Abstract
Accumulation of heavy metals in environment may cause series potential risk in the living system. This study was carried out to investigate heavy metal contamination in sand samples and sediments along the beach near to disposal site of reject brine from Alkhobar desalination plant, which is one of the oldest and largest reverse osmosis desalination plants in eastern Saudi Arabia, Arabian Gulf. Fourteen heavy metals (U, Ca, Fe, Al, Ti, Sr, Rb, Ni, Pb, Cd, Cr, Cu, As, and Zr) were measured using gamma-ray spectrometry, atomic absorption spectrometer (AAS) and energy dispersive X-ray fluorescence spectrometer (EDX). The obtained data revealed that the concentrations of these metals were higher than the values in sediment and soil for other studies in Arabian Gulf. Furthermore, the mean values of Fe, Mn, Cr, Cu, As, Sr, and Zr concentrations in sand and sediments were higher than the geochemical background values in shale. The contamination factor (CF), modified degree of contamination (mCd) and pollution load index (PLI) were assessed. According to contamination factors (CF > 1), the results showed elevated levels of Cu, Cr, Mn, Zr, and As in all samples. The highest value of contamination factor was found for As. Based on PLI (PLI > 1), the values of all sampling sites indicate a localized pollution in the study area. Current study could be useful as baseline data for heavy metals in sand and sediments nearby a desalination plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The desalination process is one of the mankind’s earliest designs to separate freshwater from a salt-water solution (Einav et al. 2002). Desalination involves several processes to remove the excess salt and other minerals from the water to obtain potable water for human usage. Desalination of sea or ocean water is a widespread technology used in many countries around the world.
Essentially, a desalination plant is a system to separate saline water into two streams: one with a low concentration of dissolved salts and inorganic materials and the other containing the remaining dissolved salts (brine). The amount of flow discharged to waste as a brine discharge varies from 20 to 70 % of the feed flow, depending on the technology used in the plant. Brine discharge is the fluid waste from a desalination plant, which contains a high percentage of salt and dissolved minerals, such as Cu, Cd, Pb, Hg, U, Ni, Cr, and As (Mohamed et al. 2005). The brine returns to the sea and distributes according to different aspects. Desalination plants could have several impacts on the surrounding environment. The major concern of these impacts surrounds the outfall brine discharge due to its physical and chemical features (Younos 2005; Abdul-Wahab 2007; WHO 2007).
Toxic metals might be produced if the discharge brine has contact with the metallic materials used in the plant facilities (Mohamed 2005; Abdul-Wahab 2007). The release of high amounts of heavy metals into water bodies creates serious health and environmental problems. The impacts of toxic metals on animals (marine life) include reduced growth, development, cancer, organ damage, nervous system damage, and, in extreme cases, death (Ogoyi et al. 2011; Rahman et al. 2012; Kim et al. 2015).
Saudi Arabia is the world’s largest producer of desalinated seawater. The Saline Water Conversion Office (SWCO) constructed 24 desalination plants across the Saudi Arabian coasts, including the 12 major plants on the western coast on the Red Sea and another three on the eastern coast on the Arabian Gulf (Murakami 1995). There are three major desalination plants in Saudi Arabia; Al-Jubail and Al-Khobar plants are located on Arabian gulf coast and Shoaiba plant on the Red Sea coast.
The beach near to Al Khobar desalination plant is famous for its large number of palms. In addition, this beach is constantly frequented by general population and many fishermen. Therefore, this study aims to achieve the following objectives:
-
Determine uranium concentration and heavy metals in sand and sediment.
-
Identify areas that may be chemically hazardous for population.
-
Evaluate the environmental pollution in this public area.
Finally, the data of this study will be useful in future to provide an environmental baseline information to evaluate and control the increase of heavy metals in the study area.
Materials and methods
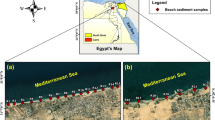
Beach sand and sediment samples were collected from different locations along Al Azizia beach near to Al Khobar desalination plant with a length interval of 6500 m (Fig. 1). From different locations near to desalination plant, 24 sand samples were collected at 2 m from the shoreline (Table 1). These samples were taken from surface and at 30 cm depth from the surface. From the same locations, 12 sediments were collected from sea water during low tide. In this study, three techniques were used for the analysis: a gamma-ray spectrometer for uranium concentration, atomic absorption spectrometry (AAS), and energy dispersive X-ray fluorescence spectrometer (EDX) for trace metals.
Gamma-ray analysis
Sample preparation
For gamma-ray measurements, sand samples were bulked as a single sample and dried in an oven at 70 °C for 24 h. After that, sand samples were prepared for radiation counting by sieving through 2 mm mesh size. Each sand sample was packed into 152-ml standard size beakers and tightly sealed and stored for 28 days to acquire secular equilibrium. Two reference materials were packed into the same standard size beakers for efficiency calibration.
Experimental setup
A Hyper pure Germanium detector (HPGe), coaxial type, P-type with relative efficiency of 20 % was exploited. The detector was shielded with a low-level background lead shield. The HPGe was calibrated for efficiency using the reference material RGU-1 from IAEA. The certified activity of uranium is 400 ppm which refers to 4960 Bq kg−1. The energy transitions of the 226Ra daughters (214Pb and 214Bi) were used to develop the efficiency calibration curve. A fourth degree polynomial fitting was performed to reach the best R 2 value (≈0.987).
Out of the three naturally occurring isotopes of uranium (234U, 235U, and 238U), 99 % is 238U which has a half-life of 4.46 × 109 years. Therefore, 238U was measured. After subtracting the background, uranium concentration was measured using its progenies 214Pb with energy 351.93 keV (37.6 %) and 214Bi with energies 609.31 keV (46.1 %), 1120.29 keV (15.1 %), and 1764.49 keV (15.4 %). Uranium was determined based on the above-mentioned energy transitions after achieving secular equilibrium for 28 days after sample packing. The average values were calculated in ppm unit (mg/kg).
Heavy metal analysis
For AAS analysis, the sand samples and sediments were digested in 10 % HNO3 solution. The digestion solution was performed by adding 3 g of sample with 30 ml of 10 % NHO3 in a beaker. After standing for 24 h, the solution was heated at 75 °C for 3 h. Then, 25 ml of 5 % HNO3 was added again to the remaining solution. After digestion, the obtained extract was filtered and transferred to 40-ml polyethylene volumetric flask for analysis. The trace metals were determined in the obtained filtrate using atomic absorption spectrometer (AAS).
The elements with high concentrations such as Ca, Fe, Al, Ti, Sr, and Rb were measured using EDX-8000 spectrometer. EDX-8000 is rapid, non-destructive technique, and sensitive for heavy and light elements. The system has a large box-shaped chamber, high visibility led lamp, X-ray tube, semiconductor camera, high-performance silicon drift detector (SDD), and PCEDX Navi software. The supply voltage is 100–240 V AC and a frequency of 50/60 Hz. Before EDX measurements, the samples were prepared in a cylindrical container of polyethylene (cell) with a diameter of 3 cm. After the sample is irradiated with X-rays inside the chamber, the qualitative and quantitative analysis can be performed by measuring the amount of X-rays at the wavelength specific to each elements.
Results and discussion
Uranium and heavy metal concentrations in sand samples and sediments collected along the beach near to disposal site of reject brine
The concentrations of heavy metals in sand samples and sediments at 12 sites near to a disposal site of reject brine and the average values of geochemical background in shale are given in Tables 2, 3, and 4. These metals included U, Ca, Fe, Al, Ti, Sr, Rb, Ni, Pb, Cd, Cr, Cu, As, and Zr. Heavy metals are a special group of trace metals which have been shown to create definite health hazards (Singare et al. 2013). The obtained results are recorded as follows:
Uranium
For sand samples, the average concentrations of uranium in sand ranged between 0.98 and 2.94 mg/kg for surface samples and between 1.58 and 2.54 mg/kg for subsurface samples. For sediment samples, uranium concentrations ranged from 1.48 to 3.04 mg/kg. The mean values of uranium in sand and sediment were within the global average in soil (0.3–11.7 mg/kg) (UNSCEAR 1993).
Nickel and copper
The concentrations of Ni and Cu ranged between 1.16 and 16.2 mg/kg and between 623 and 1022 mg/kg, respectively, for surface sand and ranged between 0.91 and 2.72 mg/kg and between 680 and 1001 mg/kg, respectively, for subsurface sand. Similarly, the concentrations of Ni and Cu for sediments ranged between 1.99 and 4.19 mg/kg and between 620 and 964 mg/kg, respectively. The results show high concentrations of Cu which were higher than the permissible value in sediment (18.7 mg/kg) (USEPA 1996). The highest concentration of Cu was found in site 7 for surface sand. Copper was recorded high concentrations in all sites for sand and sediments. High levels of Cu in sediments and sand along the shoreline may due to the use of copper-nickel alloys as heat exchanger materials in desalination plant. Brine contamination with copper is due to corrosion in these materials (Dawoud and Al Mulla 2012). Copper is an essential micronutrient for most organisms, and it might only become toxic if excess amounts become biologically available (Hoepner and Lattemann 2002).
Lead and chromium
The content of Pb and Cr in sand samples ranged between 5.37 and 16.2 mg/kg and between 1100 and 7800 mg/kg, respectively, for surface sand and ranged between 6.89 and 12.2 mg/kg and between 1550 and 9860 mg/kg, respectively, for subsurface sand. Similarly, the contents of lead and chromium for sediment ranged between 4.52 and 26.5 mg/kg and from 1040 to 5818 mg/kg, respectively. The highest value of Cr was in site 10 from subsurface sand. High contents of Cr were observed in all sand and sediment samples which are higher than the permissible value in sediment (25 mg/kg) (WHO 2004), while low contents of Pb were observed in most of the samples. Generally, concentrations of Pb in all samples were lower than the permissible value of 40 mg/kg in sediment (USEPA 1999). However, all lead compounds are potentially harmful or toxic (Jenkins 1989). The high concentrations of Cr may be due to the corrosion products in brine water resulting from the effect of water flow, dissolved gases, and treatment chemicals (acids) on the alloys utilized in the construction of desalination pipes and equipment (Dawoud and Al Mulla 2012).
Arsenic and zirconium
The concentrations of As and Zr for sand samples ranged from 377 to 1054 mg/kg and from 390 to 3211 mg/kg, respectively, for surface samples and ranged from 399 to 989 mg/kg and ranged from 424 to 1401 mg/kg, respectively, for subsurface samples. The concentrations of As and Zr for sediment ranged from 86.6 to 466 mg/kg and from 241 to 1172 mg/kg, respectively. The highest value of As was in site 2 for sand sample from surface. Generally, high concentrations of As were observed in all samples which are higher than the allowed limit (1–40 mg/kg) (WHO 2001).
Cadmium and manganese
From Tables 2, 3, and 4, it can be observed that Cd was not detected in all samples. The concentrations of Mn in sand samples ranged from 900 mg/kg for surface sand and ranged from 570 to 1900 mg/kg for subsurface sand, whereas the average concentration for sediments ranged from 990 to 1800 mg/kg. The highest concentration of Mn was observed in surface sand at site 3. However, the concentrations of Mn in sand and sediment for all sites were higher than the average of background in shale (Turekain and Wedepohl 1961) except two sites in subsurface sand (sites 9 and 4).
Iron and aluminum
In case of Fe and Al, the concentrations of Fe in sand ranged between 20,600 and 63,470 mg/kg for surface samples and ranged between 30,180 and 68,000 mg/kg for subsurface samples, while the concentrations of Al in sand ranged between 17,350 and 32,590 mg/kg for surface sand and ranged between 22,100 and 42,620 mg/kg for subsurface samples. For sediment samples, the concentrations of Fe and Al ranged between 13,780 and 67,820 mg/kg and between 18,480 and 41,460 mg/kg, respectively. The highest value of Fe was found in subsurface sand at site 6.
The mean values of Ca, Ti, Sr, and Rb were 35.5, 0.98, 0.38, and 0.028 %, respectively, for sand samples from surface, and the mean values for subsurface samples were 40.2, 1.13, 0.43, and 0.027 %, respectively. Similarly, the mean values of Ca, Ti, Sr, and Rb for sediments were 36.3, 0.77, 0.38, and 0.03 %, respectively. These results show high concentrations of Ca and Ti in sand and sediment samples which constitute major elements in soils. This study found high concentrations of Sr, which may be due to the high concentration of Sr in reject brine (Mohamed et al. 2005).
From Tables 2, 3, and 4, the mean values of Fe, Mn, Cr, Cu, As, and Zr concentrations for sand samples were higher than the geochemical background values in shale (Turekain and Wedepohl 1961). Similarly, the result for sediments is close with that in sand, except the mean value of Fe which was lower than the background value in shale.
Generally, the data shows a variation in the concentrations of heavy metals in surface and subsurface sand near to disposal site of reject brine that could be attributed to the intrinsic nature of its geological formations and the different depositional processes of heavy metals along the shoreline during the high tide. Depositional processes of heavy metals in surface and subsurface sand may be affected by sand processes such as dissolution, sorption, migration, precipitation, binding by organic matter, absorption, and volatilization. In addition to depositional processes, the accumulation of heavy metals in sand depends on different physicochemical factors such as type of pH, organic matter contents, grain size, and others. These parameters are thought to play an important role in controlling the concentration of heavy metal in surface and subsurface sand (Al-Abdali et al. 1996).
Comparison of Fe, Al, Mn, Pb, Cr, Cu, As, and Ni concentrations with other local and regional studies in Arabian Gulf coast
The concentrations of heavy metals in sand and sediment samples of this study were compared with other local and regional studies in Arabian Gulf as given in Table 5. By comparing this study with the other studies in Arabian Gulf coast, it is evident that the concentrations of Fe, Al, Mn, Cr, Cu, and As were higher than the values of those studies. The mean values of Ni in sand and sediment were lower than the values in all coastal sediment and soil from Bahrain, Qatar, Iran, United Arab Emirates and Kuwait, Arabian Gulf, whereas it was higher than the values of local studies, Saudi Arabia. The mean values of Pb in sediment and sand were higher than the values along the shoreline of the other countries in Arabian Gulf, except the concentrations of Pb in the sediment of Bahrain and Kuwait coast. From the local studies, the data of Fe, Al, Mn, Pb, Cr, Cu, and As were within the limits of geochemical background values, while the current study found high concentrations of the selected metals in sediment and sand samples at the immediate vicinity of the disposal site of reject brine from desalination plant. However, AlKahtany et al. (2015) and Almahasheer et al. (2014) found these metals in low levels away from the outfall, which indicates a localized pollution in the study area.
High concentrations of Fe, Al, Mn, Pb, Cr, Cu, and As in beach sand and sediments near to desalination plant, pollutant source, may be associated with the fluid waste discharges from desalination plant which contains elevated levels of heavy metals (Sadiq 2002; Naser 2013). These metals were deposited from the seawater during high tide (Mohamed et al. 2005; Dawoud and Al Mulla 2012). Even the essential trace elements create a toxicity problem if high levels are present in the environment (Dissanayake and Chandrajith 2009).
Generally, most of the local and regional studies indicated that levels of heavy metals in the Arabian Gulf for sediments are within the natural background levels found in offshore and onshore areas. However, elevated levels of heavy metals in Arabian Gulf coast are associated with the human activities such as oil refiners and desalination plants (Naser 2013).
Assessment of environmental pollution
To evaluate the degree of contamination of metals in sediments and sand in the study area, contamination factor (CF) for each metal in the site, the modified degree of contamination (mCd) and pollution load index (PLI) for each site were calculated. The contamination factor (CF) is calculated using the following equation:
where Cm sample is the concentration metal in sample and Cm background is the background value of metal in shale (Turekian and Wedepohl 1961).
The modified degree of contamination (mCd) was calculated using the following relationship (Abrahim and Parker 2008):
The CF and mCd categories are listed in Table 6 (Vaezi et al. 2015; Hakanson 1980; Turekian and Wedepohl 1961).
Pollution load index (PLI) was calculated with the following formula (Tomlinson et al. 1980; Ra et al. 2013):
where n is the number of metals. The PLI > 1 means the presence of pollution, whereas PLI ≤ 1 suggests the absence of pollution (Cabrera et al. 1999).
Contamination factor (CF)
Contamination factors (CFs) of Fe, Al, Mn, Pb, Cr, Cu, As, Zr, Ni, and U in sediments and sand are shown in Figs. 2, 3, and 4. For all sites, the values of CF were less than 1 for U, Ni, Pb, and Al in sand and sediment, with the exception of Pb in sediment at site 8. Based on Hakanson classification in Table 6, the classification of U, Ni, Pb, and Al in all sites was low contamination except site 8 for Pb in sediment that showed moderate contamination. The contamination factors of Mn, Zr, Cu, Cr, and As ranged between 1.06 and 466 for all sites, with the exception of Mn in subsurface sand at two sites 4 and 9. The classification of Mn, Zr, Cu, Cr, and As for sand and sediment samples in all sites ranged between moderate contamination and very high contamination except sites 4 and 9 that showed low contamination. The results showed that the contamination levels of Cu, Cr, and As in all sites are very high contamination. The CF values of Fe were higher than 1 for surface sand in all site except sites 1, 4, 7, 8, and 11which were lower than 1. Similarly, the contamination levels of Fe in subsurface samples were moderate contamination in all sites except sites 3, 4, 9, 11, and 12 which showed low contamination. The CF values of Fe in sediment indicate low contamination in most of the sites. The highest value of contamination factor was found in sediment for As. Consequently, the high contamination levels for Cu, Cr, and As in sediments and sand along the shoreline indicate the presence of a pollutant source.
Modified degree of contamination (mCd)
The values of modified degree of contamination of the studied metals in sand and sediment for all sites are shown in Table 7. According to Hakanson classification in Table 6 (1980), the results showed that the modified degree of contamination ranged from very high degree to ultra high degree in all sites. The highest degree of contamination was recorded in sediment at site 9. The values of mCd in sand and sediment indicate high level of pollution in the area of study.
Pollution load index (PLI)
Figure 5 shows the variations of pollution load indices for sediment and sand in all sampling sites. The values of PLI ranged between 1.1 and 3.0 for surface sand and between 1.6 and 2.6 for subsurface sand. Similarly, PLI ranged between 1.6 and 3.0 for sediment. The highest value of PLI was in sites 3 and 9 (PLI > 3) for surface sand and site 9 for sediment. PLIs were found to be higher than 1 in all sites. According to the results of PLI, all sites are considered polluted. The main contributors in PLI values are Cu, Cr, As, and Mn as it is revealed by contamination factors.
Conclusions
In this study, the concentrations of U, Ca, Fe, Al, Ti, Sr, Rb, Ni, Pb, Cd, Cr, Cu, As, and Zr were measured in 36 sand and sediment samples at different sites along Al Azizia beach near to disposal site of reject brine from desalination plant, Arabian Gulf, using gamma-ray spectrometry, atomic absorption spectrometer (AAS), and energy dispersive X-ray fluorescence spectrometer (EDX). The data showed that the mean values of Fe, Mn, Cr, Cu, As, Sr, and Zr concentrations in sand and sediments were higher than the geochemical background values in shale. Based on the contamination factor, modified degree of contamination (mCd), and pollution load index (PLI), the results showed elevated levels of Cu, Cr, Mn, As, and Zr in the studied area. These toxic elements have a wide range of health issues associated with their contamination. Furthermore, long-term exposure to the naturally occurring radioactive isotopes of Sr and Zr may have serious health implications if living organisms are exposed to them.
In view of the current study, the analysis of heavy metals in sand and sediments near to disposal site of reject brine from desalination plant provided evidence of a localized pollution in the study area.
The regulations and policies related to reject brine discharges and chemical composition disposal must be implemented and enforced. Generally, the available information from this study indicates the need for an environmental management plan to reduce and control the input of high concentrations of Cu, As, Cr, Mn, and Zr from further pollution in the environment nearby a desalination plant. However, if the pollution problem is neglected in the area of study, Arabian Gulf, this will be a cause of great concern that can cause for irreparable ecological damage in the long term.
Abbreviations
AAS, Atomic absorption spectrometry; EDX, energy dispersive X-ray fluorescence spectrometer; UNSCEAR, United Nations Scientific Committee on the Effects of Atomic Radiation; WHO, World Health Organization; USEPA, United States Environmental Protection Agency
References
Al-Abdali F, Massoudb MS, Al-Ghadban AN (1996) Bottom sediments of the arabian gulf-iii. Trace metal contents as indicators of pollution and implications for the effect and fate of the Kuwait oil slick. Environ Pollut 93(3):285–301
Abdul-Wahab SA (2007) Characterization of water discharges from two thermal power/desalination plants in Oman. Eng Sci 24:321–337
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki estuary, Auckland, New Zealand. Environ Monit Assess 136(1–3):227–238
Akhter MS, Al-Jowder O (1997) Heavy metal concentrations in sediments from the coast of Bahrain. Int J Environ Health Res 7:85–93
AlKahtany K, Youssef M, El-Sorogy A (2015) Geochemical and foraminiferal analyses of the bottom sediments of Dammam coast, Arabian gulf, Saudi Arabia. Arab J Geosci 8:11121–11133
Almahasheer HB, Al-Taisan WA, Mohamed MK (2014) Metals accumulation in Grey mangrove (Avicennia marina (Forsk.) Vierh.) inhabiting Tarut Bay, eastern Saudi Arabia. J Agric Sci 1:137–149
Almasoud FI, Usman AR, Al-Farraj AS (2015) Heavy metals in the soils of the Arabian gulf coast affected by industrial activities: analysis and assessment using enrichment factor and multivariate analysis. Arab J Geosci 8:1691–1703
Al-Naimi HA, Al-Ghouti MA, Al-Shaikh I, Al-Yafe M, Al-Meer S (2015) Metal distribution in marine sediment along the Doha Bay, Qatar. Environ Monit Assess 187:130
Cabrera F, Clemente L, Dıaz Barrientos E, López R, Murillo J (1999) Heavy metal pollution of soils affected by the Guadiamar toxic flood. Sci Total Environ 242(1):117–129
Dawoud MA, Al Mulla MM (2012) Environmental impacts of seawater desalination: Arabian gulf case study. Int J Environ Sustain 1(3):22–37
de Mora S, Fowler SW, Wyse E, Azemard S (2004) Distribution of heavy metals in marine bivalves, fish and coastal sediments in the Gulf and gulf of Oman. Mar Pollut Bull 49:410–424
Einav R, Harussi K, Perry D (2002) The footprint of the desalination processes on the environment. Desalination 152:141–154
Hakanson L (1980) An ecological risk index for aquatic pollution control a sedimentological approachs. Water Res 14:975–1001
Hoepner T, Lattemann S (2002) Chemical impacts from seawater desalination plants - a case study of the northern Red Sea. Desalination 152:133–140
Jenkins D (1981) Biological monitoring of toxic trace elements. EPA Report 600/S3–80–090: 1–9
Kim HK, Jang TI, Kim SM, Park SW (2015) Impact of domestic wastewater irrigation on heavy metal contamination in soil and vegetables. Environ Earth Sci 73(5):2377–2383
Mohamed AMO, Maraqa M, Al Handhaly J (2005) Impact of land disposal of reject brine from desalination plants on soil and groundwater. Desalination 182:411–433
Murakami M (1995) Managing water for peace in the Middle East: alternative strategies. The United Nations University, New York
Naser HA (2013) Assessment and management of heavy metal pollution in the marine environment of the Arabian gulf: a review. Mar Poll Bull 72:6–13
Ogoyi DO, Mwita CJ, Nguu EK, Shiundu PM (2011) Determination of heavy metal content in water, sediment and microalgae from Lake Victoria, East Africa. Open Environ Eng J 4:156–161
Ra K, Kim ES, Kim KT, Kim JK, Lee JM, Choi JY (2013) Assessment of heavy metal contamination and its ecological risk in the surface sediments along the coast of Korea. J Coast Res SI65:105–110
Rahman SH, Khanam D, Adyel TM, Islam MS, Ahsan MA, Akbor MA (2012) Assessment of heavy metal contamination of agricultural soil around Dhaka export processing zone (DEPZ), Bangladesh: implication of seasonal variation and indices. Appl Sci 2:584–601
Sadiq M (2002) Metal contamination in sediments from a desalination plant effluent outfall area. Sci Total Environ 287:37–44
Singare PU, Talpade MS, Dagli DV, Bhawe VG (2013) Heavy metals in sediment ecosystem of Bhavan’s college lake of Andheri, Mumbai. Int Lett Chem Phys Astr 13:105–112
Tomlinson D, Wilson J, Harris C, Jeffrey D (1980) Pro- blems in the assessment of heavy-metal levels in Estu-aries and the formation of a pollution index. Helgol Mar Res 33(1–4):566–575
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the Earth's crust. Geol Soc Am Bull 72:175–192
UNSCEAR (1993) Sources effects and risks of ionizing radiation. United Nations Scientific Committee on the effects of Atomic Radiation. Report to the general Assembly, with annexes, United Nations, New York
USEPA (1996) Report: recent Developments for In Situ Treatment of Metals contaminated Soils, U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response
USEPA (1999) US Environmental Protection Agency: Screen-ing Level Ecological Risk Assessment Protocol for Hazardous Waste Combustion facilities. Appendix E: Toxicity Reference Values, Vol. 3
Vaezi AR, Karbassi AR, Fakhraee M (2015) Assessing the trace metal pollution in the sediments of Mahshahr Bay, Persian gulf, via a novel pollution index. Environ Monit Assess 187:613
WHO (2007) Desalination for safe water supply, guidance for the health and environmental aspects applicable to desalination. World Health Organization, Geneva http://www.who.int/water_sanitation_health/en
WHO (2004) Guidelines for drinking water quality, 3rd edn. World Health Organization, Geneva, p. 515
WHO (2001) Geneva, environment health criteria, arsenic and arsenic compounds. World Health Organization, Geneva, p. 224
Younos T (2005) Environmental issues of desalination. Journal of Contemporary Water Research & Education. Universities Council on Water Resources UCOWR132, USA, pp. 11–18
Acknowledgments
The author greatly appreciated the work performed by Nora Alnaim and Ameena Alahmari from research units, University of Dammam. Also, the author is grateful to Dr. Hanadi Baghdadi, director of Research Units, University of Dammam, for her support and assistance in laboratory analysis. Great thanks are extended to Mr. Abdullah Al amri and the workers in Al Khobar desalination plant for assistance in sampling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Alshahri, F. Heavy metal contamination in sand and sediments near to disposal site of reject brine from desalination plant, Arabian Gulf: Assessment of environmental pollution. Environ Sci Pollut Res 24, 1821–1831 (2017). https://doi.org/10.1007/s11356-016-7961-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7961-x