Abstract
Phosphate compounds and related materials are effective amendments for immobilization of heavy metals in contaminated soils. A greenhouse pot experiment with ryegrass (Lolium perenne) as the test plant was conducted to explore the impact of nanoparticle hydroxyapatite (HAP) on the immobilization and bioavailability of Cu and Zn in a heavy metal-polluted soil. The addition of nanoparticle HAP significantly decreased the uptake of Cu and Zn by ryegrass. As a result, the biomass of ryegrass increased as the rate of nanoparticle HAP increased. The toxicity characteristic leaching procedure (TCLP) and physiologically based extraction test (PBET) results of the treatments showed that the leachable and bioaccessible concentrations of Cu and Zn were significantly reduced after the soil stabilized with nanoparticle HAP. The XRD pattern of nanoparticle HAP was not changed by the presence of Cu and Zn, which suggests that Cu and Zn were immobilized by the formation of solid amorphous phosphate. Nanoparticle HAP was an effective material to immobilize heavy metals in contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphate compounds and related materials are effective amendments for in situ immobilization of heavy metals in contaminated soils or solid wastes (Ma et al. 2002; Liu and Zhao 2007; Wang et al. 2008; Cao et al. 2009; Chen et al. 2009; Wang et al. 2014). Calcium hydroxyapatite (HAP), Ca10(PO4)6(OH)2, is the main inorganic component of hard tissues of vertebrates such as bones and teeth and is widely used in environmental pollution remediation because it is environmental friendly and is highly efficient. It plays an important role in the purification of wastewaters (Corami et al. 2007; Yan et al. 2014; Wei et al. 2014) and the remediation of heavy metal-contaminated soils (Chaturvedi et al. 2006; Chen et al. 2006; Liu and Zhao 2007; Li et al. 2013; Wang and Zhang 2014). The possible reaction mechanisms for the immobilization of heavy metals by HAP may include ion exchange, surface complexation, and precipitation as new metal phosphates (Choy and McKay 2005; Smiciklas et al. 2006; Corami et al. 2007).
Rock phosphate with smaller grain size was more effective than a larger grain size in decreasing the bioavailabilities of Cd(II), Pb(II), and Zn(II) in soils. The rock phosphate with size <35 μm was not only highly effective in reducing Cd(II), Pb(II), and Zn(II) uptake by plants but also enhanced the transformation of large amounts of heavy metals from nonresidual fractions to residual ones in the soil (Chen et al. 2006). This enhancement is possibly due to its high specific surface area. Nano-sized particles possess a higher adsorption capacity for metal ions than normal-sized particles (Liu and Zhao 2007). Immobilization of heavy metals by HAP nanoparticles in water has been reported, but little is known about the effects of these nanoparticles on metal immobilization in soils and their effect on the uptake of heavy metals by plants. He found that the addition of nHAP reduced the water-soluble, bioaccessible, and phytoavailable Pb/Cd by forming the Pb/Cd phosphate (e.g., hydroxypyromorphite-like mineral). The immobilization mechanism mainly involved both cation exchange and partial dissolution of the nHAP amendment and precipitation of heavy metal-containing phosphates (He et al. 2013). Jin also found that nanoparticle HAP significantly reduced the Pb concentrations in ryegrass grown in Pb-contaminated soils. The reduction of the Pb content in the ryegrass could be attributed to the formation of pyromorphite in the soil, which reduced the metal ion activity in the soil solution and reduced the plants’ absorptive capacity (Jin et al. 2016).
Soil heavy metal pollution is very serious in China. According to the survey results of soil pollution in China released by MEP and MLR in 2014, up to 16.1% of the total survey sites fail to attain soil environment quality standards, and among those nonattainment sites, 82.8% are caused by inorganic pollutants. Of the survey samples, 2.1 and 0.8% exceed the national environmental quality standards for Cu and Zn, respectively (MEP 2014). In the present study, Cu and Zn was chosen as the objective compounds. The main objective of this study is to investigate the immobilization of heavy metals in a nanoparticle HAP-amended soil and the effect of nanoparticle hydroxyapatite on the uptake of heavy metals by ryegrass. The results will help us to find an effective material to alleviate heavy metal toxicity to plants in contaminated soils.
Materials and methods
Soil characteristics
The test soil was collected from a residential area (0–20 cm in depth) in Guixi City, Jiangxi Province, China. The soil was contaminated by a nearby Cu smelting factory. The soil sample was air-dried and then ground to pass through a 2-mm sieve for the pot trial. The total Cu and Zn and other physicochemical properties of the soil sample were determined using standard methods recommended by the Chinese Society of Soil Science (Lu 1999). Some soil properties were pH of 4.80, organic matter content of 26.7 mg kg−1, and total Cu and Zn content of 547 and 70.4 mg kg−1, respectively.
Pot experiment
The nanoparticle HAP was added to the soil at rates of 0, 1, 3, and 5% (w/w, on air-dry weight basis), and the weight of the soil and nanoparticle HAP mixture was 0.5 kg per pot with four replications of each treatment. Ryegrass was planted with 1.0 g seeds per pot. During the experiment, soil water content was maintained at about 70% of field capacity. The aboveground part of the ryegrass was harvested using stainless steel scissors at the end of the first and second month. The harvested plant material was weighed for fresh weight and then rinsed with deionized water before drying in an oven at 75 °C for 48 h to obtain dry weight and then ground to pass a 100-mesh screen. Heavy metal concentrations in the plant materials were determined via flame atomic absorption spectrometry (AAS) after wet digestion. Briefly, 0.200 g plant samples were weighed and digested with 15 mL concentrated HNO3 and 1 mL concentrated HClO4. The digested solution was then transferred into a volumetric flask and brought to a fixed volume for metal determination by AAS.
Bioavailability of heavy metals by CaCl2 extraction
The plant available soil Cu and Zn were determined by 0.01 M CaCl2 extraction. Briefly, 5.0 g of soil was extracted with 25 mL of 0.01 M CaCl2 for 2 h at 25 °C and filtered. Both Cu and Zn concentrations in the extract were determined by AAS.
Modified Community Bureau of Reference three-step sequential extraction procedure
The metal distribution in soils was evaluated by using the modified Community Bureau of Reference (BCR) three-step sequential extraction procedure (Hwang et al. 2008). First, a 1 g soil sample was extracted with 40 mL of 0.11 mol L−1 acetic acid solution by shaking in a mechanical, end-over-end shaker at 30 ± 10 rpm at 22 ± 5 °C for 16 h to determine the exchangeable and weak acid-soluble fraction (BCR1). The extract was separated by centrifugation at 9000×g for 10 min, collected in polyethylene bottles, and stored at 4 °C until analysis. The residue was washed by shaking for 15 min with 20 mL of double deionized water and then centrifuged to discard the supernatant. Then, 40 mL of 0.5 mol L−1 hydroxylammonium chloride solution was added to the residue from the first step, and the mixture was shaken at 30 ± 10 rpm at 22 ± 5 °C for 16 h to extract the reducible fraction (BCR2). The acidification of this reagent was carried out by the addition of 2 mol L−1 HNO3 solution. The extract was separated, and the residue was washed as in the first step. Finally, 10 mL of 8.8 mol L−1 hydrogen peroxide solution was carefully added to the residue from the second step to quantify the oxidizable fraction (BCR3). The mixture was digested for 1 h at 22 ± 5 °C and for 1 h at 85 ± 2 °C, and the volume was reduced to less than 3 mL. A second aliquot of 10 mL of H2O2 was added; the mixture was digested for 1 h at 85 ± 2 °C, and the volume was reduced to about 1 mL. The residue was extracted with 50 mL of 1 mol L−1 ammonium acetate solution (adjusted to pH 2.0), after shaking at 30 ± 10 rpm and 22 ± 5 °C for 16 h. The extract was separated, and the residue was washed as in previous steps. The residual fraction (BCR4) was obtained by subtracting the sum of the three fractions described above from the soil total heavy metal.
Modified toxicity characteristic leaching procedure
Immobilization efficiency was evaluated by using the no. 2 solution of the toxicity characteristic leaching procedure (TCLP) (Liu and Zhao 2007; Wang et al. 2008). A 5.7 mL aliquot of glacial acetic acid was diluted to 1 L with deionized water. The pH of the extracting solution was 2.88. Twenty milliliters of extracting solution was added to a 50-mL centrifuge tube containing 1 g of test material. The centrifuge tubes were then placed on an end-to-end shaker at 180 excursions per minute for 18 h at 23 °C. The supernatant was separated from the adsorbent by centrifuging at 9000×g for 10 min, filtered through a 0.45-μm membrane filter, and analyzed for Cu and Zn by AAS.
Modified physiologically based extraction test
The bioavailability of heavy metals was also determined by a modified physiologically based extraction test (PBET) procedure (Morrison and Gulson 2007; Tang et al. 2008). The gastric solution for the PBET was prepared by adding 5 g of pepsin (P7000, Sigma Chemical Co.), 2 g of anhydrous citric acid, 2 g of DL-malic acid, 1.68 mL of DL-lactic acid, and 2 mL of glacial acetic acid to 4 L of deionized water. Trace metal grade concentrated HCl was added to the gastric solution to maintain the soil-gastric solution mixture pH near 2.0. Two hundred milliliters of prewarmed gastric solution (37 °C) was combined with 2 g soil in a 250-mL wide-mouth HDPE bottle covered with a cap fitted with a rubber septum. The headspace was replaced with Ar gas, and the bottle was shaken for 1 h in a controlled temperature chamber maintained at 37 °C. The pH was maintained at 2.00 ± 0.2 throughout this period. After 1 h, a 10 mL aliquot of gastric solution was removed for analysis using a cellulose acetate membrane filter attached to a 10-mL disposable syringe. The 10 mL aliquot was replaced with 10 mL of fresh simulated gastric solution at 37 °C. The suspension of the bottle was adjusted to pH 6.5 by adding NaHCO3, 0.345 g of bile extract (porcine, 8008-63-7; Sigma, St. Louis, MO), and 0.1 g of pancreatin (porcine pancreas-4X 8049-47-6; Sigma) were added. The bottle was recapped; the headspace was replaced with argon gas, and the bottle was shaken for 1 h at 37 °C. Another 10 mL of aliquot was removed as before for analysis. A drop of trace metal grade concentrated HCl acid was added to each aliquot to acidify the sample. The samples were stored at 4 °C prior to analysis by AAS. All in vitro tests were performed in duplicate for each soil sample.
XRD analysis
Soil samples amended with 5% nanoparticles were examined by a Rigaku X-ray diffractometer equipped with a stepping motor and graphite crystal monochromator. Scans were conducted from 2° to 60° at a rate of 2°θ per minute. Nanoparticle HAP saturated with Cu and Zn was used as references. The preparation of nanoparticle HAP saturated with Cu and Zn followed the method: Aliquot of nanoparticle HAP was added into 1 mol L−1 of Cu and Zn solution at pH 6 for 24 h at 25 °C; after the equilibrium, the solid was separated from the equilibrium solution by centrifuging at 9000×g for 10 min and then washed with deionized water several times until the conductivity of the filtrate was less than 2 mS m−1. The solid was freeze-drying for the further XRD analysis.
Results
The effect of nanoparticle HAP on plant biomass and the uptake of Cu and Zn
The effect of nanoparticle HAP on the biomass of ryegrass is shown in Table 1. Nanoparticle HAP significantly increased the biomass of ryegrass, and the more nanoparticle HAP that was added, the more biomass of ryegrass produced. Compared with the control, the fresh weight of ryegrass increased by 297, 629, and 644% for the first harvest and by 650, 814, and 890% for the second cut with the addition of 1, 3, and 5% nanoparticle HAP, respectively. However, the biomass increase from 3 to 5% nanoparticle HAP amendment was not significant. As shown in Table 2, the addition of nanoparticle HAP significantly decreased the uptake of Cu and Zn by ryegrass from compared to the control soil. The contents of Cu and Zn in the aboveground part of ryegrass decreased by 45.5 and 24.8% amended with 1% nanoparticle HAP in the first harvest and decreased by 25 and 15.4% in the second harvest, respectively. Adding more than 1% nano-HAP, however, did not further reduce Cu and Zn uptake.
The effect of nanoparticle HAP on the bioavailability of heavy metals evaluated by CaCl2 extraction
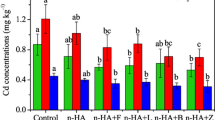
In order to explore the mechanism of the effect of nanoparticle HAP on the uptake of heavy metals, the bioavailability of Cu and Zn in soil using CaCl2 extraction was determined and is shown in Fig. 1. The addition of nanoparticle HAP decreased the bioavailability of Cu and Zn markedly. The reduction of bioavailability increased as the nanoparticle HAP concentration increased. The mean Cu and Zn concentrations in the CaCl2 extract were reduced from 37.9 in the control to 12.2, 2.6, and 1.0 mg kg−1 (67.8, 93.0, and 97.3% reduction) and from 7.7 to 2.8, 0.7, and 0.1 mg kg−1 (63.1, 91.5, and 98.5% reduction) after stabilization of the contaminated soils with 1, 3, and 5% nanoparticle HAP, respectively. The uptake of Cu and Zn by ryegrass was positively correlated with the concentrations of Cu and Zn in the soil extracted with CaCl2.
The effect of nanoparticle HAP on the fractionation of metals
A sequential extraction was conducted on both the control and the treated soils in order to investigate the efficiency of the tested nanoparticle HAP. It is clear that the effects of nanoparticle HAP on the species transformation of Cu and Zn were quite different (Table 3). In the absence of nanoparticle HAP, the exchangeable/acid-soluble fraction (BCR1) of Cu was maintained at about 41.4% and the residual fraction (BCR4) reached 32.4%. However, in the case of Zn, the acid-soluble fraction was at about 21.0% and the residual fraction reached 61.8%. Most of the Cu was in the acid fraction phase which was more bioavailable. This may be why the ryegrass grew poorly without nanoparticle HAP. In the presence of nanoparticle HAP, the acid-soluble fraction of Cu decreased significantly. The acid-soluble fraction of Cu decreased by 9.3, 24.4, and 35.7% with the treatment of 1, 3, and 5% nanoparticle HAP, respectively. Meanwhile, the reducible fraction and oxidizable fractions (BCR3) were increased significantly with nanoparticle HAP treatment. When 5% nanoparticle hydroxyapatite was added, Cu in the reducible fraction (BCR2) and oxidizable fractions increased about 5.5 and 83.5%, respectively.
The effect of nanoparticle HAP amendment on metal leachability
The presence of nanoparticle HAP caused a pronounced reduction of the TCLP leachable Cu and Zn concentrations compared to that of the control sample (Fig. 2). The mean Cu and Zn leachable concentrations decreased from 79.3 to 57.2, 40.6, and 29.5 mg kg−1 (27.9, 48.8, and 62.8% reduction) and from 8.3 to 8.15, 6.40, and 6.06 mg kg−1 (1.8, 22.9, and 27.0% reduction) after stabilization of the contaminated soils with 1, 3, and 5% nanoparticle HAP, respectively.
The effect of nanoparticle HAP on the bioaccessibility of Cu and Zn
Table 4 shows the bioaccessibility of Cu and Zn for the soil amended with and without nanoparticle HAP as evaluated by using the PBET procedure. The bioaccessibility of Cu in the soil was 69.9 and 57.7% of the total soil Cu in the gastric and intestinal phases, respectively. The bioaccessibility of Cu in gastric phase was higher than that in the intestinal phase, which may be due to the lower pH of the gastric solution. Heavy metals are more soluble in soils with lower pH. The presence of nanoparticle HAP decreased bioaccessibility of Cu in the soil. The mean Cu bioaccessibility concentrations were reduced from 380.7 to 375.7, 358.2, and 346.2 mg kg−1 (1.3, 5.9, and 9.1% reduction) in the gastric phase after stabilization of the contaminated soils with 1, 3, and 5% nanoparticle HAP, respectively. We also found similar results for the intestinal phase when nanoparticle HAP was added.
Discussions
Due to the quick industrialization and urbanization, heavy metal pollution in urban soils, agricultural soils, especially soils around the mining area becomes serious during the last two decades in China (Wei and Yang 2010). Zhou et al. (2007) found that the paddy soil in the vicinity of the mining areas was seriously polluted by Cu, Zn, Cd, and Pb with the concentration of 567, 1140, 2.48, and 191 mg kg−1, respectively, and tailings and acid mine drainage were the main pollution sources. Fernández-Caliani et al. (2009) also found that the soils nearby the mining areas were seriously polluted with heavy metals. In the present study, the soil was also seriously polluted with Cu of 547 mg kg−1. Zhou et al. (2008) found that the surface water and the rice were polluted with heavy metal at the same area where we collected the soil sample. Of rice samples, 38.5% were polluted by Cu. High concentration of Cu in rice will affect people’s health by the food chain. How to reduce the uptake of heavy metal in plant and decrease the bioavailability of metals in soils has been considered by many scientists.
Soil heavy metal cannot be destroyed degraded like organic contaminants but only be relocated from one place, such as landfilling. The high cost of traditional soil remediation techniques (excavation and landfilling) prompted the development of alternative techniques that are cost-effective and less disruptive to the environment such as soil stabilization (Kumpiene et al. 2008). The stabilization of trace element-contaminated soils is a remediation technique used to reduce element mobility in soils by adding immobilizing agents. Contaminant immobilizing amendments decrease trace element leaching and their bioavailability by inducing various sorption processes: adsorption to mineral surfaces, formation of stable complexes with organic ligands, surface precipitation, and ion exchange (Kumpiene et al. 2008).
There are a lot of low-cost materials used to stabilize heavy metals in soils such as industrial by-product, agricultural by-product, some clays, and phosphate-related materials (Babayan et al. 2012; Kim and Owens 2010; Kumpiene et al. 2008; Pandey et al. 2009). Phosphate compounds and related materials are very effective amendments for stabilization of heavy metals in contaminated soils. Chen et al. (2009) studied the effects of different phosphate amendments on lead (Pb) uptake in cauliflower (Brassica oleracea L.) in Pb-contaminated soils by pot experiments. They found that lead concentration of shoot and root of the plant was reduced markedly after the addition of different phosphate amendments when compared to controls. There was a positive correlation between the Pb content and the activities of SOD/the MDA content in plant tissues and the activities of SOD; the MDA content significantly decreased in the presence of HAP; HAP enhanced the plant resistance to lead stress in contaminated soils and decreased the plant stress induced by lead toxicity. Their results are very similar to our study; the addition of nanoparticle HAP significantly increased the biomass ryegrass and decreased the uptake of Cu and Zn from soil.
It was widely accepted that total soil heavy metal concentration alone was not a good measure for its bioavailability and also was not a very useful tool to determine its potential environmental and human health risks from soil contamination. In fact, it is well-known that metals exist in a number of different soluble and particulate forms, which influence their reactivity and hence their mobility and bioavailability. Several methods have been used to evaluate bioavailability of trace elements in soils, which are based mainly on extractions by various solutions. Single extraction is used generally to provide a rapid evaluation of the bioavailability of metal fraction in soils and sediments such as mineral acids, chelating agents, buffered salts, neutral salts, and other extractants proposed for routine soil testing (Zhang et al. 2010). On the other hand, sequence extraction is used to provide more detailed information regarding different metal-phase associations, such as four-step BCR (Whalley and Grant 1994), five-step Tessier et al. (1979), and six-step extraction method (Kersten and Forstner 1986).
The addition of nanoparticle HAP significantly decreased the bioavailability of Cu and Zn. And the fraction of Cu and Zn in soils also changed markedly. In the presence of nanoparticle HAP, the acid-soluble fraction of Cu decreased significantly, and the reducible fraction and oxidizable fractions (BCR3) were increased markedly. The increasing reducible and oxidizable fractions were mainly due to the decrease of acid-soluble fraction, because the presence of nanoparticle HAP did not affect the residual fraction during the course of the experiment. The decrease of acid-soluble fraction led to an increase in ryegrass biomass demonstrating the effectiveness of nanoparticle HAP treatment. Chemical fractionation was used to evaluate the efficacy of the decontamination treatment. We assumed that the nonresidual metal fraction (sum of the exchangeable, carbonate, Fe–Mn oxides, and organic fraction) is more bioavailable than the residual fraction. The effectiveness of in situ remediation of metal-contaminated soils can be improved with more practical treatments that convert greater amounts of metal from the nonresidual to the residual fraction or from more bioavailable to less bioavailable forms.
Modified TCLP and modified PBET are the common methods to study the stabilization and the bioaccessibility of metals in soils (Hettiarachchi et al. 2000; Marschner et al. 2006; Morrison and Gulson 2007; Tang et al. 2008). Liu and Zhao (2007) studied the feasibility of using a new class of iron phosphate (vivianite) nanoparticles as a stabilizer for in situ immobilization of Cu(II) in soils. They found that when the soils were amended with the nanoparticle for 1 day, the Cu leachability in the acidic soil decreased from original 58 to 35%, or a 39% decrease. Hettiarachchi et al. (2000) studied the effects of P on bioaccessible Pb in five metal-contaminated soils by a modified PBET; they found that the addition of P reduced bioaccessible Pb in all five materials. Compared to the unamended control, reductions in bioaccessible Pb in stomach-phase extractions upon ranged from 15 to 41%. Their results confirm our study that the addition of phosphate materials can reduce the leachability of metals in soil. In our study, the bioaccessibility of Cu in stomach was high than that in intestinal phase, which may due to lower pH in stomach solution than that in intestinal solution. Heavy metal can be dissolute from soils in lower pH. The presence of nanoparticle HAP decreased bioaccessibility of Cu in soil.
Hydroxyapatite has strong ability to fix heavy metals and has been used for purification of wastewaters and soil remediation. The reaction mechanisms for metal ion immobilization include ion exchange processes, surface complexation, dissolution of HAP to form new metal phosphates, and substitution of Ca in HAP by other metal during recrystallization. In order to explore the mechanism of nanoparticle HAP on the bioavailability of Cu and Zn, soil samples amended with 1, 3, and 5% nanoparticles were examined by XRD. As shown in Fig. 3, saturation with Cu and Zn did not change the XRD pattern of nanoparticle HAP, which suggests that Cu and Zn were immobilized by the formation of amorphous phosphate instead of crystalline compounds. Sugiyama et al. (2003) found that calcium hydrogen phosphate was able to effectively remove Cu(II) as well as Pb(II), Cd(II), and Co(II) from solution and suggested that dissolution–precipitation was responsible for the heavy metal removal. They also found that Cu(II) was immobilized by solid phosphate through the formation of amorphous phases as evidenced by XRD analysis. Ma et al. (1994) also failed to find new XRD peaks of a copper solid phase on the hydroxyapatite surface in the presence of Cu, but they observed different shaped precipitates on the surface using SEM, confirming the formation of amorphous copper phosphate precipitate(s). When nanoparticle HAP was added to soils, we found the characteristic peak of nanoparticle HAP, and the intensity of the peak was related to the amount of nanoparticle HAP added. Nanoparticle HAP can immobilize heavy metals in soil solutions and reduce the availability of Cu and Zn to plants by adsorption and/or precipitation.
Conclusions
Pot experiments with ryegrass as the test plant were conducted to explore the mechanism of using nanoparticle HAP to reduce bioavailability of Cu and Zn in heavy metal-polluted soils. Adding as little as 1% of nanoparticle HAP significantly decreased the uptake of Cu and Zn by ryegrass and increased the biomass of ryegrass. More nanoparticle HAP addition can result in more biomass increase. The addition of nanoparticle HAP decreased the acid-soluble fraction of Cu and Zn and increased the reducible fraction and oxidizable fractions in soils. The TCLP and PBET tests revealed that the leachable and bioaccessible concentrations of Cu and Zn were significantly reduced after the soil was stabilized with nanoparticle HAP. Copper and Zn were immobilized by solid phosphate through the formation of amorphous phases. Nanoparticle HAP was an effective material to alleviate heavy metal toxicity to plants in contaminated soils.
References
Babayan M, Javaheri M, Tavassoli A, Esmaeilian Y (2012) Effects of using wastewater in agricultural production. Afr J Microbiol Res 6:1–6
Cao X, Wahbi A, Ma L, Li B, Yang Y (2009) Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J Hazard Mater 164:555–564
Chaturvedi PK, Seth CS, Misra V (2006) Sorption kinetics and leachability of heavy metal from the contaminated soil amended with immobilizing agent (humus soil and hydroxyapatite). Chemosphere 64:1109–1114
Chen SB, Zhu YG, Ma YB (2006) The effect of grain size of rock phosphate amendment on metal immobilization in contaminated soils. J Hazard Mater 134:74–79
Chen S, Chen L, Ma Y, Huang Y (2009) Can phosphate compounds be used to reduce the plant uptake of Pb and resist the Pb stress in Pb-contaminated soils? J Environ Sci 21:360–365
Choy KKH, McKay G (2005) Sorption of metal ions from aqueous solution using bone char. Environ Int 31:845–854
Corami A, Mignardi S, Ferrini V (2007) Copper and zinc decontamination from single- and binary-metal solutions using hydroxyapatite. Environ Int 146:164–170
Fernández-Caliani JC, Barba-Brioso C, Gonzalez I, Galan E (2009) Heavy metal pollution in soils around the abandoned mine sites of the iberian pyrite belt (Southwest Spain). Water Air Soil Poll 200:211–226
He M, Shi H, Zhao XY, Yu Y, Qu B (2013) Immobilization of Pb and Cd in contaminated soil using nanocrystallite hydroxyapatite. Procedia Environ Sci 18:657–665
Hettiarachchi GM, Pierzynski GM, Ransom MD (2000) In situ stabilization of soil lead using phosphorus and manganese oxide. Environ Sci Technol 34:4614–4619
Hwang A, Ji W, Kweon B, Khim J (2008) The physico-chemical properties and leaching behaviors of phosphatic clay for immobilizing heavy metals. Chemosphere 70:1141–1145
Jin Y, Liu W, Li XL, Shen SG, Liang SX, Liu CQ, Shan LY (2016) Nano-hydroxyapatite immobilized lead and enhanced plant growth of ryegrass in a contaminated soil. Ecol Eng 95:25–29
Kersten M, Forstner U (1986) Chemical fractionation of heavy-metals in anoxic estuarine and coastal sediments. Water Sci Technol 18:121–130
Kim KR, Owens G (2010) Potential for enhanced phytoremediation of landfills using biosolids—a review. J Environ Manag 91:791–797
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of as, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manag 28:215–225
Li LP, Xing WQ, Scheckel KG, Xiang GQ, Ji HH, Li H (2013) Lead retention in a calcareous soil influenced by calcium and phosphate amendments. J Hazard Mater 262:250–255
Liu R, Zhao D (2007) In situ immobilization of Cu(II) in soils using a new class of iron phosphate nanoparticles. Chemosphere 68:1867–1876
Lu RK (1999) Analysis method of the soil agriculture chemistry. Chinese Agriculture Science Press, Beijing
Ma QY, Traina SJ, Logan TJ, Ryan JA (1994) Effects of aqueous Al, Cd, Cu, Fe(II), Ni, and Zn on Pb immobilization by hydroxyapatite. Environ Sci Technol 28:1219–1228
Ma QY, Traina SJ, Logan TJ, Ryan JA (2002) In situ lead immobilization by apatite. Environ Sci Technol 27:1803–1810
Marschner B, Welge P, Hack A, Wittsiepe J, Wilhelm M (2006) Comparison of soil Pb in vitro bioaccessibility and in vivo bioavailability with Pb pools from a sequential soil extraction. Environ Sci Technol 40:2812–2818
Ministry of Environmental Protection of the People's Republic of China. 2014. The investigation communique on national soil pollution condition from ministry of environmental protection of the People’s Republic of China and Ministry of Land and Resources of the People’s Republic of China. Beijing, China
Morrison AL, Gulson BL (2007) Preliminary findings of chemistry and bioaccessibility in base metal smelter slags. Sci Total Environ 382:30–42
Pandey VC, Abhilash PC, Singh N (2009) The Indian perspective of utilizing fly ash in phytoremediation, phytomanagement and biomass production. J Environ Manag 90:2943–2958
Smiciklas I, Dimovic S, Plecas I, Mitric M (2006) Removal of Co2+ from aqueous solutions by hydroxyapatite. Water Res 40:2267–2274
Sugiyama S, Ichii T, Fujisawa M, Kawashiro K, Tomida T, Shigemoto N, Hayashi H (2003) Heavy metal immobilization in aqueous solution using calcium phosphate and calcium hydrogen phosphates. J Colloid Interf Sci 259:408–410
Tang XY, Cui YS, Duan J, Tang L (2008) Pilot study of temporal variations in lead bioaccessibility and chemical fractionation in some Chinese soils. J Hazard Mater 160:29–36
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Wang X, Zhang Y (2014) Enhanced adsorption and transformation of Cu and Zn in sediments by hydroxyapatite. Asian J Chem 26:186–190
Wang B, Xie Z, Chen J, Jiang J, Su Q (2008) Effects of field application of phosphate fertilizers on the availability and uptake of lead, zinc and cadmium by cabbage (Brassica chinensis L.) in a mining tailing contaminated soil. J Environ Sci 20:1109–1117
Wang L, Li YH, Li HR, Liao XY, Wei BG, Ye BX, Zhang FY, Yang LS, Wang WY, Krafft T (2014) Stabilize lead and cadmium in contaminated soils using hydroxyapatite and potassium chloride. Environ Monit Assess 186:9041–9050
Wei BG, Yang LS (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Wei W, Cui J, Wei ZG (2014) Effects of low molecular weight organic acids on the immobilization of aqueous Pb(II) using phosphate rock and different crystallized hydroxyapatite. Chemosphere 105:14–23
Whalley C, Grant A (1994) Assessment of the phase selectivity of the European-community bureau-of-reference (BCR) sequential extraction procedure for metals in sediment. Anal Chim Acta 291:287–295
Yan YB, Wang YP, Sun XY, Li JS, Shen JY, Han WQ, Liu XD, Wang LJ (2014) Optimizing production of hydroxyapatite from alkaline residue for removal of Pb2+ from wastewater. Appl Surf Sci 317:946–954
Zhang MK, Liu ZY, Wang H (2010) Use of single extraction methods to predict bioavailability of heavy metals in polluted soils to rice. Commun Soil Sci Plan 41:820–831
Zhou JM, Dang Z, Cai MF, Liu CQ (2007) Soil heavy metal pollution around the Dabaoshan mine, Guangdong Province, China. Pedosphere 17:588–594
Zhou J, Cui J, Liang JN (2008) Assessment of heavy metals pollution on water quality and rice around the spoil area of the smeltery. Acta Agriculturae Boreali-Sinica 23:349–352 (In Chinese with English abstract)
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grants No. 41422105) and the National Basic Research and Development Program (2013CB934303) and the Natural Science Foundation of Jiangsu Province (No. BK20130050).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Sun, RJ., Chen, JH., Fan, TT. et al. Effect of nanoparticle hydroxyapatite on the immobilization of Cu and Zn in polluted soil. Environ Sci Pollut Res 25, 73–80 (2018). https://doi.org/10.1007/s11356-016-8063-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8063-5