Abstract
Three different soils were spiked with 12 different concentrations of inorganic mercury (Hg). Sub-chronic Hg toxicity tests were carried out with Eisenia fetida in spiked soils by exposing the worms for 28 days following standard procedures. The toxicity studies revealed that Hg exerted less lethal effect on earthworms in acidic soil with higher organic carbon (S-3 soil) where water soluble Hg recovery was very low compared to the water soluble Hg fractions in soils with less organic carbon and higher pH (S-1 and S-2 soils). The concentrations of total Hg that caused 50 % lethality to E. fetida (LC50) after 28 days of exposure in S-1, S-2 and S-3 soils were 152, 294 and 367 mg kg−1, respectively. The average weight loss of E. fetida in three soils ranged from 5 to 65 %. The worms showed less weight loss in the organic carbon-rich soil (S-3) compared to less organic carbon containing soils (S-1 and S-2). The bioconcentration of Hg in E. fetida increased with increased Hg concentrations. The highest bioaccumulation took place in the acidic soil with higher organic carbon contents with estimated bioaccumulation factors ranging from 2 to 7.7. The findings of this study will be highly useful for deriving a more robust soil ecological guideline value for Hg.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) does not have any known biological function; however, in its toxic forms, it is one of the most hazardous metals that can be bioaccumulated and biomagnified (Driscoll et al. 2013; Garcia et al. 1999). The toxicity of Hg to human health and ecosystems has been recognized and addressed by many national and international policy developers (Sloss 2012). A recent estimate has reported that 7527 Mg of Hg is released into the environment annually, of which 5207 Mg is released by natural sources such as oceanic and volcanic emissions, agricultural activities and biomass burning, and the rest of the Hg is emitted from anthropogenic activities such as power plants, gold mining, metal manufacturing and other industrial productions (Pirrone et al. 2010).
After emission, metallic Hg is oxidized in the atmosphere. Almost 60 % of oxidized atmospheric Hg is deposited to land and 40 % to water, although land represents only 30 % of the Earth’s surface (Douglas et al. 2012; Mason et al. 1994). Till now, most of the studies on Hg toxicity have focused on aquatic organisms. Thus, information on the toxicity of Hg to terrestrial organisms is scant. The conventional methods for risk assessment of Hg in soil are based on the comparison of measured concentrations of total and extractable Hg with the precautionary or regulatory values which are set by different countries as safe levels of Hg (CCME 1997; NEPM 2013). But analysis of total or extractable pollutants does not necessarily reflect the real toxicity. Different international organizations and environmental agencies have agreed that soil risk assessments cannot be based only on chemical analysis of pollutants (Sanchez-Hernandez 2006).
In the terrestrial environment, much of the mercury is methylated under anaerobic conditions (Furutani and Rudd 1980; Stein et al. 1996). Methylated mercury is neurotoxic but it is also bioaccumulated in organisms such as earthworms which constitute up to 80 % of the total biomass of soil fauna in terrestrial ecosystems (Kabata-Pendias 2010). Since earthworms are a food source to a wide variety of organisms, mercury bioaccumulates and then is passed further up the food web (Gnamuš et al. 2000; Nevado et al. 2012). Furthermore, earthworms contribute to the decomposition of soil organic matter and cycling of nutrients; hence, toxic levels of mercury in soil have ramifications for soil fertility (Lionetto et al. 2012).
Earthworms can accumulate many pollutants including heavy metals and play an important role in the biomagnification processes of soil pollutants. Earthworms are considered as a reliable bioindicator of soil pollution (Hinton and Veiga 2009) and are an excellent model for studying Hg biochemistry and toxicity in terrestrial ecosystems (Calisi et al. 2013). However, the information on terrestrial toxicity of Hg to this important soil invertebrate is still scarce which is required for the establishment of safe Hg limits in soil. Therefore, the earthworm Eisenia fetida (Oligochaeta, Annelida) was chosen for this study. In the present investigation, total and water-soluble Hg was measured in soils spiked with gradually increasing concentrations of Hg. Survival rate and weight loss of E. fetida were monitored. Bioaccumulation of Hg in E. fetida in different soils was estimated. Toxicity data were compared with the previous investigations (Álvarez et al. 2014; Burton et al. 2006; Hinton and Veiga 2009; Lock and Janssen 2001; Rieder et al. 2011; Zagury et al. 2006).
Materials and methods
Soil analysis
Top soils (0–15 cm) differing in their properties, pH in particular, were collected from three different sites in South Australia, Australia. Soil samples S-1 and S-2 were residential soils and sample S-3 was forest soil. All soil samples were sieved through a 2-mm sieve. Soil texture was determined by the micro-pipette method (Miller and Miller 1987). Soil pH was determined electrometrically on a 1:5 dry soil/water suspension after 2 h stirring using a glass membrane electrode at 25 °C. Electrical conductivity (EC) was determined with an EC probe in the aqueous extract of a 1:5 soil/water suspension and recorded in deciSiemens per metre at 25 °C. Maximum water holding capacities (WHC) of three soils were determined by gravimetry with the oven-dry method (Gardner and Klute 1986). Total organic carbon was determined by dry combustion at 1250 °C using a Tru Mac (LECO, Japan) CNS elemental analyzer. Total nitrogen of the soils was determined using a Tru Mac (LECO, Japan) CNS elemental analyzer according to the manufacturer’s protocol.
Soil spiking, and recovery of total and water-extractable mercury
The collected soil samples were spiked with 12 different concentrations of inorganic mercury. A stock solution of 5000 mg L−1 Hg was prepared by dissolving HgCl2 in sterile de-ionized water which was utilized to spike 3 kg of each soil in different polyethylene containers to obtain desired Hg concentrations as well as to maintain 70 % of the soil’s total water holding capacity. The spiked and control soils were mixed thoroughly in a soil-mixing machine, stored in covered polyethylene containers and aged for 90 days at 20 °C in order to mimic the environmental conditions. For a contaminant to reach an equilibrium between the solid and dissolved phases within the soil can take from days to several months depending on the nature of contaminant and soil. We assumed a 90-day ageing is sufficient for Hg to reach an equilibrium between soil solution and solid phases. In field-contaminated soils, majority of Hg fractions are complexed with soil organic carbon contents or clay minerals due to ageing (Skyllberg 2012); therefore, using freshly spiked soils for toxicity testing can overestimate the toxicity. The final Hg concentrations in spiked soils were intended to be 0, 5, 10, 50, 100, 150, 200, 250, 300, 400, 500, 600 and 700 mg kg−1. These concentrations were chosen to obtain proper dose-response curve to estimate LC values and these are the concentrations found in different Hg-contaminated sites. The highest concentration of 700 mg kg−1 was determined based on preliminary screening which resulted 95 % mortality of tested earthworms in at least two soils. A microwave digestion system (model: MARS 5, CEM) was employed for the digestion of soil samples with aqua regia after 90 days of ageing. The USEPA method 3051 was employed for sample digestion (Melgar et al. 2009). For water-soluble Hg analysis, 5 g soil was taken following 90 days of ageing process and mixed with 50 mL de-ionized water, and then shaken overnight in an end-over-end shaker. The soil extract was collected by centrifugation at 2000g for 20 min followed by filtration with 0.45 μm filter (Millipore). The water extract and microwave-digested soil samples were examined for Hg using inductively coupled plasma triple quad mass spectrometry (ICP-QQQ-MS, Agilent Technologies 8800) following 100× dilution with 1 % HCl. The detection limit of Hg for this instrument was 0.5 μg L−1.
Test organism
The adult experimental organism E. fetida was purchased from Bunnings, South Australia. The worms were acclimatized for 2 months by maintaining in natural soil in the laboratory at 20 °C under controlled light (500 lx)—dark cycle of 8:16 h (Ramadass et al. 2015). The worms were not fed during the experimental duration since the soils are natural and contain sufficient organic matter.
Sub-chronic toxicity testing
The sub-chronic mercury toxicity testing was carried out following the OECD procedure with some modifications (Duan et al. 2016; OECD 1984; Ramadass et al. 2015). Briefly, adult worms with well-developed clitellum and weighing between 0.32 and 0.36 g were selected for toxicity testing. The worms were removed from the culture bed, rinsed with de-ionized water to remove any adhering soil particles and then gently blotted with fresh tissue paper. The washed worms were depurated overnight on moistened filter paper. The depurated worms were again washed and excess water was removed with tissue paper. Ten worms were taken as a group, weighed and released into each glass jar containing 500 g of Hg-spiked soil. The glass jars were covered with perforated plastic film to prevent the test medium from drying and incubated for 28 days at 20 °C under constant illumination (Cáceres et al. 2011; Duan et al. 2016). The moisture contents of all soils were kept constant throughout the experiment to 55 % of the soil’s WHC with de-ionized water. Each treatment was set up in triplicates (Duan et al. 2016). After 28 days of exposure, live worms from triplicate jars were recorded to estimate percent survival rate. Mobility, light sensitivity and discolouration of earthworms were observed to provide indication of toxic response. The live worms were then depurated overnight on moistened filter paper, washed, wiped and weighed from each jar to calculate percent weight loss after 28 days compared to the controls.
After depuration and washing, the live worms were sacrificed by keeping them in −20 °C for 24 h. Whole tissue of all individual worms from each jar were collected, mixed together and homogenized. Then, 0.5 g worm tissue from homogenized worms was digested with aqua regia in a microwave digestion system (model: MARS 5, CEM). Digested worms were filtered with a 0.45-μm filter and subjected to ICP-QQQ-MS (Agilent Technologies 8800) analysis for Hg quantification. Bioaccumulation was established by subtracting the Hg concentrations in worms exposed to control soils from Hg concentrations in worms exposed to spiked soils. The bioaccumulation factor (BAF) was calculated by the following formula:
Statistical analysis
All observations of the earthworm experiments were recorded from triplicate jars and averaged. The measured Hg concentrations were log transformed and then dose-survival rate data were fitted to the four parametric logistic models, and the concentrations of Hg that required to kill 10 % (LC10), 20 % (LC20) and 50 % (LC50) of E. fetida were estimated by the same model using IBM SPSS Statistics 22. Linear regression tool in Microsoft Excel 2010 was employed to test the correlation between spiked and recovered concentrations of Hg at 95 % confidence interval. One-way ANOVA was employed to reveal correlation between Hg concentrations in soil and earthworm’s weight loss and Hg concentrations in soil and in earthworms by IBM SPSS Statistics 22.
Results
Soil properties
The physicochemical properties of the experimental soils from three different locations are presented in Table 1. EC of the three soils indicated that S-1 and S-2 soils contained more dissolved salts/ions than S-3 soil. Water holding capacities were higher in S-1 and S-2 than S-3. Total nitrogen content was almost the same in the three soils. S-1 and S-2 soils had neutral and alkaline pH. S-3 soil was different from the other two soils as it was much lower in pH (4.2) and higher in organic carbon content (4 %). S-3 soil had the highest percentage of sand and lowest clay content compared to the other two soils.
Total and bioavailable Hg in spiked soils
Total Hg content after 90 days of ageing in spiked soils varied from 0.2 to 644 mg kg−1 (80 to 120 % recovery, Fig. 1). The concentrations of total Hg in three control soils which were spiked only with de-ionized water were close to the background level of Hg (~0.5 mg kg−1) in soil. Recovery of total Hg in all three spiked soils was ~100 % in the lower concentrations, i.e. 5, 10 and 50 mg kg−1. In the higher concentrations, the recovery was ~90 %. The water-soluble fraction of Hg in experimental soils varied from 0.05 to 1.5 % of the total spiked amount (Fig. 1). In the present investigation, the water-soluble Hg portion in S-3 soils was found approximately two to three times lower than the water-soluble Hg fraction in other soils spiked with 50 to 700 mg kg−1.
Survival rate of E. fetida in mercury-spiked soils
After 28 days of incubation, all worms survived in the control soils which fulfilled the criteria (<10 % mortality in the control) of OECD guideline 207 (1984). All worms exposed to the control and spiked soils burrowed in the first 24 h of exposure. Mortality was observed from ~5 mg kg−1 Hg-spiked soils in S-1 and S-2. But in S-3 soil samples, there was no mortality of earthworms in at least duplicate jars up to ~262 mg kg−1. In S-1 soil samples, 100 % mortality was recorded in triplicate jars at ~528 mg kg−1 Hg; in S-2 soil samples, all worms died at ~638 mg kg−1 Hg. Whereas in S-3 soil samples, 100 % mortality was observed in one replicate of ~660 mg kg−1 Hg-containing soil. The non-linear regression analysis of log-transformed total Hg concentrations and mean of survival percentages revealed a sigmoidal pattern of toxicity (Fig. 2). The effect of total Hg on earthworm survival rate was significant in all three soils (P < 0.0001). The estimated LC50, LC20 and LC10 values after 28 days are presented in Table 2. The total Hg concentration that exerted 50 % mortality (LC50) in S-1 soil was estimated to be 152 mg kg−1; in S-2 and S-3 soils, this concentration was found to be 294 and 367 mg kg−1, respectively. The LC20 values in S-1, S-2 and S-3 soils were 56, 141 and 281 mg kg−1, respectively. Based on LC values, it is apparent that the earthworms had better survival rate in Hg-contaminated soil with a lower pH and higher organic carbon contents (in S-3 soil). This could be due to Hg being in less toxic forms as a result of chelating with organic matter in the more acidic soil.
Earthworm survival rate after 28 days in three different soils spiked with different concentrations of inorganic Hg (log transformed). Survival rate is the mean of percentages of live earthworms in triplicate pots. Error bars represent standard error of mean. Fitted lines show the dose-response trends in three soils—S-1 (continuous line), S-2 (broken line) and S-3 (dotted line)
Weight loss of E. fetida in mercury-spiked soils
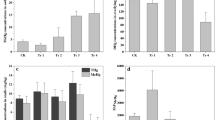
After 28 days of exposure, earthworm’s body weight was recorded in soils where at least 30 % of the worms were still alive (in 500 mg kg−1). In control soils, 3–6 % weight loss was observed which may be due to adaptation to the new environment. Weight loss increased with increasing Hg concentrations in all the three soils (Fig. 3). The highest weight loss was observed in S-1 soil (65 %). In S-2 soil, 50 % weight loss was recorded for the highest spiked Hg concentration. In S-3 soil, the experimental worms did not lose as much weight as the worms lost in S-1 and S-2 soils. The weight loss and measured total Hg contents in the worms were positively correlated and showed significant dose-effect relationship (P < 0.05) which could be due to the Hg stress in soils. The R 2 values for weight loss vs Hg gradients in S-1, S-2 and S-3 were found to be 0.6, 0.7 and 0.6, respectively, with estimated slope values 19.8, 10.7 and 8.6, respectively. The slope of the fitted lines indicated that Hg had larger effect on earthworm weight loss in S-1 soil, followed by S-2 and S-3 soils.
Bioaccumulation of mercury in E. fetida
Total Hg contents in the experimental worms varied in the three different soils after 28 days of exposure. Worms exposed from 0 to 300 mg kg−1 Hg-spiked soils were considered for accumulation study, but not for soils with >300 mg kg−1 Hg because there were insufficient live worms for extraction. Hg was below detection limit in the earthworms from control soils and soils with ~5 and ~10 mg kg−1 Hg. The average Hg concentrations in earthworms varied from 237 to 529 μg g−1 earthworm. One-way ANOVA analysis confirmed that the total Hg contents in all three soils and the Hg accumulated in the worms were significantly correlated (P < 0.05). Hg accumulation increased with increasing Hg level in soil which was fitted to the linear regression model (Fig. 4a). Higher concentrations of Hg were observed in the organic carbon-rich S-3 soils (slope 193, R 2 0.8) compared to those in S-1 (slope 152, R 2 0.8) and S-2 (slope 138, R 2 0.8) soils. The BAF decreased with increasing Hg concentrations in all three soils and was fitted to a linear regression model (Fig. 4b). The highest average BAF of 7.7 was observed in S-3 soil (slope 13, R 2 0.92) with ~53 mg kg−1 Hg, whereas in S-1 (slope 8, R 2 0.95) and S-2 (slope 8, R 2 0.99) soils, the highest BAFs were 4.4 and 4.5, respectively, for similar Hg concentrations. The bioaccumulation study revealed that experimental worms accumulated much higher concentrations of Hg when they were exposed to S-3 soil, which is lower in pH and higher in organic carbon content compared to the S-1 and S-2 soils.
Discussion
To date, most of the investigations on Hg toxicity and bioaccumulation have focused on the aquatic ecosystems (Du et al. 2015; Rodrigues et al. 2013; Wu and Wang 2014). Conversely, there are few studies on the toxicity and biogeochemistry of mercury in terrestrial ecosystem. As earthworms are one of the most important components in the soil ecosystem in addition to being considered reliable bioindicators of soil pollution, it was important to investigate their response to Hg toxicity and estimate lethal concentrations.
The lower water-soluble Hg in acidic S-3 soil with the higher organic carbon contents compared to S-1 and S-2 soils observed in this study is consistent with other researchers’ findings (Gu et al. 2011; Mahbub et al. 2016; Skyllberg et al. 2006). As discussed previously, this is due to the chelation of Hg with soil organics and the decreased solubility of Hg and its complexes at lower pHs (Busto et al. 2012; Neculita et al. 2005; Reis et al. 2014). There is a positive correlation between organic matter and total Hg concentrations in soil (Skyllberg 2012; Yuan et al. 2012). Therefore, Hg solubility and its bioavailability in organic rich soils are generally low (Tipping et al. 2010).
The reduced Hg toxicity of S-3 soils compared to S-1 and S-2 soils was demonstrated by higher survival rates of the earthworms. Similar toxicity patterns were found in the earthworm weight loss study where the least weight loss was observed in organic carbon-rich S-3 soil. During their movement through soils, earthworms interact with available metals through their dermal contact and/or ingestion of soil organic matter. Therefore, such behaviour and routes of exposure will affect the toxicity as well as bioaccumulation. The ingested soil organic matter is subjected to chemical reactions in the earthworm’s intestine which results in different proportions of the pollutants being absorbed through the intestinal membrane (Belfroid et al. 1996; Lanno et al. 2004). Since the water-soluble fraction of Hg was higher in S-1 and S-2 soils, it can be speculated that the earthworms more readily absorbed soluble Hg through dermal contact and ingestion than S-3, which resulted in greater mortality and weight loss. This was clearly demonstrated by the lower LC values recorded for survival rates in S-1 and S-2 compared to S-3 soils. The data from this study is very significant because there is little published data of this type. The only other study on survival rates of earthworms in the presence of Hg was done by Lock and Janssen (2001) who found 100 % survival at 100 mg Hg kg−1 soil and a strong effect on reproduction (measured as cocoon production) already at much lower concentrations (EC50 9.16 mg kg−1). The effects on mortality do not contradict our findings, but indicate that for the derivation of protective soil values, chronic endpoints should be used.
BAFs for Hg in different species of earthworms were reported to be <1 to ~16 in various studies (Table 3). In the present research, the BAFs were observed to be larger in low Hg-containing soils and the absolute Hg bioconcentrations were higher in higher Hg-containing soils; similar observations were made in earlier studies (Burton et al. 2006; Sample et al. 1999). Studies demonstrated that a compound can accumulate in earthworms without exerting any toxicity because of the sequestration of the compound or accumulation in the non-essential part of tissues (Lanno et al. 2004; McCarty et al. 1993). The present study showed that Hg might not kill the earthworms at certain concentration (because of earthworm’s behaviour, mode of exposure and resistance mechanisms), but the worms could accumulate Hg in their body even when the contamination is low. After 28 days of exposure, the bioconcentration of Hg in earthworms was two to eight times higher than the concentrations in soils which confirmed the augmentation of Hg in the earthworms (Fig. 4a).
Since earthworms are food sources for a large number of terrestrial organisms, the bioaugmented Hg can enter the food chain and exert toxicity to the organisms which are not part of the contaminated site. In the present study, Hg accumulation in earthworm was almost double in an acidic soil with higher organic carbon content compared to the soils with less organic matter and higher pH. In a 28-day study, Hinton and Veiga (2009) also reported similar phenomena when the bioaccumulation of Hg in E. fetida was more than 100 times greater in gold mining soils with larger organic matter contents compared to the soils with less organic matter and higher Hg. The similar observation of higher Hg accumulation in earthworms exposed to soils containing high organic carbon was reported in few other studies (Table 3). Burton et al. (2006) found high Hg BAF in E. fetida after 28 days when exposed to soil with the higher organic carbon content and very low Hg concentrations; BAF was very low when soil Hg level was higher and organic carbon content was low. Similar trend was reported in another 28-day study with Lumbricus terrestris using historically Hg-contaminated soils (Álvarez et al. 2014). In contrast, another study involving Eisenia anderi exposed to alkaline soils with low Hg and high organic carbon contents showed low Hg BAF (Zagury et al. 2006). There are few studies of Hg accumulation in earthworms using un-contaminated soils; for example, in a study using forest soils where Hg contents were close to background level, the Hg bioaccumulation in different species varied due to soil properties, importantly soil organic carbon contents (Rieder et al. 2011).
Most of the earlier studies of earthworm bioaccumulation were conducted using long-term or historical contaminated soils (Álvarez et al. 2014; Burton et al. 2006; Zagury et al. 2006). In long-term contaminated soils, the metal bioavailability is very less compared to spiked or recently contaminated soil. Therefore, the acute effect of immediate toxicity may not be reflected in a long-term contaminated soil. The ageing period in the present experiment was 90 days, which is less than some long-term contaminated soils; therefore, bioavailability of Hg could be higher (272–529 μg g−1 earthworms) than the other reports (Table 3). But for regression analysis, soils spiked with Hg gradients are more appropriate than naturally contaminated soils where appropriate control soils are difficult to obtain.
The lethal concentrations observed in the present study can be used as important information to enrich current precautionary values for inorganic Hg. The current guidelines of inorganic Hg for residential use in different industrialized countries are 1 mg kg−1 in Australia (NEPM 2013), 6.6 mg kg−1 in Canada (CCME 1999), 170 mg kg−1 in the UK (EA 2009), 2.3 mg kg−1 in the USA (USEPA 2015) and 10 mg kg−1 in the Netherlands (GON 2000). These values were set higher while considering industrial land use in some countries, such as 50 mg kg−1 in Canada (CCME 1999) and 350 mg kg−1 in the USA (USEPA 2015). Other than these precautionary values set by the regulatory bodies in different countries, there are few published reports in the literature on safe Hg values obtained through species sensitivity distribution (SSD) approach. For example, Jänsch et al. (2007) proposed 1.18 mg Hg kg−1 in soil as safe level, which is the fifth percentile of SSD, calculated from already published data. In a similar investigation, the critical limit of Hg in soil was suggested as 0.13 μg g-1 soil (Tipping et al. 2010). The LC10 values estimated based on earthworm mortality in three soils in the present study reflect that the current available safe limits in different countries may be protective to earthworms in soils. However, more studies involving different earthworm species and more contrasting soils are required to predict significant Hg toxicity in the terrestrial environment. It is also important to study the chronic effect of inorganic Hg gradients on earthworm’s reproduction and cellular biochemistry. Derivation of ecological regulatory pre-cautionary values using SSD model requires more toxicity data involving a number of different species. Earthworms are one of the sentinel species included for risk assessment of chemicals. In view of very limited available data on the Hg toxicity to earthworms, the results of this study will be highly useful in developing/improving the soil ecological guidelines for Hg using SSD approach.
References
Álvarez CR, Moreno MJ, Bernardo FG, Martín-Doimeadios RR, Nevado JB (2014) Mercury methylation, uptake and bioaccumulation by the earthworm Lumbricus terrestris (Oligochaeta). Appl Soil Ecol 84:45–53
Belfroid A, Sijm D, Gestel CV (1996) Bioavailability and toxicokinetics of hydrophobic aromatic compounds in benthic and terrestrial invertebrates. Environ Rev 4:276–299
Burton DT, Turley SD, Fisher DJ, Green DJ, Shedd TR (2006) Bioaccumulation of total mercury and monomethylmercury in the earthworm Eisenia fetida. Wat Air Soil Pollut 170:37–54
Busto Y, Tack F, Cabrera X (2012) Mercury mobility and availability in highly contaminated solid wastes from a chlor-alkali plant. International J Environ Sust Dev 11:3–18
Cáceres TP, Megharaj M, Naidu R (2011) Toxicity and transformation of insecticide fenamiphos to the earthworm Eisenia fetida. Ecotoxicol 20:20–28
Calisi A, Zaccarelli N, Lionetto M, Schettino T (2013) Integrated biomarker analysis in the earthworm Lumbricus terrestris: application to the monitoring of soil heavy metal pollution. Chemosphere 90:2637–2644
CCME (1997) Recommended Canadian soil quality guidelines. http://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines/index.html
CCME (1999) Canadian soil quality guidelines for the protection of environmental and human health. http://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines/index.html
Dang F, Zhao J, Greenfield BK, Zhong H, Wang Y, Yang Z, Zhou D (2015) Soil geochemistry and digestive solubilization control mercury bioaccumulation in the earthworm Pheretima guillemi. J Hazard Mater 292:44–51
Douglas TA, Loseto LL, Macdonald RW, Outridge P, Dommergue A, Poulin A, Amyot M, Barkay T, Berg T, Chetelat J (2012) The fate of mercury in Arctic terrestrial and aquatic ecosystems, a review. Environ Chem 9:321–355
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ. Sci. Technol. 47:4967–4983
Du M, Wei D, Tan Z, Lin A, Du Y (2015) Predicted no-effect concentrations for mercury species and ecological risk assessment for mercury pollution in aquatic environment. J Environ Sci 28:74–80
Duan X, Xu M, Zhou Y, Yan Z, Du Y, Zhang L, Zhang C, Bai L, Nie J, Chen G, Li F (2016) Effects of soil properties on copper toxicity to earthworm Eisenia fetida in 15 Chinese soils. Chemosphere 145:185–192
EA (2009) Soil guideline values for mercury in soil: science report SCO50021/Mercury SGV. Environment Agency. https://www.gov.uk/government/publications/land-contamination-soil-guideline-values-sgvs.
Furutani A, Rudd JW (1980) Measurement of mercury methylation in lake water and sediment samples. Appl Environ Microbiol 40:770–776
Garcia E, Orta M, Suarez P (1999) Toxicity assays and bioconcentration of mercury in bacteria selected from marine environments. Bull Environ Contam Toxicol 62:79–86
Gardner WH, Klute A (1986) Water content. Methods of soil analysis. Part 1. Physical and mineralogical methods, 2nd edn. SSSA Inc. and ASA Inc., Madison, WI
Gnamuš A, Byrne AR, Horvat M (2000) Mercury in the soil-plant-deer-predator food chain of a temperate forest in Slovenia. Environ. Sci. Technol. 34:3337–3345
GON (2000) Dutch Target and Intervention Values (the New Dutch List). Government of the Netherlands, Netherlands
Gu B, Bian Y, Miller CL, Dong W, Jiang X, Liang L (2011) Mercury reduction and complexation by natural organic matter in anoxic environments. Proc Nat Acad Sci 108:1479–1483
Hinton JJ, Veiga MM (2009) Using earthworms to assess Hg distribution and bioavailability in gold mining soils. Soil Sed Contam 18:512–524
Jänsch S, Römbke J, Schallnaß H-J, Terytze K (2007) Derivation of soil values for the path ‘soil-soil organisms’ for metals and selected organic compounds using species sensitivity distributions. Environ Sci Pollut Res 14:308–318
Kabata-Pendias A (2010) Trace elements in soils and plants. CRC Press, Boca Raton
Lanno R, Wells J, Conder J, Bradham K, Basta N (2004) The bioavailability of chemicals in soil for earthworms. Ecotoxicol Environ Saf 57:39–47
Lionetto MG, Calisi A, Schettino T (2012) Earthworm biomarkers as tools for soil pollution assessment. INTECH Open Access Publisher
Lock K, Janssen C (2001) Ecotoxicity of mercury to Eisenia fetida, Enchytraeus albidus and Folsomia candida. Biol Fert Soils 34:219–221
Mahbub KR, Krishnan K, Megharaj M, Naidu R (2016) Mercury inhibits soil enzyme activity in a lower concentration than the guideline value. Bull Environ Contam Toxicol 96:76–82
Mason RP, Fitzgerald WF, Morel FM (1994) The biogeochemical cycling of elemental mercury: anthropogenic influences. Geochim Cosmochim Acta 58:3191–3198
McCarty L, Mackay D, Smith A, Ozburn G, Dixon D (1993) Residue-based interpretation of toxicity and bioconcentration QSARs from aquatic bioassays: polar narcotic organics. Ecotoxicol Environ Saf 25:253–270
Melgar M, Alonso J, García M (2009) Mercury in edible mushrooms and underlying soil: bioconcentration factors and toxicological risk. Sci Tot Environ 407:5328–5334
Miller W, Miller D (1987) A micro-pipette method for soil mechanical analysis. Commun Soil Sci Plant Anal 18:1–15
Neculita C-M, Zagury GJ, Deschênes L (2005) Mercury speciation in highly contaminated soils from chlor-alkali plants using chemical extractions. J Environ Qual 34:255–262
NEPM (2013) National Environmental Protection Measure 1999. Schedule B1: guideline on investigation levels for soil and groundwater. doi:http://www.comlaw.gov.au/Details/F2013C00288/Html/Volume_2
Nevado JJB, Martín-Doimeadios RCR, Mateo R, Fariñas NR, Rodríguez-Estival J, Ropero MJP (2012) Mercury exposure and mechanism of response in large game using the Almadén mercury mining area (Spain) as a case study. Environ Res 112:58–66
OECD (1984) OECD guidelines for testing of chemicals n 207, “Earthworm acute toxicity tests”, adopted: 4 April 1984
Pirrone N, Cinnirella S, Feng X, Finkelman RB, Friedli HR, Leaner J, Mason R, Mukherjee AB, Stracher GB, Streets DG (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10:5951–5964
Ramadass K, Megharaj M, Venkateswarlu K, Naidu R (2015) Ecological implications of motor oil pollution: earthworm survival and soil health. Soil Biol Biochem 85:72–81
Reis AT, Lopes CB, Davidson CM, Duarte AC, Pereira E (2014) Extraction of mercury water-soluble fraction from soils: an optimization study. Geoderma 213:255–260
Rieder SR, Brunner I, Horvat M, Jacobs A, Frey B (2011) Accumulation of mercury and methylmercury by mushrooms and earthworms from forest soils. Environ Pollut 159:2861–2869
Rodrigues AC, Jesus FT, Fernandes MA, Morgado F, Soares AM, Abreu SN (2013) Mercury toxicity to freshwater organisms: extrapolation using species sensitivity distribution. Bull Environ Contam Toxicol 91:191–196
Sample BE, Suter GW, Beauchamp JJ, Efroymson RA (1999) Literature-derived bioaccumulation models for earthworms: development and validation. Environ Toxicol Chem 18:2110–2120
Sanchez-Hernandez J (2006) Earthworm biomarkers in ecological risk assessment, Rev. Environ. Contam. Toxicol. Springer, Berlin Heidelberg New York, pp. 85–126
Skyllberg U (2012) Chemical speciation of mercury in soil and sediment, Environmental Chemistry and Toxicology of Mercury. Wiley, New York
Skyllberg U, Bloom PR, Qian J, Lin C-M, Bleam WF (2006) Complexation of mercury (II) in soil organic matter: EXAFS evidence for linear two-coordination with reduced sulfur groups. Environ Sci Technol 40:4174–4180
Sloss L (2012) Legislation, standards and methods for mercury emissions control CCC/195 London, UK, IEA Clean Coal Centre 43
Stein ED, Cohen Y, Winer AM (1996) Environmental distribution and transformation of mercury compounds. Crit Rev Environ Sci Technol 26:1–43
Tipping E, Lofts S, Hooper H, Frey B, Spurgeon D, Svendsen C (2010) Critical limits for Hg (II) in soils, derived from chronic toxicity data. Environ Pollut 158:2465–2471
USEPA (2015) Regional Screening Level (RSL) Summary Table (TR = 1E-6, HQ = 1) June 2015 (revised). USA
Wu Y, Wang W-X (2014) Intracellular speciation and transformation of inorganic mercury in marine phytoplankton. Aq Toxicol 148:122–129
Yuan Q, Guoqing Z, Wenxiang H (2012) Effects of Hg on soil enzyme. J Northwest Agric Forest Univ 40:191–198
Zagury GJ, Neculita CM, Bastien C, Deschênes L (2006) Mercury fractionation, bioavailability, and ecotoxicity in highly contaminated soils from chlor-alkali plants. Environ Toxicol Chem 25:1138–1147
Zhang ZS, Zheng DM, Wang QC, Lv XG (2009) Bioaccumulation of total and methyl mercury in three earthworm species (Drawida sp., Allolobophora sp., and Limnodrilus sp.). Bull Environ Contam Toxicol 83(6):937–942
Acknowledgments
The research was funded by the Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CRC-CARE) in collaboration with Global Centre for Environmental Remediation, Faculty of Science and Information Technology, The University of Newcastle. The funding was provided as PhD scholarship to KRM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Mahbub, K.R., Krishnan, K., Naidu, R. et al. Mercury toxicity to Eisenia fetida in three different soils. Environ Sci Pollut Res 24, 1261–1269 (2017). https://doi.org/10.1007/s11356-016-7869-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7869-5