Abstract
Cytostatics are part of the forefront research topics due to their high prescription, high toxicity, and the lack of effective solutions to stop their entrance and spread in the environment. Among them, 5-Fluorouracil (5-Fu) has received particular attention because is one of the most prescribed active substances in chemotherapy worldwide. The degradation of 5-Fu by advanced oxidation processes (AOPs) is a poorly addressed topic, and this work brings valuable inputs concerning this matter. Herein, the efficacy of Fenton’s process in the degradation of 5-Fu is explored for the first time; the study of the main variables and its successful application to the treatment of real wastewaters is demonstrated. Moreover, hydrogen peroxide-based and photo-assisted techniques (direct photolysis, photodegradation with H2O2 and photo-Fenton) are also investigated for purposes of comparison. Under the best operation conditions obtained (T = 30 °C, [Fe2+]0 = 0.5 mM; [H2O2]0 = 240 mM and pH = 3 for [5-Fu]0 = 0.38 mM), 5-Fu was completely eliminated after 2 h of Fenton’s reaction and about 50 % of mineralization was reached after 8 h. The best performance was obtained by the photo-Fenton process, with 5-Fu mineralization level as high as 67 %, using an iron dose within the legal limits required for direct water discharge. Toxicity (towards Vibrio fischeri) of the effluents that resulted from the application of the above-mentioned AOPs was also evaluated; it was found that the degradation products generated from the photo-assisted processes are less toxic than the parent compound, putting into evidence the relevance of such technologies for degradation of cytostatics like 5-Fu.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water resources contaminated with pharmaceutical compounds have received substantial public and scientific attention (Klavarioti et al. 2009, Wang et al. 2009, Zhang et al. 2013). Among the various classes of pharmaceuticals, cytostatic drugs, widely used in chemotherapy, are of particular environmental concern because of their high consumption rate (it is expected that anti-cancer treatment will double from 2010 to 2020 (Hoppe-Tichy 2010)), their adverse effects to humans and to the environment (Li et al. 2015, Lin and Lin 2014, Lutterbeck et al. 2015), as well as to their low degradation by wastewater treatment plants (WWTPs). On the other hand, it is worth mentioning the scarcity of valuable alternatives for the effective remediation of cytostatic-containing effluents (Garcia-Ac et al. 2010, Klavarioti et al. 2009, Lin et al. 2014, Lutterbeck et al. 2015, Mahnik et al. 2007, Negreira et al. 2014, Zhang et al. 2013).
5-Fluorouracil (5-Fu) combined with its prodrug capecitabine is one of the most prescribed cytostatic drugs (Franquet-Griell et al. 2015, Johnson et al. 2013, Longley et al. 2003). The high resistance of 5-Fu towards biodegradation, its low adsorption to suspended solids present in water matrices, and its very low vapor pressure suggest that this drug will be extremely persistent in the aqueous environment (Zhang et al. 2013). Meanwhile, taking into account that 5-Fu is not completely metabolized (10–30 % is excreted in the parent form) and that current technologies available at wastewater treatment facilities are not prepared to efficiently degrade such hazardous substance, it is anticipated that 5-Fu can reach surface and ground waters (Li et al. 2015, Zhang et al. 2013). Franquet-Griell and co-workers predicted high environmental concentrations for 5-Fu and capecitabine because of being among the most prescribed cytostatics and of their stability at WWTPs (Franquet-Griell et al. 2015).
Because the degradation and removal of 5-Fu by conventional WWTPs is inefficient and incomplete, other alternatives need to be investigated. Advanced oxidation processes (AOPs) are recommended for the treatment of wastewaters contaminated with compounds of high chemical stability and/or low biodegradability. Up to now, a very limited number of studies have been published regarding the degradation of 5-Fu by AOPs (Lin and Lin 2014, Lutterbeck et al. 2015, Yu-Chen Lin et al. 2015). The most promising results have been reported by Lutterbeck et al. (Lutterbeck et al. 2015), reaching 73.1 % of 5-Fu mineralization after ca. 4 h of photo-Fenton reaction.
Until now, the performance of the dark Fenton’s process in terms of 5-Fu degradation is completely unknown, although this technique has been effectively used in the degradation of many hazardous compounds. Moreover, it is important to emphasize that Fenton’s process may constitute a simpler and a more cost-effective alternative than the photo-assisted approaches (Neyens and Baeyens 2003, Santos et al. 2011). Fenton’s oxidation involves the reaction between H2O2 and ferrous (Fe2+) or ferric (Fe3+) ions via a free radical chain reaction (Eqs. (1) and (2)) (Bokare and Choi 2014), which produces highly active species, mainly non-selective hydroxyl radicals (HO•), which oxidize the target compounds (Rahim Pouran et al. 2014, Santos et al. 2011).
The main advantages of Fenton’s process are related with the fact that it can be carried out at atmospheric pressure and room temperature, requires reagents that are readily available, easy to store and handle, being also safe and environmental friendly; moreover, the high mineralization efficiencies of this technology enable the transformation of organic pollutants into non-toxic carbon dioxide (Babuponnusami and Muthukumar 2014, Bokare and Choi 2014).
The aims of this study were therefore (1) to study the effectiveness of the dark Fenton’s process as a potential technology for 5-Fu degradation in water matrices, including the study of the main variables that affect the process and the treatment of a contaminated real wastewater and (2) to compare dark Fenton’s oxidation with photo-assisted technologies (direct photolysis, photodegradation with hydrogen peroxide and photo-Fenton) in 5-Fu degradation, including the toxicity assessment of generated effluents against Vibrio fischeri.
Materials and methods
Reagents
5-Fluorouracil, 97.7 % purity (w/w), was purchased from Sigma–Aldrich (St. Louis, MO, USA). For the homogenous Fenton and photo-Fenton processes, it was used iron (II) sulfate heptahydrate (FeSO4.7H2O) with 99.0 % purity (w/w) as catalyst, hydrogen peroxide (H2O2, 30 % v/v) as oxidant and anhydrous sodium sulphite (Na2SO3, 98 % w/w) as quenching agent; all of them were purchased from Merck (Darmstadt, Germany). Sulphuric acid (H2SO4, 96 % v/v) was acquired from José M. Vaz Pereira, Lda (Lisbon, Portugal), while sodium hydroxide (NaOH, 98.7 % w/w) was purchased from José Manuel Gomes dos Santos, Lda (Odivelas, Portugal); both were used to adjust medium pH. Formic acid (98 % v/v) (analytical grade) was from Sigma Aldrich (St. Louis, MO, USA) and methanol (99.9 % v/v), HiPerSolv CHROMANORM, was acquired at VWR. Syringe filters with 0.2 μm PTFE membrane were purchased from VWR (Wester Chester, USA).
Standard preparation
A 5-Fu stock solution (1.9 mM) was prepared dissolving an appropriate amount of 5-Fu analytical standard in 250 mL of distilled water. Working solutions of 5-Fu were prepared daily by dilution of the previous stock solution in water and were kept in the refrigerator until their use.
Analytical procedures
Quantification of 5-Fu
The 5-Fu degradation was followed by high-performance liquid chromatography coupled with a diode array detector (HPLC-DAD). The HPLC-DAD was a Hitachi Elite Lachrom apparatus, consisting in an L-2130 pump, an L-2200 auto-sampler, and an L-2455 diode array detector. The chromatographic separation was achieved by a Purospher® Star RP-C18 endcapped column (250 mm × 4 mm, 5 μm) using a mobile phase composed of 97 % (v/v) distilled water and 3 % (v/v) of methanol, both acidified with 0.01 % of formic acid (v/v), at isocratic conditions, with a flow rate of 0.2 mL min−1. The injection volume was 20 μL. The spectra acquisition was done from 220 to 400 nm and 5-Fu was quantified at 266 nm, with a retention time of 23.80 ± 0.02 min. The method performance was assessed by evaluating the linearity, repeatability, and accuracy (recovery data); results are presented in the Supplementary Material section. The calibration curve for 5-Fu in water was performed by direct injection of 8 standards from 0.00038 to 0.38 mM of 5-Fu. The coefficient of determination obtained was 0.9999 and the linearity tests revealed an excellent fitness. A limit of detection (LOD) of 4.6 × 10−5 mM and a limit of quantification (LOQ) of 3.8 × 10−4 mM were reached. The recovery results showed that the analytical methodology allows a reliable quantification of 5-Fu in all tested conditions (cf. Supplementary Material section).
Total organic carbon
Mineralization was monitored through total organic carbon (TOC) losses by measuring the total carbon (TC) and the inorganic carbon (IC) in a Shimadzu 5000A analyzer according to the standard method 5310 D of the American Public Health Association (American Public Health Association 1998). The TOC of each sample was calculated by subtracting the IC to the TC. The reported values of TOC represent the average of at least two measurements; in most cases each sample was injected three times or more in order to obtain a coefficient of variation (CV) lower than 2 %.
Quantification of hydrogen peroxide
The hydrogen peroxide concentration was determined by molecular absorption spectrophotometry after the reaction of hydrogen peroxide with titanium (IV) ions, according to the method developed by Sellers (Sellers 1980).
Toxicity
The toxicity assays were carried out in a Microtox Model 500 Analyzer by measuring the inhibition of V. fischeri bioluminescence according to the standard DIN/EN/ISO 11348–3 (International Organization for Standardization 2005). The toxicity of the initial solution and of the generated effluents was determined based on the changes in V. fischeri luminescence after 15 min of exposure to those solutions.
Degradation processes
The oxidation experiments were conducted with an initial target compound concentration of 0.38 mM, which corresponds to an organic carbon concentration of 1.5 mM. This initial concentration was chosen taking into account the high LOD of the TOC analyzer (0.020 mM of 5-Fu, which corresponds to a carbon concentration of 0.083 mM). Only two experiments were carried out with a lower 5-Fu initial concentration (0.038 and 0.0038 mM), but the TOC was not recorded for the above reasons.
Regarding the dark Fenton’s oxidation, a parametric study was performed considering the main variables that affect the process: the temperature, the concentration of Fenton’s reagents and the concentration of 5-Fu (Table 1). The reaction pH was fixed at 3.0, the optimum value commonly reported for the degradation of many organic compounds by this process (Babuponnusami and Muthukumar 2014, Bagal and Gogate 2014, Santos et al. 2011). Moreover, the Fenton’s process performance was evaluated in a real wastewater spiked with 5-Fu, under the best conditions achieved.
After that, Fenton’s oxidation was compared with photo-assisted technologies (directed photolysis, photodegradation with hydrogen peroxide and photo-Fenton). This comparison was based (i) on the performance of the different processes to achieve the same 5-Fu degradation percentage and mineralization level, (ii) on the toxicity of the generated effluents towards V. fischeri, and (iii) on a preliminary operating costs estimation considering the costs of reagents and energy.
Fenton’s oxidation
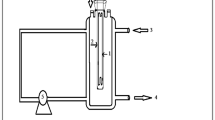
The Fenton’s process was carried out in a 250-mL batch jacketed reactor filled with 200 mL of 5-Fu aqueous solution prepared with distilled water. The temperature was kept constant using a Huber thermostatic bath (Polystat CC1 unit). After the temperature stabilization, the solution pH was adjusted to pH 3.0 using H2SO4 (1 M). To measure the solution temperature and pH, a thermocouple and a pH electrode (WTW, SenTix 41 model), connected to a pH-meter from WTF (model Inolab pH Level 2), were used. Afterwards, a predetermined amount of FeSO4.7H2O was added and dissolved under constant agitation. The H2O2 was added when FeSO4.7H2O was completely dissolved, this representing the initial instant (t = 0) of the experiments. Samples were periodically taken from the reactor along the reaction. In order to eliminate the residual H2O2 for HPLC analysis, the pH was adjusted to 9 ± 1 with NaOH (0.1 mol L−1). The remaining H2O2 in samples for TOC analysis was eliminated by adding an excess of Na2SO3. All samples were filtered with 0.2-μm PTFE syringe filters and stored at −20 °C until further analysis.
Photo-assisted processes
The degradation of 5-Fu in the presence of radiation was investigated exposing 250 ml of a 5-Fu aqueous solution (0.38 mM) to a 150 W medium-pressure mercury vapor lamp (Heraeus TQ 150), whose more intense line is at 366 nm; the photonic flux of the lamp was estimated to be 6.22 × 10−7 Einsteins s−1. Figure S1 of the supplementary material shows the emission spectrum of the lamp. A quartz (or glass) jacket with water recirculation was employed to cool the irradiation source and filter the infrared radiation, thus preventing any heating of the solution. Depending on the kind of experiment performed, the degradation reaction was left to occur only in the presence of radiation (direct photolysis), in the presence of radiation and hydrogen peroxide or combining radiation with Fenton’s reagents (photo-Fenton process). The samples were treated as described in the previous section.
The influence of visible light radiation was evaluated through the cut-off the UV-B and UV-C emission lines in direct photolysis experiments. For that, a DURAN 50® glass cooling jacket was placed around the UV-Vis lamp, resulting emission lines at λexc = 365, 405, 436, 646, and 678 nm (cf. Fig. S2 of the supplementary material).
Results and discussion
Fenton’s oxidation
Parametric study
In this study, the conditions for which the dark Fenton’s process has its best performance were determined using a classical methodology consisting in the variation of one parameter at a time while keeping the others constant. The studied parameters were the temperature, and the concentrations of H2O2, Fe2+ and 5-Fu, as reported in Table 1. The effect of the water matrix was studied as well.
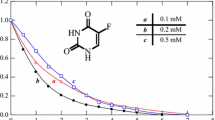
Temperature plays an important role in the degradation of pollutants by Fenton’s process because it affects the rate of all reactions involved, particularly between H2O2 and Fe2+, as well as the rate of organic compounds attack by hydroxyl radicals (HO•) (Babuponnusami and Muthukumar 2014, Rahim Pouran et al. 2014). The results obtained showed that, as expected and predicted by Arrhenius law, an increase of the temperature led to an increase of the 5-Fu degradation rate, as shown in Fig. 1a. For example, after 1 min of reaction 5-Fu removals of 67, 74, and 78 % were reached at 15, 30, and 45 °C, respectively, revealing a very quick initial degradation rate, which is higher as the temperature increases. As time goes on oxidation rate slows down, but still best performances are reached at the highest temperature tested. Although the temperature of 45 °C has been found as the most effective, a temperature of 30 °C was selected to proceed with the study. Since the process is still fast and effective at 30 °C, leading to complete 5-Fu degradation within 1 h, the additional energetic cost associated to the process operation at the highest temperature (45 °C) is not justified.
The study of the catalyst load effect is necessary as an overdose of iron would decrease the efficiency of the process (by HO• scavenging) and would produce iron sludge (Cortez et al. 2011). Figure 1b shows the effect of Fe2+ dose on the degradation of 5-Fu in water. The results reveal that the 5-Fu degradation increases with the dose of catalyst. After 60 min of reaction, 5-Fu was completely removed for a concentration of catalyst of 0.5 mM. Lower performances were recorded for lower catalyst doses (0.1 and 0.25 mM). Concentrations of Fe2+ higher than 0.5 mM were not considered because iron precipitation occurs (Santos et al. 2011). It is again observed an initial rapid decrease in 5-Fu concentration followed by a more gradual decline, which can be explained by the inter-conversion of Fe2+ into Fe3+. In the Fenton’s reaction, Fe2+ is quickly converted into Fe3+ (k1 = 63–76 M−1 s−1,cf. Eq. (1)) and 5-Fu is degraded rapidly. However, Fe3+ is converted into Fe2+ (k2 = 0.001–0.01 M−1 s−1,cf. Eq. (2)) more slowly, resulting in the decrease of 5-Fu degradation rate. A similar degradation profile was previously obtained by Jiang and co-workers for phenol degradation by Fenton’s reaction (Jiang et al. 2010) and by Santos et al. for paraquat degradation (Santos et al. 2011).
The loading of H2O2 plays a crucial role in the efficiency of the degradation process and the main operating costs associated with the Fenton’s process are often due to H2O2 (Cortez et al. 2011). Thus, the loading of H2O2 should be adjusted in order to maximize the efficiency of its use. The results shown in Fig. 2a demonstrate that in the range tested, the degradation of 5-Fu was only slightly enhanced when the H2O2 concentration was increased from 1 to 240 mM. However, different mineralization degrees were achieved when the different H2O2 concentrations were tested. The degree of mineralization increased from about 0 to 50 % after 8 h of reaction when the concentration of H2O2 was increased from 1 to 240 mM (Fig. 2b). These results can be explained by the increase of HO• concentration that is responsible for the 5-Fu and its oxidation by-products degradation (Santos et al. 2011). However, 5-Fu mineralization was only slightly enhanced when the H2O2 concentration increased from 15 to 240 mM. Although the generation of hydroxyl radicals is higher in the experiments using higher initial concentrations of H2O2, scavenging reactions start to get relevance too (Eqs. (3)–(5)); particularly through Eq. (5), as HO• reacts with the excess H2O2 to produce hydroperoxyl radicals (with less oxidative power than HO•).
In order to prove that hydroxyl radicals are essential to degrade 5-Fu, dark Fenton reaction was performed in the presence of the radical scavenger dimethyl sulfoxide (DMSO), in a DMSO:H2O2 molar ratio of 10, following the procedure reported before (Rodrigues et al. 2016). The results show that 5-Fu cannot be degraded in the presence of DMSO (Fig. 2a), which supports the existence of a radical-based mechanism behind the 5-Fu degradation by the Fenton’s process.
In Fig. 2b, it is possible to observe that the mineralization rate is faster for the first 2 h of reaction and afterwards slows down, achieving 50 % after 8 h for an initial H2O2 concentration of 240 mM. Therefore, two different phases can be distinguished in 5-Fu mineralization. In the first one, a fast conversion of 5-Fu into its degradation by-products is observed. In the second phase, the generated by-products were more slowly degraded, which may be due to their recalcitrance character or their less reactivity towards hydroxyl radicals. A similar two-stage mineralization profile was previously reported by Lutterbeck and co-workers (Lutterbeck et al. 2015) for 5-Fu degradation by photo-Fenton process.
The parametric study of the variables affecting the Fenton’s reaction was up to now done with a 5-Fu concentration of 0.38 mM. Since 5-Fu has been detected in the aquatic environment at concentrations ranging from ng L−1 up to μg L−1 (Hartmann et al. 1998, Kovalova et al. 2009, Lin et al. 2013, Lin et al. 2014, Lutterbeck et al. 2015, Mahnik et al. 2007, Straub 2010, Tauxe-Wuersch et al. 2006, Yu-Chen Lin et al. 2015), the best operating conditions obtained for a concentration of 0.38 mM were also tested for 5-Fu initial concentrations of 0.038 and 0.0038 mM (decrease by a factor of 10 and 100, down to 0.5 mg/L), in order to assess the performance of the process under different pollutant concentrations. It is noteworthy that as the initial pollutant concentration was decreased, the dose of oxidant and catalyst were also decreased in the same proportion to keep the ratio 5-Fu:H2O2:Fe2+ constant in all experiments. The results presented in Fig. 3 indicate that the best degradation performance was attained for the highest initial concentration because the reaction kinetics is accelerated. In all cases, the typical two stages of the Fenton’s process are again observed.
Effect of the aqueous matrix
In this section, the effect of the aqueous matrix on the 5-Fu degradation performance by Fenton’s process was assessed. For that, degradation experiments using distilled water and an effluent from a WWTP were performed, employing two different H2O2 doses (60 and 240 mM). High H2O2 concentrations were chosen because dissolved organic matter present in WWTP effluent may probably act as radical scavenger (Rahim Pouran et al. 2015). The results presented in Fig. 4a showed that 5-Fu is quickly removed by Fenton’s reaction. The best performance was obtained for the highest H2O2 concentration, for which 5-Fu was completely removed after 2 min of reaction in water from the WWTP. The improvement on the 5-Fu degradation rate in real wastewater may be explained by the presence of some inorganic ions such as carbonate and bicarbonate (one of the most common ions in the aquatic environment). Carbonate and bicarbonate radicals have lower oxidizing powers than hydroxyl radicals, but due to their selectivity, they could constitute a valuable contribution to the overall degradation of certain compounds in real wastewaters. Umschlag and Herrmann (1999) (Umschlag and Herrmann 1999) found that the carbonate and bicarbonate radicals significantly enhance the degradation of a series of aromatic contaminants (e.g., benzene, 4-methyl anisole, toluene, p-xylene, nitrobenzene, etc.) in polluted wastewaters. Similarly, the degradation of 5-Fu, which is also an aromatic compound, could be strengthened due to the presence of bicarbonate and carbonate ions in real wastewaters. Another justification relies on the reactivity of carbonate and bicarbonate radicals towards 5-Fu molecules. Some evidences of the specificity of carbonate and bicarbonate radicals to attack 5-Fu molecule have been reported (Lin et al. 2013). Lin et al. (Lin et al. 2013) showed that 5-Fu is quickly removed in the presence of bicarbonate and attributed such behavior to the scavenging of HO• by bicarbonate. The scavenging of HO• by bicarbonate results in a higher generation rate of CO3 − • (Eq. (6)), which is more selective than HO• and attacks preferentially 5-Fu molecules (Eq. (7)) (Lin et al. 2013).
This particular behavior was already noticed for other organic compounds. For example, Merouani and co-workers (Merouani et al. 2010) concluded that carbonate radicals present more reactivity towards rhodamine B molecules than hydroxyl radical.
Direct photolysis
A limited number of studies about 5-Fu degradation in water by direct photolysis is available in the literature and contradictory conclusions have been reported. Lutterbeck et al. (Lutterbeck et al. 2015), Kosjek et al. (Kosjek et al. 2013), and Lin et al. (Lin et al. 2013) proved a rapid degradation of 5-Fu in water by direct photolysis. Lin et al. (Lin et al. 2013) also reported that 5-Fu degradation in water by direct photolysis depends on the pH of the solution. On the other hand, Lin and Lin (Lin and Lin 2014) showed that 5-Fu did not undergo direct photolysis in water, when they performed experiments with UV radiation (UV lamp, 8 W, 254 nm). Thus, to get further insight regarding the stability of 5-Fu molecule towards radiation, two different tests were performed. Firstly, the degradation of 5-Fu, exposing a contaminated water to UV-Vis and visible radiation only, was inspected at pH 3.0. Afterwards, the photodegradation performance was investigated under different pHs.
To evaluate the effect of irradiation wavelength, direct photolysis of 5-Fu was carried out at two different irradiation conditions: polychromatic light in the range 200–600 nm (UV-Vis) and polychromatic light at λ ≥ 300 nm (mostly visible light irradiation, Vis). The results presented in Fig. 5a indicate that when the radiation source emits light with wavelengths between 200 and 600 nm, 5-Fu is completely degraded after 15–30 min of irradiation by direct photolysis. However, when UV radiation is almost completely removed (cf. transmission spectrum of the Duran glass filter used in Fig. S2), the degradation rate of 5-Fu decreases noticeably. These results can be explained by 5-Fu’s absorbance spectrum (Fig. 5b). 5-Fu exhibits maximum absorbance at 200 and 266 nm (and its absorbance is quite reduced at wavelengths above 300 nm); therefore, 5-Fu degradation rate decreases due to the decrease in the light absorption.
Effect of different irradiation wavelengths on the 5-Fu degradation in water by direct photolysis at pH 3.0 (a) and of the initial pH on 5-Fu absorbance spectrum (b), 5-Fu degradation (c), and 5-Fu mineralization (d) in water by direct photolysis at 200–600 nm ([5-Fu]0 = 0.38 mM; T = 30 °C in all cases)
Several studies have shown that the pH of the solution influences the stability of chemical compounds towards direct photolysis, increasing or decreasing the degradation rate (Jung et al. 2009). In this work, the pH effect was evaluated at three different values: 3, 5, and 7. The results presented in Fig. 5c show that the initial pH of the solution did not have influence on the direct photolysis rate of 5-Fu; 5-Fu was completely degraded after 15–30 min of irradiation and nearly the same degradation profile is observed. Moreover, about 50 % of the 5-Fu is mineralized after 8 h of reaction whatever the reaction pH in the range studied (Fig. 5d). Actually, all pH values tested were below the dissociation constant of 5-Fu (pKa = 8.0), meaning that the molecule is mainly in the same form for all experiments and consequently having the same absorption spectrum (Fig. 5b).
Processes comparison between dark Fenton and photo-assisted technologies
Radiation has been often coupled with powerful oxidants (such as H2O2, including in some cases a catalyst), resulting in various kinds of important photochemical AOPs. These processes are able to degrade and/or destroy pollutants by means of several possible reactions, including direct photodecomposition (based on irradiation, excitation of pollutant molecules) and photocatalytic oxidation via formation of HO• radicals. The principal advantage of photo-assisted technologies compared to dark Fenton’s oxidation is the reduction of iron sludge generation. However, the use of radiation energy increases the process costs (Rahim Pouran et al. 2015). In this section, the effectiveness of photo-assisted technologies for 5-Fu degradation is analyzed and compared with Fenton’s oxidation.
Lutterbeck et al. (Lutterbeck et al. 2015) compared different AOPs (photodegradation with H2O2, photo-Fenton and heterogeneous photocatalysis with TiO2) and concluded that photo-Fenton oxidation is the most effective method for 5-Fu degradation, allowing 74.7 % of 5-Fu mineralization after 256 min of reaction ([5-Fu]0 = 0.15 mM; [Fe2+]0 = 1.6 mM; [H2O2]0 = 13 mM; pH = 5; T = 20 °C). In the same line, a comparative study was herein performed considering some of these processes, but also including direct photolysis and the Fenton’s oxidation, whose performance in terms of 5-Fu degradation in water is for the first time investigated.
The comparison of Fenton and photo-Fenton processes was done under two different conditions. Firstly, degradation experiments were performed using the maximum possible concentration of catalyst ([Fe2+]0 = 0.5 mM) because above this concentration iron precipitation occurs. Afterwards, 5-Fu degradation behavior was analyzed using a much lower Fe2+ concentration ([Fe2+]0 = 0.04 mM) and keeping the Fe2+:H2O2 molar ratio constant. One should remark that 0.04 mM (2 mg L−1) is the maximum value of Fe2+ allowed in a effluent ready for discharge, according to Portuguese legislation (Ordinance No. 236/98 of 1st August) and EPA guidelines (Environmental Protection Agency 2001).
As can be observed from Fig. 6, a strong effect of the catalyst and oxidant doses on the degradation and mineralization of 5-Fu is observed for Fenton’s process. Although very poor performance was achieved under lower catalyst and oxidant doses, fast and complete 5-Fu degradation was attained at higher chemical doses (Fig. 6a). Additionally, a significant improvement on the mineralization degree is observed when increasing the chemicals concentration (null against 50 % of mineralization after 8 h)—Fig. 6b. Regarding the photo-Fenton process, no significant differences were registered for different doses of chemicals, neither in terms of 5-Fu degradation nor in terms of mineralization. Lutterbeck et al. (Lutterbeck et al. 2015) achieved a comparable 5-Fu mineralization degree by photo-Fenton in the following conditions: [Fe2+]0 = 1.60 mM, [H2O2]0 = 13 mM, pH = 5, T = 20 °C and with a photon flow rate of 5.71 × 106 mol photons/cm2 s−1 for [5-Fu]0 = 0.15 mM. Although Lutterbeck et al. (Lutterbeck et al. 2015) used a lower initial concentration of 5-Fu and H2O2 as compared to those employed in the current study, the concentration of iron was 45 times above the legal limit. Herein, it is proved that similar 5-Fu mineralization degrees by photo-Fenton (~70 %) can be attained without downstream additional costs regarding the treatment of iron sludge.
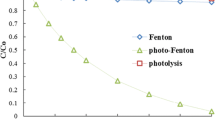
To assess the contribution of each pathway in the photo-Fenton process, the performance of direct photolysis and photodegradation with H2O2 (thus without the iron catalyst) in the removal of 5-Fu from water was also investigated (Fig. 7). In terms of 5-Fu degradation, photo-Fenton, and photodegradation with H2O2 were the fastest methods, followed by direct photolysis. In photo-Fenton, 5-Fu was completely eliminated in less than 8 min, while only 15 min after the same result was achieved by direct photolysis and photodegradation with H2O2 (cf. inset of Fig. 7). The much faster degradation of 5-Fu in photo-Fenton and photodegradation with H2O2 compared to dark Fenton can be attributed to the quicker hydrogen peroxide splitting (Fig. S4 of the supplementary material). In photodegradation with H2O2, 5-Fu degradation occurs due to direct photolysis and attack by radicals that are generated from H2O2. In photo-Fenton, there is also the contribution of the catalytic reduction, in H2O2 aqueous solution, of Fe3+ into Fe2+, which increases the formation of hydroxyl radicals (Eqs. (8) and (9)). The same trends reported for 5-Fu degradation are observed in terms of mineralization (Fig. 7b).
The toxicity was assessed for a 5-Fu solution (0.38 mM) and for samples obtained after 8 h of Fenton’s reaction, direct photolysis, photodegradation with hydrogen peroxide and photo-Fenton (the latter at different doses of chemicals). The experiments showed a decrease on the toxicity for samples collected after 8 h of photo-assisted technologies and an increase on the toxicity for the sample collected after 8 h of Fenton’s reaction (Fig. 8). As 5-Fu was completely removed (at least below the detection limit) with all AOPs tested, the results indicate that the degradation products are less toxic than the parent compound for photo-assisted technologies. On the other hand, the degradation products generated during the dark Fenton’s reaction are more toxic than the parent compound. The mineralization degree achieved in Fenton’s reaction was lower than the one obtained for photo-assisted technologies, which may indicate that during the Fenton’s reaction more recalcitrant and toxic by-products are generated. Among all AOPs tested, photo-Fenton provided a higher degree of mineralization and inherently an effluent that exhibits lower toxicity towards V. fischeri. A smaller inhibition was even so obtained in the photo-Fenton experiment with lower dose of chemicals, which might be a consequence of the very similar TOC reduction (Fig. 6b) while providing less residual hydrogen peroxide and dissolved iron. However, more tests should be performed in order to evaluate the toxicity of 5-Fu and of the degradation products once these results correspond to short term tests, which have low environmental relevance due to the pseudo-persistent character of 5-Fu.
Ecotoxicological assay tests with V. fisheri after 15 min of contact using 5-Fu sample and effluents after treatment with different AOPs: Fenton’s reaction (Fe2+/H2O2, [Fe2+]0 = 0.5 mM; [H2O2]0 = 240 mM), direct photolysis (UV/Vis), photodegradation with H2O2 (UV/Vis/H2O2, [H2O2]0 = 17 mM), photo-Fenton (UV/Vis/ Fe2+/H2O2*, [Fe2+]0 = 0.5 mM; [H2O2]0 = 240 mM) and photo-Fenton (UV/Vis/Fe2+/H2O2**, [Fe2+]0 = 0.04 mM; [H2O2]0 = 17 mM)
The evaluation of the treatment costs is another important aspect to be considered in the processes comparison. The overall costs of treatment processes are represented by the sum of the capital costs, the operating costs and maintenance costs (Rodrigues et al. 2013). In this work, a preliminary cost estimation for each process was done considering only the costs of the reagents and of the energy necessary to achieve 100 % of 5-Fu degradation and/or 50 % of 5-Fu mineralization (Table 2). The costs of the treatment/processing of sludge generated and the maintenance costs are not included in this approach. According to the results present in Table 2, photo-Fenton process is the best option if the main goal is to achieve 100 % of 5-Fu degradation. Although Fenton’s oxidation is the most cost effective process if the goal is 5-Fu mineralization, the generated effluent is the most toxic. Moreover, it should be pointed out that the costs can considerably decrease for photo-assisted processes if solar light is used.
Conclusions
Reduction of the input and dispersion of cytostatics in the environment is an increasing challenge and constitutes one current hotspot subject. Therefore, the search for effective degradation technologies is a priority. In this sense, the performance of Fenton’s process to degrade 5-Fu, which is one of the most prescribed cytostatics worldwide, is investigated for the first time. This approach revealed to be very effective in the degradation of the cytostatic (providing complete degradation within 30 min or less), being achieved 50 % of mineralization after 8 h of reaction. The performance of the dark Fenton’s process was also assessed in a real WWTP effluent; it was found that the process is accelerated, probably due to the presence of some inorganic ions, which are more selective for 5-Fu than HO• radicals. Concerning the photo-assisted technologies tested (direct photolysis, photodegradation with hydrogen peroxide and photo-Fenton), the photo-Fenton process proved to be the most promising technique (providing 67 % of mineralization after only 2 h of reaction). It is noteworthy that, although similar mineralization degrees were published in the literature, an iron concentration in compliance with the legislation for direct water discharge was used herein. The toxicity assays showed a strong decrease on the toxicity against V. fischeri for the samples after direct photolysis, photodegradation with H2O2 and photo Fenton, putting into evidence the relevance of these AOPs for implementation towards and effective degradation of cytostatics like 5-Fu. Finally, the estimation of treatment costs suggested that photo-Fenton oxidation is the most cost-effective process if the main goal is to achieve complete 5-Fu degradation.
References
American Public Health Association (1998) Standard Methods for the Examination of Water and Wastewater, Washington, DC
Babuponnusami A, Muthukumar K (2014) A review on Fenton and improvements to the Fenton process for wastewater treatment. Journal of Environmental Chemical Engineering 2(1):557–572
Bagal MV, Gogate PR (2014) Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: a review. Ultrason Sonochem 21(1):1–14
Bokare AD, Choi W (2014) Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J Hazard Mater 275(0):121–135
Cortez S, Teixeira P, Oliveira R, Mota M (2011) Evaluation of Fenton and ozone-based advanced oxidation processes as mature landfill leachate pre-treatments. J Environ Manag 92(3):749–755
Environmental Protection Agency (2001) Parameters of water quality, interpretation and standards
Franquet-Griell H, Gómez-Canela C, Ventura F, Lacorte S (2015) Predicting concentrations of cytostatic drugs in sewage effluents and surface waters of Catalonia (NE Spain). Environ Res 138:161–172
Garcia-Ac A, Broséus R, Vincent S, Barbeau B, Prévost M, Sauvé S (2010) Oxidation kinetics of cyclophosphamide and methotrexate by ozone in drinking water. Chemosphere 79(11):1056–1063
Hartmann A, Alder AC, Koller T, Widmer RM (1998) Identification of fluoroquinolone antibiotics as the main source of umuC genotoxicity in native hospital wastewater. Environ Toxicol Chem 17(3):377–382
Hoppe-Tichy T (2010) Current challenges in European oncology pharmacy practice. J Oncol Pharm Pract 16(1):9–18
International Organization for Standardization (2005) Water quality-determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (Luminescent Bacteria Test) Part Three
Jiang C, Pang S, Ouyang F, Ma J, Jiang J (2010) A new insight into Fenton and Fenton-like processes for water treatment. J Hazard Mater 174(1–3):813–817
Johnson AC, Oldenkamp R, Dumont E, Sumpter JP (2013) Predicting concentrations of the cytostatic drugs cyclophosphamide, carboplatin, 5-fluorouracil, and capecitabine throughout the sewage effluents and surface waters of europe. Environ Toxicol Chem 32(9):1954–1961
Jung YS, Lim WT, Park JY, Kim YH (2009) Effect of pH on Fenton and Fenton-like oxidation. Environ Technol 30(2):183–190
Klavarioti M, Mantzavinos D, Kassinos D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int 35(2):402–417
Kosjek T, Perko S, Žigon D, Heath E (2013) Fluorouracil in the environment: analysis, occurrence, degradation and transformation. J Chromatogr A 1290:62–72
Kovalova L, McArdell CS, Hollender J (2009) Challenge of high polarity and low concentrations in analysis of cytostatics and metabolites in wastewater by hydrophilic interaction chromatography/tandem mass spectrometry. J Chromatogr A 1216(7):1100–1108
Li W, Tanumihardja J, Masuyama T, Korshin G (2015) Examination of the kinetics of degradation of the antineoplastic drug 5-fluorouracil by chlorine and bromine. J Hazard Mater 282(0):125–132
Lin HH-H, Lin AY-C (2014) Photocatalytic oxidation of 5-fluorouracil and cyclophosphamide via UV/TiO2 in an aqueous environment. Water Res 48(0):559–568
Lin AY-C, Wang X-H, Lee W-N (2013) Phototransformation determines the fate of 5-fluorouracil and cyclophosphamide in natural surface waters. Environmental Science & Technology 47(9):4104–4112
Lin, H.H.-H., Lin, A.Y.-C. and Hung, C.-L. (2014) Photocatalytic oxidation of cytostatic drugs by microwave-treated N-doped TiO2 under visible light. Journal of Chemical Technology & Biotechnology, n/a-n/a
Longley DB, Harkin DP, Johnston PG (2003) 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3(5):330–338
Lutterbeck CA, Wilde ML, Baginska E, Leder C, Machado ÊL, Kümmerer K (2015) Degradation of 5-FU by means of advanced (photo)oxidation processes: UV/H2O2, UV/Fe2+/H2O2 and UV/TiO2—comparison of transformation products, ready biodegradability and toxicity. Sci Total Environ 527–528(0):232–245
Mahnik SN, Lenz K, Weissenbacher N, Mader RM, Fuerhacker M (2007) Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere 66(1):30–37
Merouani S, Hamdaoui O, Saoudi F, Chiha M, Pétrier C (2010) Influence of bicarbonate and carbonate ions on sonochemical degradation of Rhodamine B in aqueous phase. J Hazard Mater 175(1–3):593–599
Negreira N, de Alda ML, Barceló D (2014) Cytostatic drugs and metabolites in municipal and hospital wastewaters in Spain: filtration, occurrence, and environmental risk. Sci Total Environ 497–498:68–77
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater 98(1–3):33–50
Rahim Pouran S, Abdul Raman AA, Wan Daud WMA (2014) Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J Clean Prod 64(0):24–35
Rahim Pouran S, Abdul Aziz AR, Wan Daud WMA (2015) Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J Ind Eng Chem 21:53–69
Rodrigues CSD, Madeira LM, Boaventura RAR (2013) Optimization and economic analysis of textile wastewater treatment by photo-Fenton process under artificial and simulated solar radiation. Ind Eng Chem Res 52(37):13313–13324
Rodrigues, C.S.D., Carabineiro, S.A.C., Maldonado-Hódar, F.J. and Madeira, L.M. (2016) Wet peroxide oxidation of dye-containing wastewaters using nanosized Au supported on Al2O3. Catalysis Today
Santos MSF, Alves A, Madeira LM (2011) Paraquat removal from water by oxidation with Fenton's reagent. Chem Eng J 175(0):279–290
Sellers RM (1980) Spectrophotometric determination of hydrogen peroxide using potassium titanium(IV) oxalate. Analyst 105(1255):950–954
Straub JO (2010) Combined environmental risk assessment for 5-fluorouracil and capecitabine in Europe. Integr Environ Assess Manag 6(S1):540–566
Tauxe-Wuersch A, De Alencastro LF, Grandjean D, Tarradellas J (2006) Trace determination of tamoxifen and 5-fluorouracil in hospital and urban wastewaters. Int J Environ Anal Chem 86(7):473–485
Umschlag T, Herrmann H (1999) The carbonate radical (HCO3·/CO3–·) as a reactive intermediate in water chemistry: kinetics and modelling. Acta Hydrochim Hydrobiol 27(4):214–222
Wang L, Albasi C, Faucet-Marquis V, Pfohl-Leszkowicz A, Dorandeu C, Marion B, Causserand C (2009) Cyclophosphamide removal from water by nanofiltration and reverse osmosis membrane. Water Res 43(17):4115–4122
Yu-Chen Lin A, Han-Fang Hsueh J, Andy Hong PK (2015) Removal of antineoplastic drugs cyclophosphamide, ifosfamide, and 5-fluorouracil and a vasodilator drug pentoxifylline from wastewaters by ozonation. Environ Sci Pollut Res 22(1):508–515
Zhang J, Chang VWC, Giannis A, Wang J-Y (2013) Removal of cytostatic drugs from aquatic environment: a review. Sci Total Environ 445–446:281–298
Acknowledgments
Mónica S. F. Santos is grateful to the Portuguese Foundation for Science and Technology (FCT) for her postdoctoral grant (ref. SFRH/BPD/104039/2014). This work was financially supported by Project POCI-01-0145-FEDER-006939 (Laboratory for Process Engineering, Environment, Biotechnology and Energy—UID/EQU/00511/2013) funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI)—and by national funds through FCT—Fundação para a Ciência e a Tecnologia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Vítor Pais Vilar
Electronic supplementary material
ESM 1
(DOCX 76 kb)
Rights and permissions
About this article
Cite this article
Governo, M., Santos, M.S.F., Alves, A. et al. Degradation of the cytostatic 5-Fluorouracil in water by Fenton and photo-assisted oxidation processes. Environ Sci Pollut Res 24, 844–854 (2017). https://doi.org/10.1007/s11356-016-7827-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7827-2