Abstract
The toxic effects of four powder detergents: two laundry detergents (A and B), one household detergent (C), one dishwashing detergent (D), and the surfactant alkylbenzene sulfonate (LAS) were analyzed in this study on organisms from different trophic levels (microalgae, cladocerans, ostracods, amphipods, macrophytes, and fish). LC50 and EC50 values obtained in the toxicity bioassays varied between 0.019 and 116.9 mg L−1. The sensitivity of the organisms to the detergents was (from most sensitive to least sensitive) Ostracods > microalgae > amphipods > cladocerans > fishes > macrophytes. The toxicity of the commercial products (from most toxic to least toxic) was LAS > D (dishwashing detergent) > A (laundry detergent) > B (laundry detergent) > C (household detergent). When comparing the sensitivity of organisms that inhabit temperate zones (T = 18 °C) to those that are found in tropical zones (T > 25 °C), it was clear that the species that inhabit the tropics are more sensitive to detergents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The accelerated industrialization and urbanization processes in our country, over the last six decades, have caused alterations in the aquatic ecosystems due to the input of xenobiotics (Albert 2012).
Domestic detergents are among the contaminants with the greatest impact, since it is estimated that 2 billion kg per year are used worldwide. In Mexico, around 470,000 t are produced (INEGI 2014), and the final destinations of these products are the aquatic systems (Cserhati et al. 2002; Rebello et al. 2013).
A detergent is a chemical compound of complex structure that is formed by a surfactant agent, which can be of three different types: anionic, such as alkylbenzene sulfonate (LAS), dodecyl benzene sulfonate, and alkyl lauryl sulfonate; cationic, such as cetyl trimethyl ammonium chloride, benzalkonium chloride, and ethoxylated alcohols; and nonpolar, such as alkyl polyglycosides, Triton X, and Tween 80. Additionally, detergents contain additives such as water softeners (polyphosphates and silicates), anti-redeposition agents (silicates and carbonates), bleaching agents (perborates), preservatives (sodium sulfate), corrosion inhibitors, brightening pigments, enzymes (proteases and amylases), foam stabilizers, dyes, perfumes, and minor components (Warne and Schifko 1999; Pettersson et al. 2000; Rebello et al. 2013).
LAS is one of the major anionic surfactants used in cleaning products. LAS is commonly used in all-purpose cleaning products at a typical concentration from 1 to 37 %. The powdered laundry detergents contain between 3 and 22 % and dishwashing detergents between 2 to 30 % (HERA 2012).
The additives contained in different powder detergents are poly-phosphate (20–25 %), silicate (0.2–15 %), sodium perborate (7–16 %), fluorescent pigment (0.1 %), sodium sulfate (10–20 %), and enzymes (0.007–0.1 %) (PROFEPA 2002; HERA 2012). The formulations of commercial detergents are confidential.
The detergents are found in the discharges that end up in aquatic systems in a continuous manner, causing plant and animal species to be chronically exposed to these compounds.
The toxicity caused by detergents to aquatic organisms has been evaluated since the 1960s (Lemke and Mount 1963; Abel 1974; Cserhati et al. 2002; Warne and Schifko 1999; Rebello et al. 2013); however, only the effects of the surfactants have been determined. The harmful effects of anionic compounds are inhibition of the activity of important enzymes such as esterases and phosphatases; changes in the nerve receptors of fish that induce disorders in feeding and thermoregulation; alterations in the permeability of cell membranes caused by variations in phospholipid composition, as well as inhibition or modification of the transport functions of proteins in the membrane; and alterations in the epithelial tissue of the gills, causing respiration problems in fish and molluscs (Bardach et al. 1965; Alcaraz et al. 1993; Bao-Quey and Dar-Yi 1994; Hansen et al. 1997; Rebello et al. 2013). Elevated concentrations of these compounds produce cellular lysis and cause the death of sensitive organisms (Sandbacka et al. 2000).
Additionally, it has been observed that anionic surfactants induce inflammatory reactions and oxidative stress (Susmi et al. 2010). Furthermore, there is evidence that the surfactant alkylbenzenesulfonate is bioaccumulated in crustaceans (Renaud et al. 2014).
Moreover, anionic surfactants cause important alterations in aquatic systems by producing changes in water quality and eutrophication due to the input of phosphates (Hoffman and Bishop 1994; Stow et al. 2001).
Cationic surfactants are highly toxic to aquatic species. Alkylphenols, ethoxylates, and their metabolites can act as endocrine disruptors in fish (Sonnenschein and Soto 1998; Koerner et al. 1998).
Recent studies have demonstrated that the additives present in commercial detergents have harmful effects. Sodium citrate, sodium perborates, polycarboxylates, corrosion inhibitors, and perfumes with lemon fragrances have genotoxic effects (Tükoglu 2007; Pedrazzani et al. 2012). The enzymes present in the detergents contribute to the toxicity of commercial detergents, causing tissue damage due to their lytic activity (Davis 2004; HERA 2012).
The studies carried out on commercial detergents are scarce, and their toxic effects have only been evaluated on the following microalgae: Euglena gracilis (Azizullah and Häder 2011; Azizullah et al. 2013), Plagioselmis prolonga (Aizdaicher and Markina 2006), Attheya ussurensis (Markina and Aizdaicher 2007), Dunaliella salina and Plagioselmis prolonga (Markina and Aizdaicher 2010); Cladocerans: Daphnia magna (Pettersson et al. 2000; Pedrazzani et al. 2012) and Ceriodaphnia dubia (Warne and Schifko 1999); polychaetes: Laeonereis culveri (Uc-Peraza and Delgado-Blas 2012) and Capitella sp. (Uc-Peraza and Delgado-Blas 2015); the gastropod, Physa acuta (Sobrino-Figueroa 2015); and in the zebra fish, Danio rerio (Sobrino-Figueroa 2013).
Since the studies on commercial detergents are few and there are no previous studies for species native to our country, the objective of this work was to evaluate the toxicity of four commonly used detergents, two laundry detergents, one household detergent, one dishwashing detergent, and the surfactant LAS, on organisms from different trophic levels: the microalgae: Pseudokirchneriella subcapitata and Monoraphidium sp.; the cladocerans: D. magna, Daphnia exilis, Moina macrocopa, and Simocephalus mixtus; the ostracod Cypris sp.; the amphipod Hyallela azteca; the fish: D. rerio and Chirostoma jordani; and the macrophytes: Lemna gibba and Egeria densa, in order to determine their sensitivity to these products.
Methods
Detergents
Four common brands of detergents were selected, two used for washing clothes: Ariel Oxianillos (A) (P&G) and Foca (B) (La Corona), one dishwashing powder: Salvo (D) (P&G), and one general use detergent: Roma (C) (La Corona). The compounds found in each detergent are listed in Table 1, based on the information available on the packaging and the corresponding Material Safety Data Sheets. The tensoactive agent LAS (80 %) was obtained from Sigma-Aldrich (CAS 151-21-3).
From each detergent, a stock solution of 1000 mg L−1 (ppm) in deionized water was prepared. From this solution, the test solutions were prepared and used for the toxicity bioassays. The stock solution was prepared on the same day on which the bioassays were performed. The pH of the stock solution was between 7.2 and 8.2.
Toxicity bioassays
The organisms used in this study were obtained from laboratory cultures under controlled conditions.
Toxicity bioassays were carried out. The duration of the bioassays was 48 h for the testing on cladocerans, 72 h for the assays on ostracods, and 96 h for the assays on microalgae, amphipods, macrophytes, and fishes. The organisms were exposed to six concentrations of each of the detergents, in triplicate (0.1, 0.5, 1, 2, 4, and 8 ppm for microalgae and ostracods; 2, 4, 8, 16, 32, and 64 ppm for cladocerans and amphipods, and 10, 20, 30, 40, 80, and 160 ppm for macrophytes and fishes), and a control without detergent. The test organisms were not fed during the course of the experiment. Each assay was repeated at least three times.
Static bioassays were carried out with microalgae. The conditions during the tests were continuous illumination (4000 lx) and temperature 25 ± 1 °C. The test volume was 5 mL, with a number of cells in the initial inoculum of 10,000 cells mL−1. Reconstituted water with a water hardness of 160 mg of CaCO3 and supplemented with nutrients was used as dilution water. The effect measured was population growth. At the end of the toxicity tests (96 h), an aliquot of 0.1 mL was taken and the number of cells present in each sample was counted by using a hemocytometer (American Optical). The percentage of inhibition of population growth was calculated with the formula proposed by USEPA 1992:
Toxicity tests with cladocerans were made following the recommendations of the Mexican Standard NMX-AA-087-1995-SCFI. All experiments were made with third to fifth brood neonates (18 to 24 h old) derived from a healthy parent stock. The conditions during the bioassay were reconstituted water with a water hardness of 160 mg of CaCO3, temperature 18 ± 1 °C (for tests on D. magna) and 25 ± 1 °C (for bioassays with D. exilis, M. macrocopa, and S. mixtus), Photoperiod 16:8 h light/dark, test volume 100 mL, number of organisms per test 10 neonates, and response measured: immobility at 24 and 48 h.
With juveniles (5 days old) of the H. azteca amphipod, static acute toxicity tests were performed. The tests were carried out with reconstituted water with a hardness of 160 mg of CaCO3 with 10 organisms per concentration. During the bioassay, the amphipods were maintained under the following conditions: temperature 25 ± 1 °C, photoperiod of 16:8 light/dark, test volume 100 mL, and measured effect: death of the juveniles. The criterion used to establish the death of the juvenile was the absence of a response when stimulated mechanically (Cano et al. 1996).
For the tests with ostracods, juveniles 3 days old (150 ± 12 mm length) were used. The physical and chemical conditions of the acute toxicity tests were temperature 25 ± 1 °C, total water hardness of 160 mg of CaCO3, volume of the test solution 50 mL, number of organisms per condition 20 juveniles, and measured effect immobility at 24, 48, and 72 h.
The assays with fishes were carried out following the protocol suggested by APHA (1994). Ten juveniles (0.52 ± 0.20 g) were placed in 10-L glass containers, in triplicate, and were exposed to six concentrations of each type of detergent during 4 days. Reconstituted water with hardness of 160 mg L−1 of CaCO3 was used as dilution water in the tests. Bioassays were maintained at the following conditions: temperature 25 ± 1 °C, photoperiod 12:12 (light/dark), and dissolved oxygen >4 mg L−1. Every 24 h, the water with the detergent solution was changed for each condition. The effect measured was the mortality of the fish.
The experiments with aquatic macrophytes were made with axenic cultures of L. gibba and E. densa. For each test, healthy young plants were used. Colonies of four to five leaves of L. gibba and stems (3 cm) of E. densa were placed in containers with 350 mL of test solution (modified Hoagland’s culture medium) with different concentrations of the detergents. The conditions recorded during the bioassay were temperature 25 ± 1 °C, pH 7.6, continuous illumination (6500 lx), and measured effect: increase of biomass as wet weight (OECD 2002).
In the assays with cladocerans, amphipods, ostracods, and fish, the number of dead organisms was evaluated every 24 h and these were removed from each container to avoid the production of toxic metabolites.
With the mortality data, the lethal concentration 50 (LC50) with its confidence intervals were determined, by using the Probit method (Probit-EPA, version 1.5).
In the tests on the microalgae and macrophytes, the EC50 was calculated, which corresponds to the detergent concentration which reduces the cell production number of the microalgae or the biomass (wet weight) of macrophytes by 50 %.
The calculation for comparison between the LC50 and the EC50 and the corresponding confidence intervals was carried out by using the statistical analysis described by APHA (1994) and then used to evaluate the statistical significance of the differences found between the different treatments with the detergents.
f 2 = Lower endpoint of the 95 % confidence interval f 1,2 = 1.96 SD/LC50 f 1,2
LC50 upper/LC50 lower > f 1,2 = There are significant differences.
where
- SD:
-
standard deviation
- LC50 :
-
lethal concentration 50
- f :
-
confidence limit factor
- f 1 :
-
upper endpoint of the 95 % confidence interval
Results
Validity of the tests—abiotic variables
Three bioassays were carried out for each species; in total, there were 36 assays. The mortality in the control groups of all of the tests was below 10 %, a value considered to be acceptable in standardized protocols. The values for O2 in the bioassays were above 70 % saturation; the pH values varied between 7.2 and 7.6; the temperature was maintained at 25 ± 1 °C, except for the tests on D. magna where it was 18 ± 1 °C.
The average LC50 and the confidence intervals (p < 0.05) are shown in Table 2. In all of the cases, we observed significant differences in the response of the organisms exposed to xenobiotics as compared to the control group.
In the tests performed by using detergent A, it was observed that the most sensitive organism to this product was the cladoceran D. exilis and the least sensitive was the macrophyte E. densa. Significant differences in sensitivity between the species were detected by using a Tukey test (p < 0.05), showing differences in the responses of the microalgae, cladocerans, ostracods, amphipods, and macrophytes.
The LC50 values obtained in the experiments by using detergent B varied between 0.67 and 90.6 ppm. The most susceptible species to this detergent was the ostracod, and the least susceptible was the macrophyte E. densa. There were significant differences between the responses of the microalgae, cladocerans, ostracods, fishes, and macrophytes.
The data obtained from the tests by using the detergent C showed that the most sensitive organism was the ostracod and the least sensitive was the macrophyte E. densa. No significant differences were observed between the responses shown by the macrophyte L. gibba and the cladoceran D. magna, and the fishes.
In the lethality tests by using the detergent D, the most sensitive species was the ostracod Cypris and the least sensitive was the macrophyte. There were significant differences in the response to the detergent between the microalgae, macrophytes, cladocerans, and fishes. There were no significant differences observed between D. magna and the macrophytes, and the fishes.
In the bioassays by using the tensioactive compound LAS (alkyl lauryl sulfonate), the LC50 varied between 0.263 and 79.7 ppm. LAS was most toxic to the cladoceran D. exilis and least toxic to the macrophyte E. densa. Significant differences in the responses were detected between the macrophytes and the other species.
When comparing the average values of LC50 obtained in the tests with the different brands of detergents, we observed that the most toxic product was the surfactant LAS and the least toxic was the commercial detergent C.
The toxicity of the detergents under study was
Based on the average of the calculated LC50, the sensitivity of the different species to the detergents used in this study was (from most sensitive lo least sensitive)
Cypris sp. > P. subcapitata > M. macrocopa > H. azteca > Monoraphidium sp. > D. exilis > S. mixtus > C. jordani > D. rerio > D. magna > L. gibba > E. densa.
Degree of toxicity of the detergents
Based on LC50 values obtained by the assays, the compounds were classified as established by the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) (Fig. 1):
-
(i)
Highly toxic: LC50, EC50 ≤ 1 mg L−1
-
(ii)
Toxic: LC50, EC50: 1 ≤ 10 mg L−1
-
(iii)
Harmful to aquatic organisms: LC50, EC50: 10 ≤ 100 mg L−1
-
(iv)
Nontoxic: LC50, EC50 > 100 mg L−1
The detergent A was highly toxic for the cladoceran D. exilis and the ostracods; toxic for the microalgae, the cladocerans: D. magna and M. macrocopa, and the amphipod; harmful to the cladoceran S. mixtus, the fishes, and the macrophyte L. gibba; and nontoxic to the macrophyte E. densa.
The degree of toxicity of detergent B was high for the ostracods; toxic to the microalgae, the cladocerans: D. exilis and M. macrocopa, and the amphipods; and harmful to the cladocerans: D. magna and S. mixtus, fishes, and macrophytes.
The product C was highly toxic to the ostracods; toxic to the microalgae, the cladoceran M. macrocopa, and the amphipods; and harmful to the cladocerans: D. magna, D. exilis, and S. mixtus, fishes, and macrophytes.
The toxicity of the detergent D was high for the microalgae and the ostracods; toxic for the cladocerans: D. exilis, M. macrocopa, and S. mixtus, the amphipods, and the fishes; and Harmful to the cladoceran D. magna and the macrophytes.
The surfactant LAS was highly toxic to the cladoceran D. exilis and the amphipods; toxic to the microalgae and the cladocerans: D. magna, M. macrocopa, and S. mixtus, the ostracods, and the fish; and harmful to the macrophytes (Fig. 2).
Toxicity of alkylbenzene sulfonate surfactant in tests with aquatic organisms. Highly toxic: LC50 or EC50 ≤ 1 ppm. Toxic: LC50 or EC50: 1 ≤ 10 ppm. Harmful: LC50 or EC50 10 ≤ 100 ppm. Nontoxic: LC50 or EC50 > 100 ppm. Microalgae: Pseudokirchneriella subcapitata (Pse), Monoraphidium sp. (Mo); cladocerans: Daphnia magna (Dm), D. exilis (De), Moina macrocopa (Mo), and Simocephalus mixtus (Sm); the ostracod Cypris sp. (Cy); the amphipod Hyallela azteca (Ha); the fishes: Danio rerio (Dr) and Chirostoma jordani (Cj); and the macrophytes Egeria densa (Ed) and Lemna gibba (Lg)
Discussion
As mentioned above, the toxicity of detergents to aquatic organisms has been studied since late 1960s (Rebello et al. 2013). However, the majority of the studies have only determined the toxic effects of the surfactants (Abel 1974; Lewis 1990; Lewis and Suprenant 1983; Cserhati et al. 2002; Liwarska et al. 2005) or of a few of the ingredients that are present in the detergents (Warne and Schifko 1999), and studies carried out on products of commercial use are very scarce (Pettersson et al. 2000; Azizullah and Häder 2011; Sobrino-Figueroa 2013).
The detergents used in this study have anionic surfactants, and only detergent A also contains cationic tensioactive compounds. Additionally, all of the detergents contain sodium silicate as a water softener and sodium sulfate as a preservative (to prevent clumping of the detergent). Three of the products tested (A, B, and D) contain enzymes, dyes, and perfumes. Only two detergents (A and B) contain bleaching compounds.
The difference in the degree of toxicity among the detergents used for this study is due to the composition of each product. The most toxic detergents were D and A.
Detergent D is a product used to wash dishes; it contains enzymes (proteases) as well an anionic surfactant compound, perfumes, dyes, and silicates. Detergent A is a detergent used to wash clothes. It contains anionic and cationic tensioactive compounds, whiteners, enzymes, perfumes, dyes, and silicates. A previous study by Warne and Schifko (1999) showed that the toxic components of detergents are the surfactants, silicates, whiteners, enzymes, and dyes.
In this study, it was found that the most toxic detergents were those used for washing clothes and dishes.
In other studies, it has been reported that concentrations of the surfactant LAS between 1.4 and 116 ppm are toxic to microalgae (EC50 at 96 h) (Lewis 1990). The EC50 values obtained in this study were of 2.1 ± 0.7 and 3.52 ± 2.02 ppm for tests carried out on P. subcapitata and Monorraphidium sp., respectively. This indicates that these two species are more sensitive to the surfactant than diatoms, Nitzschia fonticola (EC50 = 50 ppm), cyanophyte Microcystis aeruginosa (20 ppm) (Yamane et al. 1984), and chlorophytes Chorella vulgaris (32 to 50 ppm) (Canton and Slooff 1982) and Cladophera glomerata (100 ppm) (Whitton 1967).
Azizullah and Häder (2011) evaluated the effects of detergent A on the microalgae E. gracilis and observed that concentrations of 917 ppm produced a reduction of 50 % in the motility of this organism. The data obtained in this study indicates that P. subcapitata and Monorraphidium sp. (LC50 = 2.1 ± 0.95 and 2.9 ± 1.3 ppm, respectively) are more sensitive to the detergent A than E. gracilis, a species that is used for the tertiary treatment of residual waters (Rebello et al. 2013).
The invertebrates, especially in their juvenile phases, are extremely sensitive to surfactants, since concentrations below 1 ppm can cause alterations in their survival, conduct, and growth rate. The toxicity of the anionic surfactant LAS varies from 1 to 270 ppm in tests carried out on aquatic invertebrates (Lewis 1993; Lewis and Suprenant 1983; Cano et al. 1996; Iannacone and Alvariño 2002; Da Silva-Coelho and Rocha 2010).
The LC50 values obtained in this work, in the tests carried out on cladocerans, amphipods, and ostracods, vary from 0.91 ± 0.73 to 6.31 ± 1.21 ppm. These concentrations are found within the interval of toxic values for aquatic invertebrates, but it is evident that the organisms used in this study are more sensitive to the tensioactive LAS than the isopods (Asellus sp. LC50 = 270 ppm), nematodes (Rhabditis sp. LC50 = 16 ppm), the midge Paratanytarsus parthenogenica (LC50 = 23 ppm), and gastropods Physa heterostropha (LC50 = 34.2 ppm), Physa integra (LC50 = 9 ppm), Campeloma decisum (LC50 = 27 ppm), and Goniobasis sp. (LC50 = 92 ppm).
In another study, Pettersson et al. (2000) evaluated the toxicity of 25 commercial detergents on the cladoceran, D. magna, and found that the toxicity of these products varied between 4 and 85 ppm (LC50 to 48 h). Furthermore, Warne (1995) estimated the toxicity of 25 detergents on C. dubia obtaining LC50 values between 1.6 and 70.3 ppm. The LC50 values obtained in this study, from tests of commercial detergents on D. magna, varied between 1.6 ± 0.9 and 52.9 ± 20.5 ppm, values that are within the range on those reported by those authors.
Fishes are considered to be a bioindicator species since, due to their sensitivity to different xenobiotics, they play an important role in water quality monitoring studies (Sen and Semiz 2007). The results obtained in this study by using the fishes C. jordani and D. rerio show that their sensitivity to the surfactant LAS is similar to that found for the fish Rita rita (Debasish 1988). Omotoso and Fagbenro (2005) evaluated the toxic effect of three brands of commercial detergents on Oreochromis niloticus, finding LC50 values that varied between 12.04 and 41.8 ppm. The average LC50 values from our bioassays on C. jordani and D. rerio were 17.42 ± 8.67 and 21.12 ± 10.5 ppm, respectively. These values are within those reported by the authors cited above.
Another factor that can influence the toxicity of detergents and pollutants is the temperature.
The effects of increasing temperatures on chemical toxicity are relatively documented on aquatic species.
Mayasich et al. (1986) assessed the effects of atrazine and temperature in Nannochloris oculata microalgae; these researchers detected a significant dependence between atrazine’s toxicity and temperature, the toxicity increasing with increased temperature. Gaunt and Barker (2000) evaluated the toxic effect of atrazine herbicide on catfish (Ictalurus punctatus), and they observed that toxicity increased when the temperature rose and dissolved oxygen decreased. These authors mentioned that changes in these two parameters probably enhanced the toxicity of atrazine on some aquatic species. Ratushnyak et al. (2005) reported that the toxicity of three pesticides (fenvalerate, cypermethrin, and deltamethrin) in D. magna was enhanced as water temperature increased. Kim et al. (2010) observed that water temperature was one of important factors that influenced the toxicity of three drugs: Scetaminophen, Enrofloxacin, and Chlortetracycline on D. magna, as the temperature was increased from 15 to 25 °C; the toxicity of the drugs increased significantly from three to eight times.
The manner in which the temperature increases the toxic effect of pollutants is not completely explained, but some authors mention that when temperature rises, important changes occur in the homeostasis, the physiological and depuration rates, causing greater incorporation of compounds and higher production of toxic metabolites that affect the integrity of organisms (Noyes et al. 2009; Kim et al. 2010). Furthermore, it has been found that ectothermic organisms, such as microcrustaceans, ostracods, fishes, amphibians, and reptiles, may be most vulnerable to the temperature and contaminant interactions (Gordon 2003; Patra et al. 2007).
Additionally, the effects of temperature increase in the toxicity of detergents have been documented by many researchers: Pantani et al. (1997) observed changes in sensitivity to detergents with increasing temperature in tests performed with Gamarus sp.; in bioassays carried out at 8 °C, the LC50 for LAS is 20.5 ± 1.4 and in assays at 23 °C, the LC50 is 3.3 ± 0.5 ppm. Likewise Orathai et al. (1987) found that the toxicity of detergents in freshwater prawns Macrobrachium rosenbergii increases with increasing water temperature; these authors mention that the cause of death of prawns was probably extensive gill damage, because the solubility of detergents increases with increasing water temperature, and consequently, the rate of adsorption of the detergents by epithelial cells of the gills and the surface body of the prawns increases with increasing water temperature, thus rendering them more sensitive to detergents.
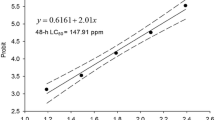
Moreover, Lewis and Horning (1991) observed an increase in sensitivity to sodium dodecyl sulfate (SDS) in assays with D. magna at a higher temperature (26 °C) (mean LC50 = 13.5 mg L−1 at 20 °C and 10.3 mg L−1 at 26 °C). These authors concluded that toxicity tests conducted at 20 and 26 °C may give significantly different results with D. magna. Likewise, Sobrino-Figueroa (2015) (unpublished data) observed that the values of LC50 for the anionic surfactant LAS ranged between 29.5 and 21.5 ppm in tests carried out at 18 °C; however, if the assays were carried out at a higher temperature (25 °C), the LC50 were 0.013 ± 0.012 ppm, almost 2300 times lower.
In Mexico, D. magna is used as a test organism to evaluate the toxicity of effluents that discharge into different aquatic systems. D. magna has a limited geographical distribution since it is found in temperate regions (Gutiérrez 2008). In our country, natural populations of this organism have not been found (Gutiérrez 2008).
The use of species from temperate climates to carry out ecotoxicological studies in subtropical and tropical environments has been questioned by various authors (Hong et al. 2004; Azad and Agard 2006; Freitas and Rocha 2011, 2012), since the majority of the test protocols are carried out at temperatures between 18 and 20 °C (OECD 1984; APHA 1994), conditions which are less than optimal in latitudes where the climates are subtropical or tropical.
In many cases, the native species that inhabit tropical and subtropical regions are more sensitive to xenobiotics than the test species (Hong et al. 2004; Freitas and Rocha 2011 ). D. magna has been reported to be one of the organisms that is most sensitive to chemical compounds (Lewis 1993; USEPA 1982; ISO 6341 1982); however, the species of cladocerans, amphipods, and ostracods used for this study were more sensitive to the commercial detergents, compared to the response observed for D. magna.
The average LC50 value obtained in our assays with D. magna exposed to the detergents was 32.65 ± 12.04 ppm, while the average LC50 values obtained from our bioassays on D. exilis, M. macrocopa, S. mixtus, Cypris sp., and H. azteca were 9.68 ± 5.5, 3.07 ± 1.71, 14.72 ± 8.46, 0.685 ± 0.49, and 5.67 ± 3.09 ppm, respectively, values between 55 and 98 % lower than those obtained in the tests on D. magna.
This data indicates that native species in our country are more sensitive to detergents as compared to the organism commonly used to assess toxicity in water (D. magna). This fact is important because native species are not being protected if the indicator organism for water toxicity is more tolerant to the compounds.
From the previous argumentation, it is evident that commercial detergents have harmful effects on aquatic organisms, and the data from this study shows that the organisms belonging to different trophic levels have different sensitivities to these xenobiotics and that the temperature has an effect on its toxicity.
Conclusions
The four commercial detergents tested in this study had LC50 values that varied from 1.4 to 8.4 ppm in the tests on microalgae, from 0.1 to 52.9 ppm in the bioassays on invertebrates (cladocerans, amphipods, and ostracods), from 4.8 to 29.9 ppm in the assays on fishes, and finally from 32.25 to 116.9 ppm in the tests on macrophytes. The most sensitive organisms to these products were the ostracods, the amphipods, and the cladocerans, with the exception of D. magna.
The most toxic detergents were the products D and A, which are used for dishwashing and laundry, respectively. The toxicity of the products that contained surfactants, silicates, bleaching agents, enzymes, and dyes was greater compared to the detergents that lacked some of these compounds. Additionally, it was evidenced that the toxicity of the detergents increases when the temperature rises.
The presence of detergents in natural waters can cause a great impact on aquatic organisms. Since wastewater treatment is limited and often detergents are discharged directly into the environment, it is important to know the potential adverse effects of these compounds in order to propose appropriate measures to reduce the risk caused by their presence in aquatic environments.
References
Abel PD (1974) Toxicity of synthetic detergents to fish and aquatic invertebrates. J Fish Biol 6:279–298
Aizdaicher NA, Markina ZV (2006) Toxic effects of detergents on the alga Plagioselmis prolonga (Cryptophyta). Russ J Mar Biol 32:45–49
Albert L (2012) Curso Básico de Toxicología ambiental. Limusa, México
Alcaraz G, Rosas C, Espina S (1993) Effect of detergent on the response to temperature and growth of grass carp, Ctenopharyngodon iclella. Bull Environ Contam Toxicol 50:659–664
APHA, AWWA, WPFC (1994) Métodos estándar para el examen de aguas y aguas de desecho, 64° Edn. Interamericana, México
Azad M, Agard J (2006) Comparative sensitivity of species (Diaphanosoma brachyurum, Ceriodaphnia rigaudii and Moinodaphnia macleayi) to six chemicals. J Environm Scien Health Part A 41:2713–2720
Azizullah A, Häder N (2011) Toxicity assessment of a common laundry detergent using the freshwater flagellate Euglena gracilis. Chemosphere 84:1392–1400
Azizullah A, Waheed PR, Imran A, Donat PH (2013) Ecotoxicity evaluation of a liquid detergent using the automatic biotest ECOTOX. Ecotoxicology 22:1043–1052
Bao-Quey H, Dar-Yi W (1994) Effects of linear alkylbenzene sulfonate (LAS) on the respiratory functions of Tigerperch (Terapon Jarbua). Zool Stud 33:205–210
Bardach JE, Fujiya M, Holl A (1965) Detergent: effect on the chemical senses of the fish Ictalurus natialis (Le Suer). Science 148:1605–1607
Cano M, Scott D, Alvaro J, Decarvalho J (1996) Effect of sediment organic carbon on the toxicity of a surfactant to Hyalella azteca. Environm Toxicol Chem 15:1411–1417
Canton JH, Slooff W (1982) Substitutes for phosphate containing washing products: their toxicity and biodegradability in the aquatic environment. Chemosphere 11:891–907
Cserhati T, Forgacs E, Oros G (2002) Biological activity and environmental impact of anionic surfactants. Environ Int 28:337–348
Da Silva-Coelho K, Rocha O (2010) Assessment of the potential toxicity of a linear alkylbenzene sulfonate (LAS) to freshwater animal life by means of cladoceran bioassays. Ecotoxicology 19:812–818
Davis B (2004) Recent developments in the technology of surfactants, critical reports on applied chemistry. Elsevier Applied Science
Debasish R (1988) Toxicity of an anionic detergent, dodecylbenzene sodium sulfonate, to a freshwater fish, Rita rita: determination of LC50, values by different methods. Ecotoxicol Environ Saf 15:186–194
Freitas EC, Rocha O (2011) Acute toxicity tests with the tropical cladoceran Pseudosida ramosa: the importance of using native species as test organisms. Arch Environ Contam Toxicol 60:241–249
Freitas EC, Rocha O (2012) Acute and chronic effects of atrazine and sodium dodecyl sulfate on the tropical freshwater cladoceran Pseudosida Ramosa. Ecotoxicol 21:1347–1357
Gaunt P, Barker SA (2000) Matrix solid phase dispersion extraction of triazines from catfish tissues; examination of the effects of temperature and dissolved oxygen on the toxicity of atrazine. Int J Environ Pollut 13:284–312
Gordon CJ (2003) Role of environmental stress in the physiological response to chemical toxicants. Environ Res 92(1):1–7
Gutiérrez, Manuel Elias. 2008. Cladócera y Copépoda de las aguas continentales de México. Guía ilustrada. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. UNAM, Facultad de Estudios Superiores-Iztacala, El Colegio de la Frontera Sur, México
Hansen B, Fotel FL, Jensen NJ, Wittrup L (1997) Physiological effects of the detergent linear alkylbenzene sulphonate on blue mussel larvae (Mytilus edulis) in laboratory and mesocosm experiments. Mar Biol 128:627–637
HERA (2012) Human and environmental risk assessment on household cleaning products. http://www.heraproject.com/
Hoffman FA, Bishop JW (1994) Impacts of a phosphate detergent ban on concentrations of phosphorus in the James River, Virginia. Water Res 28:1239–1240
Hong CDL, Slooten KB, Tarradellas J (2004) Tropical ecotoxicity testing with Ceriodaphnia cornuta. Environ Toxicol 19:497–504
Iannacone J, Alvariño L (2002) Efecto del Detergente Doméstico Alquil Aril Sulfonato de Sodio lineal (LAS) Sobre la Mortalidad de tres Caracoles Dulceacuícolas en el Perú. Ecol Aplicada 1:81–87
INEGI, Instituto Nacional de Geografía e informática (2014) Banco de información económica: México
ISO 6341(1982) Water quality-determination of the inhibition of the mobility of Daphnia magna Straus (Cladocera, Crustacea) (15 March 1982)
Kim J, Park J, Kim PG, Lee C, Choi K, Choi K (2010) Implication of global environmental changes on chemical toxicity effect of water temperature, pH, and ultraviolet B irradiation on acute toxicity of several pharmaceuticals in Daphnia magna. Ecotoxicol 19:662–669
Koerner W, Hanf V, Schuller W, Bartsch H, Zwirner M, Hagenmaier H (1998) Validation and application of a rapid in vitro assay for assessing the estrogenic potency of halogenated phenolic chemicals. Chemosphere 37:2395–2407
Lemke AE, Mount DI (1963) Some effects of alkyl benzene sulfonate on the bluegill, Lepomis Macrochirus. Trans Am Fish Soc 92:372–378
Lewis MA (1990) Chronic toxicities of surfactants and detergentes builders to algae: a review and risk assessment. Ecotoxicol Environ Saf 20:123–140
Lewis MA (1993) Chronic and sublethal toxicities of surfactants to aquatic animals: a review and risk assessment. Water Res 25:101–113
Lewis PA, Horning WB (1991) Differences in acute toxicity test results of three reference toxicants on Daphnia magna at two temperatures. Environ Toxicol Chem 10:1351–1358
Lewis MA, Suprenant D (1983) Comparative acute toxicities of surfactants to aquatic invertebrates. Ecotoxicol Environ Saf 7:313–322
Liwarska BE, Korneliusz MB, Malachowska-Juts AZ, Kalka J (2005) Acute toxicity and genotoxicity of five selected anionic and nonionic surfactants. Chemosphere 58:1249–1253
Markina ZV, Aizdaicher NA (2007) Influence of laundry detergents on the abundance dynamics and physiological state of the benthic microalga Attheya ussurensis (Bacillariophyta) in laboratory culture. Russ J Mar Biol 33:391–398
Markina ZV, Aizdaycher NA (2010) Influence of the Ariel detergent on the growth and physiological state of the unicellular algae Dunaliella salina (Chlorophyta) and Plagioselmis prolonga (Cryptophyta). Hydrobiol J 46:49–56
Mayasich JM, Karlander EP, Terlizzi ER (1986) Growth responses of Nannochloris oculata droop and Phaeodactylum Tricornutum Bohlin to the herbicide atrazine as influenced by light intensity and temperature. Aquat Toxicol 8:175–184
NMX-AA-087-1995-SCFI ANALISIS DE AGUA – EVALUACION DE TOXICIDAD AGUDA CON Daphnia magna Status (Crustacea – Cladecera) – METODO DE PRUEBA. Dirección General de Normas, México
Noyes P, McElwee M, Miller H, Clark B, Van Tiem L, Walcott K, Erwin K, Levin E (2009) The toxicology of climate change: environmental contaminants in a warming world. Environ Intl 35:971–986
OECD (1984) Guideline for testing of chemicals. "Daphnia sp., Acute Immobilisation Test and Reproduction Test" Guideline 202
OECD (2002) OECD guidelines for the testing of chemical. Lemna sp Growth inhibition test Draft Guideline 221
Omotoso FO, Fagbenro OA (2005) A comparative study on the toxicity of three commercial detergents on the survival of the Nile tilapia, Oreochromis Niloticus. J Agric Res Develop 4:139–147
Orathai L, Upatham ES, Poolsanguan B, Duangsawasdi M, Kiravanich P (1987) Effects of water hardness and temperature on toxicity of detergents to the freshwater prawn Macrobrachium rosenbergii. Nat Hist Bull Siam Soc 35:35–45
Pantani C, Pannunzio G, De Cristofaro M, Novelli AA, Salvatori M (1997) Comparative acute toxicity of some pesticides, metals and surfactants to Gammarus italicus Goedm. And Echinogammarus tibaldii pink. And stock (Crustacea: Amphipoda). Bull Environ Contam Toxicol 59:963–967
Patra RW, Chapman JC, Lim RP, Gehrke PC (2007) The effects of three organic chemicals on the upper thermal tolerances of four freshwater fishes. Environ Toxicol Chem 26:1454–1459
Pedrazzani R, Ceretti E, Zerbini I, Casale R, Gozio E, Bertanza G, Gelatti U, Donato F, Feretti D (2012) Biodegradability, toxicity and mutagenicity of detergents: integrated experimental evaluations. Ecotoxicol Environ Saf 84:274–281
Pettersson A, Adamsson M, Dave G (2000) Toxicity and detoxification of Swedish detergents and softener products. Chemosphere 41:1611–1620
PROFEPA (Procuraduría Federal de Protección al Ambiente) (2002) Analisis de calidad de detergentes. Revista del consumidor, Mexico
Ratushnyak AA, Andreeva MG, Trushin MV (2005) Effects of type II pyrethroids on Daphnia magna: dose and temperature dependences. Riv Biol-Biol Forum 98:349–357
Rebello S, Aju KA, Sathish M, Jisha MS (2013) Surfactants: chemistry, toxicity and remediation. In: Lichtfouse E et al (eds) Pollutant diseases, remediation and recycling, environmental chemistry for a sustainable world 4. Springer International Publishing, Switzerland, pp. 277–320. doi:10.1007/978-3-319-02387-8_5
Renaud F, Warnau M, Oberhänsli F, Teyssié JL, Temara A, Rouleau C, Metian C (2014) Bioconcentration of the anionic surfactant linear alkylbenzene sulfonate (LAS) in the marine shrimp Palaemonetes varians: a radiotracer study. Mar Pollution Bull 85:244–247
Sandbacka M, Christianson I, Isomaa B (2000) The acute toxicity of surfactants on fish cells, Daphnia magna and fish. A comparative study. Toxicol in Vitro 14:61–68
Sen A, Semiz A (2007) Effects of metals and detergents on biotransformation and detoxification enzymes of leaping mullet (Liza saliens). Ecotoxicol Environ Saf 68:405–411
Sobrino-Figueroa A (2013) Evaluation of oxidative stress and genetic damage caused by detergents in the zebrafish Danio rerio (Cyprinidae). Comp Biochem Physiol Part A 165:528–532
Sobrino-Figueroa A (2015) Toxic effects of emerging pollutants in juveniles of the freshwater gastropod Physa acuta (Draparnaud, 1805). Amer Malac Bull 33:1–6
Sonnenschein C, Soto AM (1998) An update review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol 65:143–150
Stow CA, Borsuk ME, Stanley DW (2001) Long-term changes in watershed nutrient inputs and riverine exports in the Neuse River, North Carolina. Water Res 35:1489–1499
Susmi TS, Rebello S, Jisha MS, Sherief PM (2010) Toxic effects of sodium dodecyl sulfate on grass carp Ctenopharyngodon Idella. Fish Technol 47(2):157–162
Tükoglu S (2007) Genotoxicity of five food preservatives tested on root tips of Allium Cepa L. Mutat Res 626:4–14
Uc-Peraza RG, Delgado-Blas VH (2012) Determinación de la concentración letal media (CL50) de cuatro detergentes domésticos biodegradables en Laeonereis culveri (Webster, 1879) (Polychaeta, Annelida). Rev Int Contam Ambient 28:137–144
Uc-Peraza RG, Delgado-Blas VH (2015) Acute toxicity and risk assessment of three commercial detergents using the polychaete Capitella sp. from Chetumal Bay, Quintana Roo, Mexico. Int Aquat Res. doi:10.1007/s40071-015-0112-z
USEPA (1982) Environmental Effects Test Guidelines, ES-1 EPA 560/6–82-002. Office of Toxic Substances, US Environmental Protection Agency, Washington DC
USEPA (1992) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms. EPA-600/4–91-022, Environmental Protection Agency, Washington DC
Warne MSJ (1995) Acute toxicity of laundry detergents to an Australian cladoceran (Ceriodaphnia dubia). Australasian J Ecotoxicol 1:127–136
Warne MSJ, Schifko AD (1999) Toxicity of laundry detergent components to a freshwater cladoceran and their contribution to detergent toxicity. Ecotoxicol Environ Saf 44:196–206
Whitton B (1967) Studies on the growth of riverain Cladophora in culture. Arch Mikrobial 58:21–29
Yamane AN, Okada M, Sudo R (1984) The growth inhibition of planktonic algae due to surfactants used in washing agents. Water Res 18:1101–1105
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Braunbeck
Rights and permissions
About this article
Cite this article
Sobrino-Figueroa, A. Toxic effect of commercial detergents on organisms from different trophic levels. Environ Sci Pollut Res 25, 13283–13291 (2018). https://doi.org/10.1007/s11356-016-7861-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7861-0