Abstract
The present study was conducted to systematically review, analyze, and interpret all the relevant evidence in the literature on the possible link between exposure to bisphenol A (BPA) and the risk of type-2 diabetes mellitus (T2DM). We developed a comprehensive search strategy and used it to search Web of Science, Scopus, PubMed, and Google Scholar up to March 31, 2016, producing 3108 hits, of which 13 original papers were included. Findings of these studies were quite controversial; few studies indicated a significant positive association between BPA exposure and T2DM, while some other failed to detect such a relationship. Overall, it can be suggested that chance is unlikely the plausible explanation for the observed association between BPA exposure and T2DM. This was mainly because even in the negative studies some clues could be found in favor of a statistically significant relationship between BPA and T2DM. Additionally, some of the studies had shortcomings in defining the exposure and outcome measures, which, if present, might have led to underestimating the relationship between BPA exposure and T2DM. The theoretical plausibility of such a relationship found earlier in animal studies also supports this point. However, more definitive answer requires the conduct of future longitudinal studies, in which the possible association between BPA exposure and T2DM is assessed over much longer periods of time with more temporally robust BPA measurements. In addition, it would be quite beneficial if future studies be conducted in areas where data is still lacking (e.g., South America, Australia/Oceania, and Europe).

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (2, 2′ − bis[4 − hydroxyphenyl]propane), also known as BPA, is a synthetic compound that is produced in very large volumes worldwide; reports indicate an annual production rate of over 2 million metric tons, with a 6–10 % annual increase in its demand (Burridge 2003). BPA is mostly used in the production of polycarbonate plastic and epoxy resins, both of which are used in drinks containers, food packaging, and dental sealants (Arenholt-Bindslev et al. 1999; Calafat et al. 2005; Howe et al. 1998; Sajiki and Yonekubo 2003), where it can rapidly leach into the surrounding environment (Vandenberg et al. 2010). Studies have also indicated that BPA is released into the atmosphere in amounts as high as 100 t per year (Vandenberg et al. 2009). Consequently, detectable levels of BPA have been found in water, wastewater, indoor and outdoor air, and dust (Loganathan and Kannan 2011; Tsai 2006; Vandenberg et al. 2007), leading to human exposure to BPA in a vast majority of different populations (Calafat et al. 2005, 2008; Vandenberg et al. 2007).
A number of epidemiological studies have so far been conducted to evaluate the possible association between exposure to BPA and the risk of T2DM in human subjects. However, results from these studies have been controversial; some studies have found a statistically significant relationship between human exposure to BPA and increased risk of developing T2DM (for example, (Silver et al. 2011)), while others have found no clear association between the two (for instance, (Kim and Park 2013)). So far, four systematic reviews have also been published exploring the relationship between BPA and T2DM (Kuo et al. 2013; LaKind et al. 2014; Rancière et al. 2015; Song et al. 2015). In the earliest work by Kuo et al. (2013), exposure to a number of chemicals, including BPA, was explored for their possible link with T2DM. This review had included four relevant studies, all of which were based on the National Health and Nutrition Examination Survey (NHNES) data. In addition, the quality of evidence and risk of bias were not considered in that review, as stated by the authors. Furthermore, their systematic search was performed in only one database, Medline, although at least two databases should be searched when conducting a systematic review. In a more recent systematic review, LaKind et al. (2014) evaluated the possible link between BPA and obesity, glucose metabolism, T2DM, and cardiovascular disease. This review included seven relevant studies for the association between BPA exposure and the risk of developing T2DM, of which five were based on the NHNES data, one was from China (Ning et al. 2011) and one was from South Korea (Kim and Park 2013). The authors concluded that data source was the likely reason for the discrepancies observed in the findings of different studies. They stated that the 2003–2004 NHNES data, in which urinary BPA concentrations were high, was responsible for observing a positive association between BPA and T2DM in the relevant studies included in their systematic review, which was not supported by NHNES data for other years as well as data from different countries. In the study by Song et al. (2015), the authors evaluated the association between exposure to endocrine disturbing chemicals (EDCs), including dioxin, polychlorinated biphenyl (PCBs), chlorinated pesticide, BPA, and phthalate, and the risk of developing T2DM and related metabolic traits. Searching up to March 2014, the authors were able to locate only five relevant papers directly evaluating the association between exposure to BPA and the risk of developing T2DM. Additionally, the quality of evidence and risk of bias were not considered in that review. Moreover, their systematic search was performed in only one database, i.e., Medline. Finally, in the most recent systematic review conducted by Ranciere et al. (2015), the authors evaluated the association between exposure to BPA and the risk of developing cardiometabolic disorders, including diabetes, hyperglycemia, measures of anthropometry, cardiovascular diseases, and hypertension. The authors performed a systematic search up to August 2014 and found eight relevant papers directly assessing the association between exposure to BPA and the risk of developing T2DM. Of the nine papers included, the meta-analysis was conducted on only three of the papers (pooled OR 1.47 (95 % CI 1.21–1.80)), mainly because inclusion of other studies significantly increased the heterogeneity of the results.
However, after these reviews, a number of original papers have been published, the association between exposure to BPA and the risk of developing T2DM in new populations, warranting a re-evaluation of current body of evidence. Therefore, in the present work, we set out to systematically review, analyze, and interpret the most recent, relevant evidence in the literature on the possible link between exposure to BPA and the risk of T2DM.
Methodology
In the present systematic review, we followed the methods used in our previous work (Sowlat et al. 2014), which was originally suggested by Khan et al. (2003). We first formulated the framing question as follows: Is there an association between exposure to BPA and the risk of developing T2DM? Based on this framing question, we then formulated a comprehensive search strategy to locate all the relevant evidence on the topic. The systematic search used in this review is as follows:
(“Bisphenol A” OR BPA OR “2,2-bis(4-hydroxyphenyl)propane” OR “4,4′-dihydroxy-2,2-diphenylpropane” OR “diphenylolpropane” OR “(CH3)2C(C6H4OH)2”) AND (“Type-2 Diabetes” OR “Diabetes Mellitus” OR T2D OR T2DM OR NIDDM OR MODY OR “Diabetes type-2” OR “Diabetes type-II” OR “type-II Diabetes” OR “Non-insulin-dependent” OR “Insulin resistance” OR hyperglyc* OR “prediabetic state”).
We searched in Web of Science, Scopus, PubMed, and Google Scholar using the abovementioned strategy up to March 31, 2016. Additional potentially relevant papers were also located by reviewing the reference list of the relevant papers (backward citation). We also searched and located the papers in which those most relevant studies had been cited (forward citation).
Afterwards, we defined a set of inclusion and exclusion criteria, based on which we screened the potentially relevant papers retrieved by the search. These criteria are presented in detail in Table 1. We included papers evaluating the association between exposure to BPA and the risk of T2DM in human subjects. We included papers published in English or any other language. We did not set any limitation regarding the time of publication or study design. We, however, excluded experimental studies conducted on laboratory animals or those evaluating health outcomes other than T2DM. Title, Abstract, and keywords of the retrieved papers were independently reviewed for relevance by two reviewers. If a decision on the relevance could not be made at this step, the paper was retrieved in full text and a decision was made based on the whole paper.
In the next step, we developed a checklist to systematically assess the quality of the relevant papers (critical appraisal). Since studies included in this systematic review were either cross-sectional or case-control (both observational) in design, we developed two quality assessment checklists based on the STROBE statement (www.strobe-statement.org), which has different checklists for critical appraisal of different types of observational studies. Tables 2 and 3 present the developed checklists used to assess the quality of the papers included in this systematic review. Each question in the checklists was given one score (yes = 1, no = 0), thereby the final score ranging from 1 to 7. We only included those papers that had acquired a score of four or above. This step was also done by two reviewers assessing the quality of the papers independently.
In the next step, we developed a comprehensive data extraction form for unbiased and impartial extraction of the relevant information from the included papers. Two reviewers separately extracted information pertaining to the study population, year of conduct, mean age and age range of the participants, sample size, measure of exposure, outcome measure, confounding variables considered in the study, and the effect size of the relationship. It is noteworthy that in all of the steps mentioned above (i.e., screening, quality assessment, and data extraction), consensus was reached in case of disagreement between the reviewers.
Results
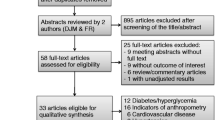
Figure 1 illustrates the flow diagram of the process we used for locating and selecting the relevant studies included in this systematic review. As shown in the figure, 3108 hits were produced by the systematic search strategy we used, of which 326 were from Web of Science, 2148 were from Scopus, 134 were from PubMed, and 500 were from Google Scholar. After a thorough review of the title, abstract, keywords, and, when necessary, the full text of the potentially relevant papers for their relevance, an overall of 13 papers were included in this review.
Tables 4 and 5 present the summary of the information extracted from the 13 papers included. As given in Tables 4 and 5, of the studies included in this systematic review, one had been conducted on a Chinese population (Ning et al. 2011), one on a Korean population (Kim and Park 2013), one on an Iranian population (Ahmadkhaniha et al. 2014), one on a Thai population (Aekplakorn et al. 2015), one on a Cypriot population (Andra et al. 2015), and eight on American populations (Casey and Neidell 2013; LaKind et al. 2012; Lang et al. 2008; Melzer et al. 2010; Sabanayagam et al. 2013; Shankar and Teppala 2011; Silver et al. 2011; Sun et al. 2014). Of the latter studies, seven were based on the data from the National Health and Nutrition Examination Survey (NHNES), spanning from 2003 to 2008 and one was based on data from Nurses’ Health Study (NHS) and NHSII cohorts (Sun et al. 2014).
In all of the included studies, except for the one in which serum BPA was measured (Aekplakorn et al. 2015), urinary BPA was considered as the exposure measure (determined from spot urine samples), while the outcome measure was somewhat different in different studies; in some of the studies, T2DM was determined based on self-reports, prior doctor diagnosis, or current use of diabetic treatments, while in some other studies, these factors were accompanied by laboratory test of fasting blood glucose or HbA1c, or either of the last two were used alone. The outcome measure even varied in the studies conducted using NHNES data. One of the studies had also considered prediabetes as the outcome measure (Sabanayagam et al. 2013). This combined with the fact that different variables were considered as confounders in different studies that suggested a high level of heterogeneity in the findings of the included studies.
Of the studies included in this review, seven were based on the NHNES data. Four of the NHNES-based studies reported a statistically significant association between BPA exposure and increased risk of T2DM for 2003–2008 data (Lang et al. 2008; Melzer et al. 2010; Shankar and Teppala 2011; Silver et al. 2011). This was due mainly to the increased BPA level in the case group of the 2003–2004 cycles, although the ORs for the years 2005–2006 and 2007–2008 were not statistically significant (due to the smaller difference in urinary BPA concentration between the diabetes and control groups and, therefore, the reduced statistical power). Sabanayagam et al. (2013) also found a statistically significant relationship between BPA exposure and prediabetes in 2003–2008 cycles of the NHNES data. LaKind et al. (2012), however, evaluated the possible link between BPA exposure and T2DM in 2003–2010 NHNES data, but found no such a relationship between the two. Casey and Neidell (2013) also used 2003–2008 NHNES data to further explore the possible association between BPA exposure and T2DM using two different approaches: first, calculation of OR based on 1-SD increase in BPA concentration; and second, OR calculation based on a ten-fold increase in BPA concentration. The first approach did not show a significant relationship (an OR and 95 % CI of 1.065 (0.973–1.166)), while the second approach revealed a positive association between BPA exposure and T2DM (an OR and 95 % CI of 1.202 (1.049–1.377)).
The study of Ahmadkhaniha et al. (2014) found a significant relationship between exposure to BPA and T2DM in an Iranian population, with the OR (95 % CI) being as high as 57.60 (21.10–157.05). Aekplakorn et al. (2015) also found a significant association between BPA exposure and T2DM, with OR and 95 % CI for the fourth quartile being 1.82 (1.12–2.95). However, Ning et al. (2011) and Kim and Park (2013) did not find a significant association between BPA exposure and T2DM. Nonetheless, it is noteworthy that in the study of Ning et al. (2011), although the test of trend was not significant, the ORs in two of the four BPA concentration categories were significantly higher than one. Additionally, in the study of Kim and Park (2013), higher prevalence of T2DM was observed in categories with higher BPA concentrations (p = 0.035). It is also worth considering that in these two studies, the difference in BPA concentration between the diabetes and control groups was marginal, especially in the case of the study of Ning et al. (2011). Sun et al. (2014) conducted a nested case-control study on the Nurses’ Health Study (NHS) and NHSII cohorts to assess the possible link between BPA exposure and T2DM. Results from this study indicated a significant positive association in the NHSII population (OR and 95 % CI for the fourth quartile 2.08 (1.17, 3.69) (p = 0.02)), while such a relationship was not found in the NHS cohort (OR and 95 % CI for the fourth quartile 0.81 (0.48, 1.38) (p = 0.45)). Finally, Andra et al. (2015) performed a similar study to evaluate the association between mono-chlorinated and total BPA and T2DM in a Cypriot population. Results from this study revealed a positive relationship between exposure to mono-chlorinated BPA and increased risk of T2DM (OR and 95 % CI 5.06 (1.29–25.4)), while the authors did not observe a significant association in case of total BPA (OR and 95 % CI 10.6 (0.36–2.79)).
In the present systematic review, we could not perform a meta-analysis, synthesizing the ORs presented in different studies on the association between BPA exposure and T2DM. As can be seen in Table 5, this was mainly due to the fact that in each study, authors had adopted a different approach to calculate the OR; for example, Kim and Park (2013) had calculated the OR for different quartiles of BPA concentration, while Lang et al. (2008) had calculated the OR based on a 1-SD increase in BPA concentration (see section 4 for a more detailed discussion).
Alternatively, we set out to perform a meta-analysis, calculating the overall standardized mean difference (SMD) of BPA concentration between the diabetes and control groups. However, the values of chi-squared (χ2), Tau-squared (τ2), and I-squared (I2) tests (P < 0.0001, 0.542 and 97.7 %, respectively) suggested considerable heterogeneity in the findings of the included studies and, therefore, the inappropriateness of synthesizing the data. In addition, the data presented in different papers did not match. For instance, in the study of Ning et al. (2011), BPA concentrations in the diabetes and the control groups were given as medium (interquartile range) rather than as mean (SD, SE, or CI). In some others, such information had not been presented at all (for example, (Sun et al. 2014)). Therefore, exclusion of such studies from the meta-analysis would have resulted in bias in the results.
Discussion
Comparison with previous review studies and implications of the findings
As mentioned above, prior to the present work, four other systematic reviews had been published exploring the relationship between BPA and T2DM (Kuo et al. 2013; LaKind et al. 2014; Ranciere et al. 2015; Song et al. 2015). In the study of Kuo et al. (2013), authors explored exposure to a number of chemicals, including BPA, for their possible link with T2DM, including four relevant studies, all of which were based on the National Health and Nutrition Examination Survey (NHNES) data. In addition, the quality of evidence and risk of bias were not considered in that review. Another drawback was that their systematic search was performed in only one database, Medline, although at least two databases should be searched when conducting a systematic review.
In a more recent systematic review, LaKind et al. (2014) evaluated the possible link between BPA and obesity, glucose metabolism, T2DM, and cardiovascular disease, including seven relevant studies for the association between BPA exposure and the risk of developing T2DM, of which five were based on the NHNES data, one was from China (Ning et al. 2011), and one was from South Korea (Kim and Park 2013). The authors concluded that data source was the likely reason for the discrepancies observed in the findings of different studies. They stated that the 2003–2004 NHNES data, in which urinary BPA concentrations were high, was responsible for observing a positive association between BPA and T2DM in the relevant studies included in their systematic review, which was not supported by NHNES data for other years as well as data from different countries.
In the study by Song et al. (2015), the authors evaluated the association between exposure to endocrine disturbing chemicals (EDCs), including dioxin, polychlorinated biphenyl (PCBs), chlorinated pesticide, BPA, and phthalate, and the risk of developing T2DM and related metabolic traits. Searching up to March 2014, the authors were able to locate only five relevant papers directly evaluating the association between exposure to BPA and the risk of developing T2DM. Additionally, the quality of evidence and risk of bias were not considered in that review. Moreover, their systematic search was performed in only one database, i.e., Medline. Finally, in the most recent systematic review conducted by Ranciere et al. (2015), the authors evaluated the association between exposure to BPA and the risk of developing cardiometabolic disorders, including diabetes, hyperglycemia, measures of anthropometry, cardiovascular diseases, and hypertension. The authors performed a systematic search up to August 2014, and found eight relevant papers directly assessing the association between exposure to BPA and the risk of developing T2DM. Of the nine papers included, the meta-analysis was conducted on only three of the papers (pooled OR 1.47 (95 % CI 1.21–1.80)), mainly because inclusion of other studies significantly increased the heterogeneity of the results.
The present systematic review was conducted to gather all the relevant evidence on the possible link between exposure to BPA and increased risk of developing T2DM in adults. Results from the included studies were quite controversial; some of the studies indicated a statistically significant association between BPA exposure and T2DM (for instance, (Ahmadkhaniha et al. 2014; Lang et al. 2008; Melzer et al. 2010; Shankar and Teppala 2011; Silver et al. 2011)), while others two failed to detect such a relationship (for example, (Kim and Park 2013; Ning et al. 2011)). This controversy even exists in case of studies performed on the NHNES data. The first four studies published in this domain using the NHNES data all indicated a significant positive association between BPA and T2DM (Lang et al. 2008; Melzer et al. 2010; Shankar and Teppala 2011; Silver et al. 2011) for 2003–2008 cycles. In fact, a significant association was observed only in case of the 2003–2004 NHNES data, while each year cycles were analyzed separately; the BPA exposure levels in the year 2003–2004 were so high that when the data for 2003–2008 were pooled, the resulting OR was significantly higher than the unity. More recently, LaKind et al. (2012) conducted the same study using the 2003–2010 NHNES data but found no significant association between BPA exposure and T2DM, even in case of the 2003–2004 cycle. The authors argued that this discrepancy was as a result of using different exclusion criteria than those of other studies, mainly whether or not individuals with prediabetes should be included in the case group. Casey and Neidell (2013) also performed a study on the 2003–2008 NHNES data using different statistical approaches to assess the possible link between BPA exposure and T2DM. The authors concluded that the results (observing or failing to observe a significant association between BPA exposure and T2DM) were sensitive to both the statistical model used and the eligibility criteria set. Therefore, the authors suggested that when the NHNES data are used for such studies, researchers should explicitly report any specification and assumption they have made to allow for recognition of the reasons behind inconsistencies observed in the results.
Studies conducted on populations other than the NHNES have also reported inconsistent results. Ahmadkhaniha et al. (2014) and Aekplakorn et al. (2015) both reported a significant positive association between exposure to BPA and increased risk of T2DM in Iranian and Thai populations, respectively. Sun et al. (2014) found a significant association between BPA exposure and T2DM in the NHSII cohort, while such a relationship was not observed in case of the NHS. Andra et al. (2015) also found a significant relationship between exposure to mono-chlorinated BPA and T2DM in a Cypriot population, while such an association was not observed for total BPA. On the other hand, Ning et al. (2011) and Kim and Park (2013) did not find a significant relationship between BPA exposure and increased risk of T2DM in Chinese and Korean populations, respectively. Nonetheless, it is noteworthy that even in the case of some of the negative studies, some clues could be found in favor of a positive relationship. For example, in the study of Ning et al. (2011), although the test of trend was not significant, the ORs in two of the four BPA concentration categories were significantly higher than one. Additionally, in the study of Kim and Park (2013), higher prevalence of T2DM was observed in categories with higher BPA concentrations (p = 0.035). Therefore, chance might not be the plausible explanation for observing such a relationship between BPA exposure and T2DM. The theoretical plausibility of such a relationship found earlier in animal studies also supports this view.
Few mechanisms have been proposed for the effect of BPA on metabolic homeostasis by laboratory studies conducted on rodents and human tissue culture models. The first proposed mechanism is that overstimulation of the estrogen receptor ERα in pancreatic β-cells can occur due to BPA exposure, which leads to alteration in the biosynthesis and secretion of insulin in pancreatic β-cells (Alonso-Magdalena et al. 2006; Nadal et al. 2009a, b). Second, studies have indicated that BPA imitates estradiol (E2), another hormone which is critical for maintaining β-cells function and insulin sensitivity (Livingstone and Collison 2002; Louet et al. 2004). It was reported that the glucose tolerance and insulin resistance of male mice treated with either BPA or E2 were altered, and the mice exhibited hyperinsulinemia (Alonso-Magdalena et al. 2006; Alonso-Magdalena et al. 2008; Ropero et al. 2008). Lastly, it is suggested that BPA is capable of suppressing adiponectin (Ben-Jonathan et al. 2009; Hugo et al. 2008). Adiponecin is another important hormone that is responsible for maintaining insulin sensitivity (Whitehead et al. 2006). It has been reported that the levels of this hormone decrease before the development of T2DM (Trujillo and Scherer 2005).
Methodological concerns on the included studies
As mentioned in the “Results” section, we faced a high level of heterogeneity in the studies included in this systematic review, which could have been caused by several factors. Firstly, as presented in Table 5, each study had included individuals with a different age range. For example, Kim and Park (2013) had included those in the age range of 40–69 years, while in the NHNES studies, individuals in the age range of 18–74 (in one case, up to 84 years old) had been included. Since age is one of the influential factors affecting the portion of the sample population having developed T2DM, this difference in the age range could have caused heterogeneity in the findings of the included studies. Although the effect of age was adjusted for in almost every single study that was included in this systematic review, the between-study difference in the age range, as one of the possible factors causing the between-study heterogeneity (shown by the value of I2 test), would still remain unsolved. This was because we tried to obtain a pooled SMD of raw (not adjusted) BPA concentrations between the two groups rather than a pooled value of ORs adjusted for confounders.
Another source of heterogeneity in the findings reported by different studies might have been the different exposure levels in the case (exposure) and control groups. Since different studies had conducted their analysis on different populations and given that BPA concentrations varied in the urine samples collected from different populations, this had resulted in a wide range in urine BPA concentrations in both exposure and control groups among different studies. For instance, as can be seen in Table 5, in the study of Kim and Park (2013) conducted on Korean adult population, the BPA concentrations were in the range of 1.92–2.14 and 2.04–2.82 ng/ml in the control and exposure groups, respectively. However, in the study of Lang et al. (2008), the corresponding ranges for the control and exposure groups were 3.8–4.9 and 5.2–10.3 ng/ml, respectively.
The different populations included in the studies included in the present systematic review can also be viewed from another perspective. There exist some interesting findings that may suggest the involvement of racial/ethnic or genetic factors in the like between BPA exposure and T2DM. As presented in Table 5, two studies had been conducted on eastern Asian populations, i.e., the study of Kim and Park (2013) conducted on a Korean adult population and the study of Ning et al. (2011) conducted on a Chinese adult population, and neither of them observed a positive like between exposure to BPA and an increased risk of developing T2DM. This may be an indication of involvement of racial/ethnic or genetic factors when exploring the possible association between BPA exposure and developing T2DM. However, no study has so far provided any evidence in support of this theory and, therefore, it is worth looking into more deeply in future studies.
Another important factor that may have contributed in the heterogeneity observed in the findings of the included studies was the inconsistency in measuring the outcome. As presented in Table 5, some of the studies had determined the outcome as self-reported or doctor-diagnosed diabetes or the current use of diabetic pills (Kim and Park 2013), while some other studies had relied on laboratory tests such as fasting blood glucose or HbA1c (Ahmadkhaniha et al. 2014; Shankar and Teppala 2011; Silver et al. 2011). This inconsistency was even observed in studies based on NHNES data. This might have resulted in different rates of T2DM in the participants and, therefore, might have resulted in heterogeneity in the findings.
Furthermore, the confounding variables whose effects were adjusted for also varied between the studies included. There were some factors, including age, sex, and BMI, whose effects were adjusted for in almost every single study (Table 5). However, there were other factors whose effects were adjusted for in some of the studies but not in others; examples of these factors include, but not limited to, education, smoking, hypertension, serum cholesterol, serum creatinine, and waist circumference. This might have been another important factor significantly contributing to the heterogeneity observed in the findings of the primary studies.
There are also a couple of more factors that need to be taken into consideration when trying to assess the possible link between exposure to BPA and increased risk of developing T2DM. Since BPA has a very fast rate of metabolism (a half-life of less than 6 h after oral intake), urine is the most appropriate body fluid that can be used for assessing the exposure to this compound (Dekant and Völkel 2008). However, since the health effects of BPA, like other compounds, are most likely due to long-term, low-level exposure, the use of spot measurements of urinary BPA for detecting significant health outcomes is being questioned (Lang et al. 2008). Studies have indicated that urinary BPA concentrations highly varies on a daily basis (Braun et al. 2011; Ye et al. 2011), thus making it impossible to address the temporal variability using single urine samples (Silver et al. 2011). This variability would most likely result in non-differential misclassification and, subsequently, underestimation of the strength of the association (Lang et al. 2008; Silver et al. 2011). Hence, future longitudinal studies with multiple sampling over longer periods of time can shed more light on the possibility of the link between BPA exposure and T2DM. In addition, longitudinal studies would also help detect the causality of the possible relationship between BPA exposure and T2DM, which could not be inferred even from the positive studies included in this review, due mainly to their cross-sectional or case-control nature.
There is also another major limitation in some of the primary studies included in this systematic review that may have disturbed their findings. Some of these studies had considered self-reported or doctor-diagnosed diabetes as the measure of outcome (Kim and Park 2013). This way, a large number of patients with T2DM may have been misclassified as normoglycemic, thus leading to an underestimation of the true association between BPA and T2DM, if present (Shankar and Teppala 2011). However, HbA1c is believed to be a better estimator of T2DM, because it is more stable than other markers of glycemic indices, including fasting blood sugar. Additionally, since it is a continuous marker, it can detect those with undiagnosed T2DM, thus reducing the misclassification of the outcome (Silver et al. 2011). These two shortcomings of the primary studies included in this systematic review were other reasons for the vantage point of the authors that chance is believed to be an unlikely explanation for the observed association between BPA exposure and the increased risk of developing T2DM.
Furthermore, when statistically assessing the association between BPA exposure and T2DM, some of the studies (for example, (Melzer et al. 2010)) had assumed a linear exposure-response relationship. However, Silver et al. (2011) indicated that the exposure-response relationship was close to log-linear rather than linear, suggesting the inappropriateness of this assumption when assessing the link between BPA exposure and T2DM.
Moreover, none of the primary studies had collected data on the diet or the dietary behaviors of the individuals included in their study. Ning et al. (2011) suggested that BPA can be considered as a marker of the consumption of sugared drinks (like soda), due primarily to its widespread use in such containers. On the other hand, intake of such drinks has already been linked to glucose dysregulation. Therefore, they concluded that when no data on the dietary behavior is collected, any study indicating a positive association between BPA exposure and T2DM may be indicating the association between higher consumption of sugared drinks and T2DM. Therefore, it is suggested that data on the diet should also be collected when conducting future studies to evaluate the association between BPA exposure and T2DM to further assess the role of this factor and prevent its possible confounding impact on the findings.
It is also noteworthy that all of the primary studies had examined the effect of BPA exposure in adult populations, so they could not offer any information on the possible health effects exposure to BPA can pose during critical growth periods. Studies have indicated that exposures during early stages of life can increase the susceptibility of individuals to developing diseases later in life (Barker 1998; Barker et al. 2002; Tang and Ho 2007). In case of BPA, an animal study suggested that maternal exposure to BPA could alter the metabolic homeostasis of mice male offsprings, significantly reducing their glucose tolerance and increasing their insulin resistance (Alonso-Magdalena et al. 2010). Therefore, it can be suggested that this is a very critical domain in which future studies seem quite necessary.
Limitations and strengths
In the present systematic review, as mentioned earlier, we failed to conduct a meta-analysis, synthesizing a pooled OR for the possible association between BPA exposure and T2DM. This was mainly due to the fact that each of the included studies had calculated the OR with a different approach. For example, Kim and Park (2013) had divided the BPA concentration into quartiles and then calculated the OR for each of the quartiles by comparison with the reference quartile. Melzer et al. (2010), on the other hand, had calculated the OR based on a 1-SD increase in BPA concentration, while Silver et al. (2011) had used a two-fold increase in BPA concentration for calculating the OR. To overcome this inconsistency, we contacted the authors of each study separately through e-mail and asked them to re-calculate the OR with the same approach, say, unit increments in BPA concentration. However, we did not receive any response from them. It is noteworthy that “author contact” is a well-established, reliable tool for systematic reviews to overcome lack of information in the primary studies included, but it requires the collaboration of authors conducting those primary researches.
Consequently, we decided, alternatively, to use another approach, which was performing a meta-analysis to obtain a pooled estimate of SMD in BPA concentration between the diabetes and control groups. However, all three of the relevant tests indicated considerable heterogeneity in the findings of the included studies, suggesting the inappropriateness of pooling the data. This high level of heterogeneity was most likely caused by the methodological issues discussed in-depth above.
Sutton et al. (2000) suggest that significant challenges arise when trying to perform a meta-analysis on the results from observational studies. This is mainly due to considerable heterogeneity in the findings, which itself can be attributed to different designs, different populations sampled, different definitions of exposure and outcome, adjustments for different confounders, and, finally, sensitivity to different types of bias from which randomized controlled trials (RCTs) are immune. Notwithstanding, Tu and Greenwood (2012) state that even if the primary studies are conducted in the same manner (i.e., regarding the factors mentioned above), the outcomes may be presented in different ways. For example, some studies may have presented the outcome as relative risks (RRs), while others might have reported ORs. For continuous exposures, RRs or ORs may have been calculated based on a unit increment of the exposure, or they may have been calculated for different categories (like quartiles) of exposure. This inconsistency, per se, prevents the conduct of meta-analysis, as observed in case of the present systematic review.
Finally, it is noteworthy that although some of the studies included in this systematic review have reported a positive association between exposure to BPA and increased risk of developing T2DM, inference on the causal relationship between the two cannot be made based solely on the current body of evidence and future longitudinal epidemiological studies are required to shed more light on the association between BPA exposure and T2DM to enable us to infer causality in the association, if there is any.
Conclusions
The present systematic review was conducted to collect and analyze all the relevant evidence in the literature on the association between BPA exposure and T2DM in adults. Findings from the primary studies in this domain were quite controversial; few studies indicated a significant positive association between BPA exposure and T2DM, while few others failed to detect such a relationship. Overall, it can at least be suggested that chance is not likely to be the plausible explanation for the observed association between BPA exposure and T2DM. The theoretical plausibility of such a relationship which was found earlier in animal studies also supports this view. However, in this review, we could not perform a meta-analysis to estimate the overall size effect of the relationship. This was mainly because the ORs from the primary studies were reported in different fashions that were not synthesizable. Nonetheless, the heterogeneity was so significant that, if done, would have made the meta-analysis scientifically unsound. This heterogeneity arose most likely due to the effect of the different populations sampled, the different age ranges considered, the different outcome measures used, and the different confounders considered. Furthermore, using single measurements of urinary BPA and the use of self-reported and doctor-diagnosed diabetes as the outcome in some of the studies may have biased the findings in favor of finding no association between BPA exposure and T2DM, while multiple measurements of urinary BPA and the use of a continuous measure for detection of T2DM (like HbA1c) can provide more accurate estimations of the measures of exposure and outcome. On the other hand, lack of data collection on dietary behavior of the individuals may have biased the findings towards observing a significant association between BPA exposure and T2DM, which in fact indicates the association between consumption of sugared drinks and T2DM. These points should be considered in, and the limitations can be resolved by conducting longitudinal studies in future to shed more light on the association between BPA exposure and T2DM. This specific study design also enables us to infer causality in the association, if there is any. It is also suggested that in the future, studies calculate the size effect in the same manner to increase their synthesizability in future systematic reviews. In addition, it is proposed that future studies use almost the same setting (i.e., characteristics of the population, measures of exposure and outcome, confounding variables considered, etc.) to decrease the level heterogeneity and, therefore, increase the chance of conducting a meta-analysis to have a pooled estimate of the size effect. Finally, an intriguing research domain that needs special attention by the researchers is the possible impact BPA exposure in critical growth periods can have on human subjects later in life, especially with respect to developing T2DM. It should be noted that the current body of evidence on the possible link between exposure to BPA and the risk of developing T2DM is not extensive enough to be the basis for making relevant policies and develop practice guidelines, again warranting further research in this domain in the areas suggested above.
References
Aekplakorn W, Chailurkit LO, Ongphiphadhanakul B (2015) Relationship of serum bisphenol A with diabetes in the Thai population, National Health Examination Survey IV, 2009. J Diabetes 7:240
Ahmadkhaniha R et al. (2014) Association of urinary bisphenol A concentration with type-2 diabetes mellitus. J Environ Health Sci Eng 12:64
Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A (2006) The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ Health Perspect 114:106
Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A (2010) Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect 118:1243
Alonso-Magdalena P et al. (2008) Pancreatic insulin content regulation by the estrogen receptor ERα. PLoS One 3:e2069
Andra SS, Kalyvas H, Andrianou XD, Charisiadis P, Christophi CA, Makris KC (2015) Preliminary evidence of the association between monochlorinated bisphenol A exposure and type II diabetes mellitus: a pilot study. J Environ Sci Health A Tox Hazard Subst Environ Eng 50:243–259. doi:10.1080/10934529.2015.981111
Arenholt-Bindslev D, Breinholt V, Preiss A, Schmalz G (1999) Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants. Clin Oral Invest 3:120–125
Barker DJ (1998) In utero programming of chronic disease. Clin Sci 95:115–128
Barker DJ, Eriksson JG, Forsen T, Osmond C (2002) Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31:1235–1239
Ben-Jonathan N, Hugo ER, Brandebourg TD (2009) Effects of bisphenol A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol Cell Endocrinol 304:49–54
Braun JM et al. (2011) Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect 119:131
Burridge E (2003) Bisphenol A: product profile. Eur Chem News 78:17
Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL (2005) Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113:391
Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL (2008) Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 116:39
Casey MF, Neidell M (2013) Disconcordance in statistical models of bisphenol a and chronic disease outcomes in NHANES 2003-08. PLoS One 8(11):e79944
Dekant W, Völkel W (2008) Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol 228:114–134
Howe SR, Borodinsky L, Lyon RS (1998) Potential exposure to bisphenol A from food-contact use of epoxy coated cans. J Coat Tech 70:69–74
Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N (2008) Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect 116:1642–1647
Khan KS, Kunz R, Kleijen J, Antes G (2003) Systematic reviews to support evidence-based medicine: how to write and apply findings of healthcare research. Royal Society of Medicine Press, London
Kim K, Park H (2013) Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: a population-based cross-sectional study. Int J Hygiene Environ Health 216:467–471
Kuo C-C, Moon K, Thayer KA, Navas-Acien A (2013) Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Curr Diab Rep 13:831–849. doi:10.1007/s11892-013-0432-6
LaKind JS, Goodman M, Mattison DR (2014) Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit Rev Toxicol 44:121–150. doi:10.3109/10408444.2013.860075
LaKind JS, Goodman M, Naiman DQ (2012) Use of NHANES data to link chemical exposures to chronic diseases: a cautionary tale. PLoS One 7:e51086
Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D (2008) Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300:1303–1310
Livingstone C, Collison M (2002) Sex steroids and insulin resistance. Clin Sci 102:151–166
Loganathan SN, Kannan K (2011) Occurrence of bisphenol A in indoor dust from two locations in the eastern United States and implications for human exposures. Arch Environ Contam Toxicol 61:68–73
Louet J-F, LeMay C, Mauvais-Jarvis F (2004) Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscleros Rep 6:180–185
Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS (2010) Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PLoS One 5:e8673
Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB (2009a) The pancreatic β-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol 304:63–68
Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I (2009b) The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol 587:5031–5037
Ning G et al. (2011) Relationship of urinary bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults: a cross-sectional analysis. Ann Intern Med 155:368–374
Rancière F, Lyons JG, Loh VHY, Botton J, Galloway T, Wang T, Shaw JE, Magliano DJ (2015) Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health 14:46
Ropero AB, Alonso-Magdalena P, Quesada I, Nadal A (2008) The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids 73:874–879
Sabanayagam C, Teppala S, Shankar A (2013) Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol 50:625–631. doi:10.1007/s00592-013-0472-z
Sajiki J, Yonekubo J (2003) Leaching of bisphenol A (BPA) to seawater from polycarbonate plastic and its degradation by reactive oxygen species. Chemosphere 51:55–62
Shankar A, Teppala S (2011) Relationship between urinary bisphenol A levels and diabetes mellitus. The J Clin Endocrinol Metabol 96:3822–3826
Silver MK, O’Neill MS, Sowers MR, Park SK (2011) Urinary bisphenol A and type-2 diabetes in US adults: data from NHANES 2003-2008. PLoS One 6:e26868
Song Y, Chou EL, Baecker A, You NCY, Song Y, Sun Q, Liu S (2015) Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: a systematic review and meta-analysis. J Diabet. doi:10.1111/1753-0407.12325
Sowlat MH, Abdollahi M, Gharibi H, Yunesian M, Rastkari N (2014) Removal of vapor-phase elemental mercury from stack emissions with sulfur-impregnated activated carbon. In: Rev Environ Contam Toxicol volume. Springer, pp 1–34
Sun Q et al. (2014) Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the nurses’ health study (NHS) and NHSII cohorts. Environ Health Perspect 122:616–623
Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F (2000) Methods for meta-analysis in medical research. J. Wiley,
Tang W-y, Ho S-m (2007) Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metabol Disord 8:173–182
Trujillo M, Scherer P (2005) Adiponectin–journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med 257:167–175
Tsai W-T (2006) Human health risk on environmental exposure to bisphenol-A: a review. J Environ Sci Health C 24:225–255
Tu Y-K, Greenwood DC (2012) Modern methods for epidemiology. Springer
Vandenberg L, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G (2010) Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118:1055–1070
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177
Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM (2009) Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30:75–95
Whitehead J, Richards A, Hickman I, Macdonald G, Prins J (2006) Adiponectin—a key adipokine in the metabolic syndrome. Diab Obes Metabol 8:264–280
Ye X, Wong L-Y, Bishop AM, Calafat AM (2011) Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-h collections. Environ Health Perspect 119:983
Acknowledgment
The present study was financially supported by the Institute for Environmental Research (IER), Tehran University of Medical Sciences (TUMS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Sowlat, M.H., Lotfi, S., Yunesian, M. et al. The association between bisphenol A exposure and type-2 diabetes: a world systematic review. Environ Sci Pollut Res 23, 21125–21140 (2016). https://doi.org/10.1007/s11356-016-7525-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7525-0