Cu (24.4 μg g−1) were determined at their highest levels in February. Cd (0.53 μg g−1) concentration reached maximum in July while the maximum Cr concentration was found as 13.3 μg g−1 in October (Table 2).
Metal concentrations in soft tissues of T. decussatus were showed a slight decrease after July which is the spawning period. Hg, Cr, and Cu concentrations in soft tissues were decreased during October and February compared to July (Fig. 3). In contrast, mean concentrations in hepatopancreas tissues exhibited some seasonal fluctuations (Fig. 3). On the other hand, metals were generally accumulated in the hepatopancreas tissues more than in the soft tissues of both bivalves in this study (Table 2, Figs. 2 and 3).
Comparison of trace metal concentrations between M. galloprovincialis and T. decussatus
The results showed that the mean concentrations of Hg, Cd, and Zn in M. galloprovincialis were relatively higher than those in T. decussatus for both tissues. On the other hand, the mean values of Cu in T. decussatus were found relatively higher than those in hepatopancreas and soft tissues of M. galloprovincialis (Figs. 2 and 3).
Mean Cr concentrations in T. decussatus were higher than those in M. galloprovincialis for soft tissues. Conversely, Cr levels in M. galloprovincialis were found higher than those in T. decussatus for the hepatopancreas. The mean concentrations of Pb were higher in soft tissues of M. galloprovincialis, while they were higher in hepatopancreas tissues of T. decussatus. The bioaccumulation patterns of metals in hepatopancreas and soft tissues of both species were Zn > Cu > Cr > Pb > Cd > Hg (Figs. 2 and 3).
Statistical analyses
Pearson’s correlation coefficients were calculated for each metal in both tissues of bivalves (p < 0.05). For M. galloprovincialis, the significant correlations between sample length and metal levels were observed for Cr (r = −0.54) and Cu (r = −0.80) in soft tissues and for Cu (r = −0.56) and Zn (r = 0.69) in the hepatopancreas. The significant correlations between sample length and metal levels were found for Hg (r = 0.62) and Cd (r = 0.75) in soft tissues and for Cd (r = 0.54) and Zn (r = −0.64) in hepatopancreas tissues of T. decussatus.
A two-way ANOVA test was performed for determination of significant differences between seasons and tissues for each bivalve. Significant differences among seasons were observed for all metals in M. galloprovincialis. Statistical differences for metal levels were found in all tissues, except Cd. For T. decussatus, concentrations of Hg, Cr, and Cu showed significant differences in tissues and seasons. Additionally, significant differences were recorded for Pb and Zn only in tissues (p < 0.05).
Three-way ANOVA tests were used for describing the significant differences among species, metal concentrations in tissues, and seasons. Both Cu and Cr levels were showed significant differences among species, tissues, and seasons. There were significant differences for Hg in both species and tissues, except seasons. Significant differences in Cd and Zn concentrations were only observed for seasons and species. Pb exhibited significant differences within seasons and tissues but no significant differences were observed within species (p < 0.05).
Human health risk assessment
PTWI levels and HQ and THI values for metals in whole body of bivalve samples were presented in Table 3. Trace metal concentrations were found below PTWI levels. The HQ values for Hg, Pb, Cu, and Zn were less than one, except Cr. Also, the HQ value of Cd was calculated as 1.30 for M. galloprovincialis. THI values were calculated as 5.10 for M. galloprovincialis and 5.12 for T. decussatus. Observation of THI values greater than 1 for both bivalves indicated a potential risk for consumers. Especially, HQ values for Cr (1.88 for M. galloprovincialis and 2.62 for T. decussatus) were main contributors to the THI of metals for each bivalve.
Discussion
Results showed that Hg, Cd, and Zn concentrations in M. galloprovincialis were generally higher than those in T. decussatus. Previous studies exhibited similar results for these bivalves (Bebianno and Serafim 1998; Irato et al. 2003; Beiras and Albentosa 2004). On the contrary, Cu concentrations in T. decussatus were relatively higher than those in M. galloprovincialis. This might be associated with specific bioaccumulation tendencies of these bivalves based on their different habitats, lifestyles, and feeding regimes. Several studies emphasized that accumulations of metals have presented different species-specific capacities for bivalves (Usero et al. 1997; Irato et al. 2003). Also, some researchers claimed that these differences were related to metabolic rates of bivalve species (Najdek and Sapunar 1987).
In selected bivalves, Hg, Pb, Cr, and Cu concentrations in hepatopancreas tissues were found higher than those in soft tissue for each season. It is suggested that toxic metals accumulate into the digestive gland for storage or detoxification. Also, it is widely known that essential elements such as Cu are involved in many metalloenzyme reactions (Bebianno and Serafim 2003; Ivanković et al. 2005; Scudiero et al. 2014). In bivalves, the gut content contains a mixture of biogenic and lithogenic materials. The filter-feeding bivalves uptake metals from particulate sources, through ingestion of suspended particulate matter (SPM), including planktonic organisms, bacteria, and inorganic particulate matter from the water column. According to previous studies, the availability of terrigenous inorganic materials such as Cr, Cd, Al, and Fe was detected in their foods (Kennedy 1986; Allison et al. 1998).
The maximum Hg concentrations for M. galloprovincialis and T. decussatus were found in February and May, respectively, which are the pre-spawning periods. It is well reported that greater accumulations of Hg and other chemicals occur in pre-spawning periods due to increasing feeding and flesh weight during gonadal development (Najdek and Sapunar 1987; Vlahogianni et al. 2007).
According to Cd levels, bioaccumulations were measured relatively higher in soft tissues of M. galloprovincialis. Similar results were reported in a previous study from the Ria Formosa Lagoon (Bebianno and Serafim 1998). The maximum concentrations of Cd were detected in October for M. galloprovincialis and in July for T. decussatus. These results are similar with those of other seasonal studies (Fattorini et al. 2008; Cravo et al. 2012). The concentrations of Pb reached the highest levels in October and February in both bivalves. Similar seasonal observations were also reported at the Brown Bay (Giarratano and Amin 2010).
In contrast to Hg, Cd, and Pb levels, Cr concentrations in T. decussatus were relatively higher than those in M. galloprovincialis. The maximum Cr levels in M. galloprovincialis and T. decussatus were detected in May and October, respectively. These months are corresponding to post-spawning periods of both species. Similar bioaccumulation profiles for Cr were found in the literature (Irato et al. 2003; Richir and Gobert 2014).
Cu levels in T. decussatus were generally higher than those in M. galloprovincialis. The maximum values were reached in February for these species. It was found that the increase in Cu levels was probably related to faster metal uptake during intense food consumption in winter season (Sokolowski et al. 2004).
The maximum Zn concentrations in M. galloprovincialis and T. decussatus were determined in February and July, respectively. These findings were similar to the results of previous studies (Regoli and Orlando 1994; Cravo et al. 2012). It was suggested that bioaccumulated metals showed variations during a spawning period due to penetration into the gonadic tissues during the reproductive cycle. Because of the species-specific differences, Zn concentrations in hepatopancreas tissues of T. decussatus were relatively higher than those in soft tissues, whereas they were lower in M. galloprovincialis in this study (Irato et al. 2003; Ivanković et al. 2005).
The bioaccumulation patterns of metals were generally similar for both species in this study. These patterns in the Homa Lagoon could be linked to the presence of anthropogenic inputs or lithogenic sources affecting the area (Uluturhan et al. 2011). Also, seasonal variations of metal concentrations in bivalves result from many factors such as large differences in water temperatures, runoff of particulate metals to coastal waters, food availability caused by transferring metals from water to filter-feeding organisms and weight changes during gonadal development, and release of biomass related to sexual production (Szefer et al. 2004; Giarratano and Amin 2010).
Effects of environmental parameters and shell size on metal concentrations
It is known that metal concentrations in bivalves are influenced from many environmental parameters such as salinity, temperature, and pH. Results showed that Hg, Cd, Pb, Zn, and Cu concentrations in only M. galloprovincialis were found higher in February with the lowest temperature and salinity values. Some studies stated that metal uptake is generally related to the effects of temperature on metabolic activities. It is reported that Cu and Zn concentrations were in inverse relationships with salinity and temperature values (Phillips 1977; Ali and Taylor 2010; Kumar et al. 2015). Also, the lower Pb concentrations were found in July with the highest dissolved oxygen levels. Similar findings were also reported in a previous study in cockle Austrovenus stutchburyi which is commonly used as metal bioindicators (Marsden et al. 2014).
According to the effects of shell size on trace metal concentrations, dominant negative relationships were obtained for M. galloprovincialis, whereas T. decussatus generally exhibited the positive relationships in this study. For bivalves, accumulation changes depend on each metal and different species or families with respect to their ability to store or/and excrete trace metals (Rainbow 2002; Marsden et al. 2014). In general, metal concentrations in bivalves increase with increasing shell size; however, in some cases, metal concentrations might decrease due to the process of growth dilution (Marsden et al. 2014).
Comparison of trace metal levels in bivalves from the Mediterranean Sea
Trace metal concentrations in whole body of bivalves were compared with those of previous studies in Table 4. Comparison of data sets revealed that Cd and Pb concentrations exhibited lower accumulation profiles at the Venice Lagoon, Balearic Islands, El Jadida coast, and Sidi Moussa Lagoon (Deudero et al. 2007; Nesto et al. 2007; Maanan 2008). On the contrary, Cr values were found higher than those of other sites (Table 4). Cu and Zn levels were detected lower in the Montenegro coast, Balearic Islands, El Jadida coast, Annaba Gulf, and Sidi Moussa Lagoon (Deudero et al. 2007; Maanan 2008; Joksimovic et al. 2011; Belabed et al. 2013). Hg concentrations in bivalves were similar to those from the Annaba Gulf, Izmir Bay, and Portonovo coast (Fattorini et al. 2008; Kucuksezgin et al. 2011; Belabed et al. 2013).
Assessment of human health risks
HQ values showed a potential hazardous impact on human consumption due to high contribution of Cr levels. Cr is one of the hazardous metals even though it is an essential trace element and plays a role in the metabolism of glucose (Mertz 1992). The biological effects of Cr are based on its oxidation state. For example, trivalent Cr is an essential trace metal, while hexavalent Cr is mutagenic, carcinogenic, and highly toxic to organisms. Also, diseases such as ulcer and lung cancer can be occurred in organisms, due to the long-term effect of Cr exposure (Eisler 2000).
HQCd in M. galloprovincialis greater than 1, while HQPb values were close to the threshold. It is known that the most serious cases of Cd poisoning could lead to severe diseases such as renal dysfunction, osteomalacia, and osteoporosis. Also, Pb is one of the main pollutant metals in the environment. Excessive Pb uptake can be ended up with mutagenic and teratogenic impacts on humans (Eisler 2000; Wright and Welbourn 2002).
As previously reported, sediments in the Homa Lagoon were enriched with Cr and Ni and considered heavily polluted (Uluturhan et al. 2011). Also, high levels of Pb, Ni, and Cr in the Homa Lagoon and Gediz Delta could be originated from domestic, agricultural, and industrial waste waters. Moreover, mining activities located at the Gediz basin affected the sediments of the Homa Lagoon and Gediz Delta (Uluturhan et al. 2011; Kucuksezgin et al. 2011).
Conclusion
This study completes a gap for valuable information about trace metal concentrations in M. galloprovincialis and T. decussatus from the Homa Lagoon. It is known that M. galloprovincialis and T. decussatus are widely utilized as bioindicators for biomonitoring studies. In this study, M. galloprovincialis is a good bioindicator in research marine environmental metal pollution; also, T. decussatus is another indicator for monitoring in the Homa Lagoon due to fact that it can accumulate metals to a considerable degree. Organic and inorganic pollutants originated from the Gediz River and the Izmir Bay strongly affect the Homa Lagoon and the Gediz Delta. The trace metal concentrations in both bivalves and the prescribed international indices (PTWI, HQ, and THI) indicated that the Cr levels exhibited a potential risk. Also, the concentrations of Cd and Pb were found at limit values for HQ. These results can be used in the evaluation of possible risks associated with the consumption of mussels and clams. Biomonitoring studies on indicator organisms should be continuously performed at ecologically protected areas such as the Homa Lagoon in the forthcoming years.
References
Ali M, Taylor A (2010) The effect of salinity and temperature on the uptake of cadmium and zinc by the common blue mussel, Mytilus edulis with some notes on their survival. Mesopot J Mar Sci 25(1):11–30
Allison N, Millward GE, Jones MB (1998) Particle processing by Mytilus edulis: effects on bioavailability of metals. J Exp Mar Biol Ecol 222(1):149–162. doi:10.1016/S0022-0981(97)00136-6
Atılgan I, Egemen Ö (2001) A comparative investigation on the levels of carbon, flammable substance and some heavy metals (Cu,Zn) accumulated in sediment of Güllük and Homa Lagoons. Ege J Fish Aqua Sci 18(1–2):225–232
ATSDR (2016) Minimal risk levels (MRLs), March 2016. http://www.atsdr.cdc.gov/mrls/mrllist.asp (accessed 25th April 2016)
Bebianno MJ, Serafim MA (1998) Comparison of metallothionein induction in response to cadmium in the gills of the bivalve molluscs Mytilus galloprovincialis and Ruditapes decussatus. Sci Total Environ 214(1):123–131. doi:10.1016/S0048-9697(98)00059-X
Bebianno MJ, Serafim MA (2003) Variation of metal and metallothionein concentrations in a natural population of Ruditapes decussatus. Arch Environ Con Tox 44(1):53–66. doi:10.1007/s00244-002-2004-7
Beiras R, Albentosa M (2004) Inhibition of embryo development of the commercial bivalves Ruditapes decussatus and Mytilus galloprovincialis by trace metals; implications for the implementation of seawater quality criteria. Aquaculture 230(1):205–213. doi:10.1016/S0044-8486(03)00432-0
Belabed BE, Laffray X, Dhib A, Fertouna-Belakhal M, Turki S, Aleya L (2013) Factors contributing to heavy metal accumulation in sediments and in the intertidal mussel Perna perna in the Gulf of Annaba (Algeria). Mar Pollut Bull 74(1):477–489. doi:10.1016/j.marpolbul.2013.06.004
Branch GM, Steffani NC (2004) Can we predict the effects of alien species? A case-history of the invasion of South Africa by Mytilus galloprovincialis (Lamarck). J Exp Mar Biol Ecol 300:189–215. doi:10.1016/j.jembe.2003.12.007
Breber P (1985) On-growing of the carpet shell clam (Tapes decussatus (L.)): two years’ experience in Venice Lagoon. Aquaculture 44:51–56. doi:10.1016/0044-8486(85)90041-9
Caçador I, Costa JL, Duarte B, Silva G, Medeiros JP, Azeda C, Castro N, Freitas J, Pedro S, Almeida PR, Cabral H, Costa MJ (2012) Macroinvertebrates and fishes as biomonitors of heavy metal concentration in the Seixal Bay (Tagus estuary): which species perform better? Ecol Indic 19:184–190. doi:10.1016/j.ecolind.2011.09.007
Catsiki VA, Florou H (2006) Study on the behavior of the heavy metals Cu, Cr, Ni, Zn, Fe, Mn and 137Cs in an estuarine ecosystem using Mytilus galloprovincialis as a bioindicator species: the case of Thermaikos gulf, Greece. J Environ Radioactiv 86(1):31–44. doi:10.1016/j.jenvrad.2005.07.005
Chapman PM, Wang F (2001) Assessing sediment contamination in estuaries. Environ Toxicol Chem 20:3–22. doi:10.1002/etc.5620200102
Cravo A, Bebianno MJ, Foster P (2004) Partitioning of trace metals between soft tissues and shells of Patella aspera. Environ Int 30:87–98. doi:10.1016/S0160-4120(03)00154-5
Cravo A, Pereira C, Gomes T, Cardoso C, Serafim A, Almeida C, Rocha T, Lopes B, Company R, Madeiros A, Norberto R, Pereira R, Araujo O, Bebianno MJ (2012) A multibiomarker approach in the clam Ruditapes decussatus to assess the impact of pollution in the Ria Formosa lagoon, south coast of Portugal. Mar Environ Res 75:23–34. doi:10.1016/j.marenvres.2011.09.012
Deudero S, Box A, March D, Valencia JM, Grau AM, Tintore J, Benedicto J (2007) Temporal trends of metals in benthic invertebrate species from the Balearic Islands, western Mediterranean. Mar Pollut Bull 54(9):1545–1558. doi:10.1016/j.marpolbul.2007.05.012
Dora EÇ, Sunlu U, Ergen Z (2007) Heavy metal concentrations in Hediste diversicolor (polychaete) and sediments from Homa Lagoon (Izmir Bay-Turkey). CIESM 38th meeting:253
Eisler R (2000) Handbook of chemical risk assessment: health hazards to humans, plants and animals. Lewis Publishers, Florida
El Nemr A, Khaled A, Moneer AA, El Sikaily A (2012) Risk probability due to heavy metals in bivalve from Egyptian Mediterranean coast. Egypt J Aqua Res 38(2):67–75. doi:10.1016/j.ejar.2012.11.001
El-Sikaily A, Khaled A, El Nemr A (2004) Heavy metals monitoring using bivalves from Mediterranean Sea and Red Sea. Environ Monit Asses 98:41–58. doi:10.1023/B:EMAS.0000038178.98985.5d
European Union (2000) Directive 2000/60/EC of the European Parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy. Official Journal L 327
FAO (2011) FAOSTAT, Food Supply-Livestock and Fish Primary Equivalent http://fenix.fao.org/faostat/beta/en/#data/CL Accessed 18 October 2016
FAO/WHO (2004) Summary of evaluations performed by the joint FAO/WHO expert committee on food additives (JECFA 1956–2003). ILSI Press International Life Sciences Institute, Washington
FAO/WHO (2007) Summary of evaluations performed by the joint FAO/WHO expert committee on food additives (JECFA 1956–2007) (first through 68th meetings). Food and Agriculture Organization of the United States and the World Health Organization. ILSI Press International Life Sciences Institute, Washington
FAO/WHO (2010) Summary of evaluations performed by the joint FAO/WHO expert committee on food additives. Seventy-third meeting, Geneva, 8–17 June 2010
Fattorini D, Notti A, Di Mento R, Cicero AM, Gabellini M, Russo A, Regoli F (2008) Seasonal, spatial and inter-annual variations of trace metals in mussels from the Adriatic Sea: a regional gradient for arsenic and implications for monitoring the impact of off-shore activities. Chemosphere 72(10):1524–1533. doi:10.1016/j.chemosphere.2008.04.071
Fernandes C, Fontainhas-Fernandes A, Cabral D, Salgado MA (2008) Heavy metals in water, sediment and tissues of Liza saliens from Esmoriz-Paramos lagoon, Portugal. Environ Monit Asses 136:267–275. doi:10.1007/s10661-007-9682-6
Giarratano E, Amin OA (2010) Heavy metals monitoring in the southernmost mussel farm of the world (Beagle Channel, Argentina). Ecotox Environ Safe 73(6):1378–1384. doi:10.1016/j.ecoenv. 2010.06.023
Guerra K, Konz J, Lisi K, Neebrem C (2010) Exposure factors handbook. USEPA, Washington DC
Irato P, Santovito G, Cassini A, Piccinni E, Albergoni V (2003) Metal accumulation and binding protein induction in Mytilus galloprovincialis, Scapharca inaequivalvis, and Tapes philippinarum from the lagoon of Venice. Arch Environ Con Tox 44:476–484. doi:10.1007/s00244-002-1262-8
Ivanković D, Pavičić J, Erk M, Filipović-Marijić V, Raspor B (2005) Evaluation of the Mytilus galloprovincialis Lam. digestive gland metallothionein as a biomarker in a long-term field study: seasonal and spatial variability. Mar Pollut Bull 50(11):1303–1313. doi:10.1016/j.marpolbul.2005.04.039
Joksimovic D, Tomic I, Stankovic AR, Jovic M, Stankovic S (2011) Trace metal concentrations in Mediterranean blue mussel and surface sediments and evaluation of the mussels quality and possible risks of high human consumption. Food Chem 127(2):632–637. doi:10.1016/j.foodchem.2011.01.057
Kennedy PC (1986) The use of molluscs for monitoring trace elements in the marine environment in New Zealand 1. The contribution of ingested sediment to the trace element concentrations in New Zealand molluscs. New Zeal J Mar Fresh 20:627–640. doi:10.1080/00288330.1986.9516183
Kucuksezgin F, Kacar A, Kucuksezgin G, Uluturhan E (2010) Monitoring metal contamination levels and fecal pollution in clam (Tapes decussatus) collected from Izmir Bay (Turkey). Environ Monit Asses 162(1–4):407–415. doi:10.1007/s10661-009-0805-0
Kucuksezgin F, Kayatekin BM, Uluturhan E, Uysal N, Acikgoz O, Gonenc S (2008) Preliminary investigation of sensitive biomarkers of trace metal pollution in mussel (Mytilus galloprovincialis) from Izmir Bay (Turkey). Environ Monit Asses 141(1–3):339–345. doi:10.1007/s10661-007-9900-2
Kucuksezgin F, Kontas A, Uluturhan E (2011) Evalutions of heavy metal pollution in sediment and Mullus barbatus from the Izmir Bay (eastern Aegean) during 1997-2009. Mar Pollut Bull 62:1562–1571. doi:10.1016/j.marpolbul.2011.05.012
Kumar V, Sinha AK, Rodrigues PP, Mubiana VK, Blust R, De Boeck G (2015) Linking environmental heavy metal concentrations and salinity gradients with metal accumulation and their effects: a case study in 3 mussel species of Vitória estuary and Espírito Santo bay, Southeast Brazil. Sci Total Environ 523:1–15
Maanan M (2008) Heavy metal concentrations in marine molluscs from the Moroccan coastal region. Environ Pollut 153(1):176–183. doi:10.1016/j.envpol.2007.07.024
María-Cervantes A, Jiménez-Cárceles FJ, Álvarez-Rogel J (2009) As, Cd, Cu, Mn, Pb, and Zn contents in sediments and mollusks (Hexaplex trunculus and Tapes decussatus) from coastal zones of a Mediterranean lagoon (Mar Menor, SE Spain) affected by mining wastes. Water Air Soil Pollut 200:289–304. doi:10.1007/s11270-008-9913-7
Marsden JD, Smith BD, Rainbow PS (2014) Effects of environmental and physiological variables on the accumulated concentrations of trace metals in the New Zealand cockle Austrovenus stutchburyi. Sci Total Environ 470-471:324–339. doi:10.1016/j.scitotenv.2013.09.085
Mertz W (1992) Chromium: history and nutritional importance. Biol Trace Elem Res 132:3–8. doi:10.1007/BF02784581
Mordoğan H, Yaramaz Ö, Alpbaz A (1990) Homa dalyanı sedimentlerinde bazı ağır metallerin (Fe, Ni, Co, Mn, Sb) derişimlerinin araştırılması. Ege J Fish Aqua Sci 8(29–30):44–50
Najdek M, Sapunar J (1987) Total and methyl-mercury content in bivalves, Mytilus galloprovincialis Lamarck and Ostrea edulis Linnaeus: relationship of biochemical composition and body size. B Environ Contam Tox 39(1):56–62. doi:10.1007/BF01691789
Nesto N, Romano S, Moschino V, Mauri M, Da Ros L (2007) Bioaccumulation and biomarker responses of trace metals and micro-organic pollutants in mussels and fish from the lagoon of Venice, Italy. Mar Pollut Bull 55(10):469–484. doi:10.1016/j. marpolbul.2007.09.009
Parlak H, Çakır A, Boyacıoğlu M, Çakal Arslan Ö (2006) Heavy metal deposition in sediments from the delta of the Gediz River (western Turkey): a preliminary study. Ege J Fish Aqua Sci 23(3–4):445–448
Pereira P, Raimundo J, Vale C, Kadar E (2009) Metal concentrations in digestive gland and mantle of Sepia officinalis from two coastal lagoons of Portugal. Sci Total Environ 407:1080–1088. doi:10.1016/j.scitotenv.2008.10.023
Pérez-Ruzafa A, Marcos C, Pérez-Ruzafa IM, Pérez-Marcos M (2011) Coastal lagoons: “transitional ecosystems” between transitional and coastal waters. J Coastal Conserv 15(3):369–392. doi:10.1007/s11852-010-0095-2
Phillips DJH (1977) Effects of salinity on the net uptake of zinc by the common mussel Mytilus edulis. Mar Biol 41:79–88
Rainbow PS (2002) Trace metal concentrations in aquatic invertebrates: why and so what? Env Poll 120:497–507. doi:10.1016/S0269-7491(02)00238-5
Rainbow PS, Philips DJH (1993) Cosmopolitan bioindicators of trace metals. Mar Pollut Bull 26:593–601. doi:10.1016/0025-326X(93)90497-8
Regoli F, Orlando E (1994) Seasonal variation of trace metal concentrations in the digestive gland of the Mediterranean mussel Mytilus galloprovincialis: comparison between a polluted and a non-polluted site. Arch Environ Con Tox 27(1):36–43. doi:10.1007/BF00203885
Richir J, Gobert S (2014) The effect of size, weight, body compartment, sex and reproductive status on the bioaccumulation of 19 trace elements in rope-grown Mytilus galloprovincialis. Ecol Indic 36:33–47. doi:10.1016/j.ecolind.2013.06.021
Roméo M, Frasila C, Gnassia-Barelli M, Damiens G, Micu D, Mustata G (2005) Biomonitoring of trace metals in the Black Sea (Romania) using mussels Mytilus galloprovincialis. Water Res 39:596–604. doi:10.1016/j.watres.2004.09.026
Scudiero R, Cretì P, Trinchella F, Esposito MG (2014) Evaluation of cadmium, lead and metallothionein contents in the tissues of mussels Mytilus galloprovincialis from the Campania coast (Italy): levels and seasonal trends. C R Biol 337(7):451–458. doi:10.1016/j.crvi.2014.05.003
Serdar S, Lök A, Köse A, Yildiz H, Acarli S, Goulletquer P (2007) Growth and survival rates of carpet shell clam (Tapes decussatus Linnaeus, 1758) using various culture methods in Sufa (Homa) Lagoon, Izmir, Turkey. Aquac Eng 37:89–99. doi:10.1016/j.aquaeng.2007.02.004
Sokolowski A, Bawazir AS, Wolowicz M (2004) Trace metals in the brown mussel Perna perna from the coastal waters off Yemen (Gulf of Aden): how concentrations are affected by weight, sex, and seasonal cycle. Arch Environ Con Tox 46(1):67–80. doi:10.1007/s00244-003-2164-0
Sridhara Chary N, Kamala CT, Samuel Suman Raj D (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotox Environ Safe 69(3):513–524. doi:10.1016/j.ecoenv.2007.04.013
Sunlu U, Egemen Ö (1998) Homa Dalyanı ve İzmir Körfezi’nin (Ege Denizi) farklı bölgelerindeki kirlenme durumu ile bazı ekonomik balık türlerinde ağır metal düzeylerinin araştırılması. Ege J Fish Aqua Sci 15(3–4):241–261
Szefer P (1986) Some metals in benthic invertebrates in Gdansk Bay. Mar Pollut Bull 17:503–507. doi:10.1016/0025-326X(86)90639-9
Szefer P, Kim CK, Kim EH, Lee CB (2004) Distribution and coassociations of trace elements in soft tissue and byssus of Mytilus galloprovincialis relative to the surrounding seawater and suspended matter of the southern part of the Korean Peninsula. Environ Pollut 106:299–314. doi:10.1016/j.envpol.2003.10.012
Uluturhan E, Kontas A, Can E (2011) Sediment concentrations of heavy metals in the Homa Lagoon (eastern Aegean Sea): assessment of contamination and ecological risks. Mar Pollut Bull 62:1989–1997. doi:10.1016/j.marpolbul.2011.06.019
UNEP (1982) Reference methods for marine pollution studies, 14
UNEP (1984) Determination of total Cd, Zn, Pb and Cu in selected marine organisms by flameless AAS, vol. 11
USEPA (2010) Risk-based concentration table. Philadelphia: United States Environmental Protection Agency, Washington DC
USEPA (2013) Reference Dose (RfD) description and using health risk assessments, Background document 1A, integrated risk information system (IRIS) United States Environmental Protection Agency, Washington DC
Usero J, Gonzalez-Regalado E, Gracia I (1997) Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic coast of southern Spain. Environ Int 23(3):291–298. doi:10.1016/S0160-4120(97)00030-5
Válega M, Lillebo A, Pereira M, Cacador I, Duarte A, Pardal M (2008) Mercury in salt marshes ecosystems: Halimione portulacoides as biomonitor. Chemosphere 73:1224–1229. doi:10.1016/j.chemosphere.2008.07.053
Viaroli P, Lasserre P, Campostrini P (2007) Lagoons and coastal wetlands: preface. Hydrobiologia 577:1–3. doi:10.1007/s10750-006-0412-9
Vlahogianni T, Dassenakis M, Scoullos MJ, Valavanidis A (2007) Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos Gulf of Greece. Mar Pollut Bull 54(9):1361–1371. doi:10.1016/j.marpolbul.2007.05.018
Wright DA, Welbourn P (2002) Environmental toxicology. Cambridge environmental chemistry series / 11. Cambridge University Press, New York
Acknowledgements
This research was funded by the Department of Scientific Research Projects of Dokuz Eylül University (project no: 2014.KB.FEN.004). Grateful thanks to especially our colleagues for their support in the field and in the laboratory. This article has been prepared within the context of Master Thesis studies of Mustafa Bilgin.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bilgin, M., Uluturhan-Suzer, E. Assessment of trace metal concentrations and human health risk in clam (Tapes decussatus) and mussel (Mytilus galloprovincialis) from the Homa Lagoon (Eastern Aegean Sea). Environ Sci Pollut Res 24, 4174–4184 (2017). https://doi.org/10.1007/s11356-016-8163-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8163-2