Abstract
Rare earths (RE), chemically uniform group of elements due to similar physicochemical behavior, are termed as lanthanides. Natural occurrence depends on the geological circumstances and has been of long interest for geologist as tools for further scientific research into the region of ores, rocks, and oceanic water. The review paper mainly focuses to provide scientific literature about rare earth elements (REEs) with potential environmental and health effects in understanding the research. This is the initial review of RE speciation and bioavailability with current initiative toward development needs and research perceptive. In this paper, we have also discussed mineralogy, extraction, geochemistry, analytical methods of rare earth elements. In this study, REEs with their transformation and vertical distribution in different environments such as fresh and seawater, sediments, soil, weathering, transport, and solubility have been reported with most recent literature along key methods of findings. Speciation and bioavailability have been discussed in detail with special emphasis on soil, plant, and aquatic ecosystems and their impacts on the environment. This review shows that REE gained more importance in last few years due to their detrimental effects on living organisms, so their speciation, bioavailability, and composition are much more important to evaluate their health risks and are discussed thoroughly as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term rare earth (RE) is actually a misnomer that shows a cursory view of these metals good enough for a 5-min review (Hurst 2010). Lanthanide series include rare earth elements (REEs) along with scandium and yttrium, being similar in chemical properties and deposited in ores same like REEs (Connelly 2005; Zdzislaw and Agnieszka 2015). Promethium occurs in trace quantities due to no long lived and stable isotope (Castor and Hedrick 2006; Borovicka et al. 2011).
For many years, human activities have been a significant source of trace metallic elements eventually contaminating the earth’s surface. Such actions overburdened the regime of ecosystem in aquatic and terrestrial environments with large quantities of trace metallic pollutants and contaminants. Mining, extraction, and utilization of trace elements in high value goods and as major component of infrastructure became cornerstone in human development civilization. Consequently, at local, national, and universal scale, human awareness and individual role on the environmental components to manage and improve degraded terrestrial and aquatic system have been enhanced (Ozdes et al. 2011; Adamu et al. 2013).
Rare earth elements (REEs) speciation in the geochemical samples provides potential hazards and pathways for remediation. Factors like time and environmental conditions affecting speciation provide challenging and great opportunity for environmental science and technology to study rare earth elements. Evaluation of contaminants, assessment, and remediation processes is recent in modern research (Adamu et al. 2013; Ashraf et al. 2015).
Different species of ecosystem have 99 % REEs bioavailable in bound to suspended matter and sediment form. Bioavailability of rare earth elements (REEs) in different environments varies with change in ionic concentration and ligands and decrease among dissolved species as in the order: free ion, organic or inorganic complexes (Sun et al. 2003; Borrego et al. 2012). It also depends on equilibrium and change when there is a disturbance in equilibrium. Sediments may become a source of available rare earths in water which also become available to other biotic organisms feeding on them. Rare earth elements (REEs) released from soil and sediments become significant as bioavailable to the algae. Sequential extraction method is commonly applied to study bioavailability of rare earths in geochemical sample analysis. Bioaccumulation of rare earth elements in organisms depends in part upon their bioavailability. Few investigations describe the relationship between rare earth elements species and plant bioavailability. Sun et al. (2003) reported that most of the rare earths in soil and sediments are present in extractable species and increase with increase in spiking of REEs. Generally, total concentration of rare earth elements (REEs) in soil is very small in milligrams per kilogram (Ebrahim et al. 2009) and in plants more than 10 mg/kg (Cao et al. 2000; Wood et al. 2004; Ebrahim et al. 2009). This concentration is quite low to detect. Still, no correlation in the studies is found between REEs species in natural soil system and their bioavailability. The current review paper provides an overview of contents relating to geochemistry, bioavailability, and speciation of REEs in the ecosystem and describes trace metal speciation and bioavailability in achieving result-oriented remediation methods and risk in approaching pollution source (Haque et al. 2014).
Mineralogy
Rare earth elements (REEs) consisting of both heavy rare earth elements (HREEs) and light rare earth elements (LREEs) exhibit in mineral form. REEs naturally occur in diversity of mineral types. HREEs and Y are mostly associated with minerals such as xenotime, euxenite, yttrotantalite, samarskite, and gadolinite, while LREEs are found in monazite, bastnasite, ancylite, lanthanite, stillwellite, allanite, cerianite, britholite, and loparite (Chakhmouradian and Wall 2012). Bastnasite, monazite, and xenotime ores are mostly extracted around the world in commercially operating mines. High concentration of REEs produces its own minerals. Despite of more than 200 REE minerals, only three ores are considered suitable for extraction: bastnasite, xenotine and monazite (Gupta and Krishnamurthy 2004; Weng et al. 2013). Bastnasite, a carbonate mineral [(Ce,La,Y)CO3F] quite similar to the mineral parasite [Ca(Ce,La)2(CO3)3F2], is found in pale white, tan, gray, brown, yellow, or pink, with a pearly, vitreous, or greasy to dull luster form and mostly found with mineral hydroxyl-bastnasite [(Ce,La)CO3(OH,F)], vein deposits, pegmatite’s and contact metamorphosis zones. It is highly enriched in LREEs such as cerium, lanthanum, and yttrium (Keith et al. 2010; Haque et al. 2014). Xenotine and Monazite, two phosphate minerals, found together, crystallize under different temperature and pressure regimes containing any of HREEs or LREEs. Monazite, an enriched LREE and thorium-bearing phosphate mineral [(Ce,La,Y,Th)PO4], is characterized by low crystallization temperature and pressure with 60–62 % RE oxides. Crystalline forms of monazite are usually yellow to brown or orange-brown with a vitreous and resinous luster. Thorium present with monazite makes it radioactive, and wastes produced from such mineral need special disposal methods, accounts serious impediment to economic extraction. Generally, minerals with radioactive properties are not easy to handle and process (Keith et al. 2010; Sprecher et al. 2014). Xenotine, an yttrium phosphate mineral (YPO4), crystallizes under high temperature and pressure with enriched HREEs and minor component of granitic and gneissic rocks. It occurs as yellowish brown to reddish brown crystals with a vitreous to resinous luster in acidic and alkaline rocks (Keith et al. 2010; Melfos and Voudouris 2012).
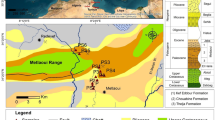
Uranium ores have also been found to contain REEs in appreciable amounts. Processing of such radioactive minerals for REEs is very challenging to the world’s producer and supplier countries. Four countries—China, USA, Australia, and Malaysia—are well-known for REE deposits. China has bastnasite and xenotime deposits, while USA principally contains bastnasite deposits. Australia has major RE-bearing minerals such as monazite and xenotime, whereas Malaysia is known for xenotime deposits only (Fig. 1) (Geoscience Australia 2012; Haxel et al. 2014).
Rare earth elements distribution in a Bayan Obo, China, b Mountain pass, USA, c Mount Weld, Australia, and d Lahat, Malyasia (USGS 2013)
Lynas corporation’s for rare earth elements (REEs) processing plant in Malaysia has become a current issue of interest in the sense that the radioactive waste generated during processing will create long-term health and environmental impacts that dates back to Asian rare earths refinery 2 decades ago. However, International Atomic Energy Agency argued that the radiations generated from the current Malaysian RE producing plant would be in certain limit not to have impact on normal life of humans and other components of environment (Heber 2013).
Geochemistry of rare earth elements
The low concentrations of REEs (ng/L or ng/kg) hinder their geochemical fate in many environmental mediums (Kynicky et al. 2012; Williams-Jones et al. 2012). Previous research work on biogeochemistry of rare earth elements (REEs) in soil/plant system and their effects on agricultural ecosystem has been widely studied (Wyttenbach and Tobler 1998; Zhang and Shan 2001; Zawisza et al. 2011). Work done can be categorized into four phases: First includes study on efficacy of rare earths-based fertilizers, second geochemical behavior of rare earth in ecosystem, third practices employment of rare earth-based fertilizer, and fourth comprises of physiological processes of enhanced yield. Ferns show high enrichment of REEs in the soil by inductively coupled plasma mass spectrometry (ICP-MS) and instrumental neutron activation analysis (INAA) which are strongly recommended techniques for such determination. Physicochemical properties of the soil affect the exchangeable fractions of speciation which ultimately put large impact on REE bioavailability. Study also indicates average content of rare earth elements (REEs) in the soil ranging 85–522.7 mg/kg. LREEs show abundance in the soil (83–95 %) with average value and mean ratio of 89 % and 8, respectively. Cerium is correlated with 42 % of all the REEs and 48 % with LREEs. Wheat seeds found with rare earth elements ranging from 10−4 to 10−8 g/g much lower than in soil but with similar distribution. Generally in roots and leaves, rare earth concentration increases with dressing (Skarpelis 2002; Tsirambides and Filippidis 2012). Lot of studies has been done on geochemistry of rare earths in different coals as potential economic value of rare earths took much attention in this century. Rare earth elements (REEs) have largely been used in modern technology as tracers to identify source and epigenetic modification especially in coal mining. Some coal deposits due to different geological settings and paleoenvironments show enrichment of HREEs as compared to LREEs. Different plies from same coal due to different depositional microenvironments have variable rare earth distribution patterns. Rare earth elements (REEs) are found bound to minerals which are associated in large extent as organics. HREEs have more organic-bound affinity than LREEs and extracted humic substances found enriched with MREEs (PePiper and Piper 2002; Wenfeng et al. 2008; Bonev et al. 2012).
Study conducted on Antaibo surface mine in China for rare earth distribution shows three patterns: shale like, LREEs enriched, and HREE rich which were recognized from peat swamps, extent of aquatic control, and rare earth resources. Results indicate that extract was relatively enriched in HREEs and depleted in middle rare earth elements (MREEs) with negative anomaly. LREEs found in organic matter of coal are mainly controlled by land-derived debris and partially with coal organic constituents, associated with hydrogen-containing functional groups which eventually influenced by seawater (Bonev et al. 2012).
Extraction methods
Since the start of REE industry in the world, China has been the major producer and supplier until nowadays. If we look on the present status of REEs in the world, limited literature is available, but it may be because apart from China, the rest of the world is not focusing on discovering new resources. One reason could be on account for its waste generation, storage, and safe disposal because of its harmful radiations that have long-lasting impacts on the whole ecosystem (Weber and Reisman 2012; Liao et al. 2013; de Boer and Lammertsma 2013).
Mining technique in the processing of REE depends on their oxide diversity. Different methods employed for REE are the following:
Surface mining: considered one of the best methods for REE extraction. For near surface deposits with lower grade, steeply dipping, or massive ore bodies, less than 100 m, open-pit mining is suitable involving digging or blasting ore, removing by truck or conveyer for stockpiling before processing. Shallow and deep parts of ores mined at single mine (combination of surface and underground mining). Bastanasite extracted by these methods. Banyan Obo deposit in China and mountain pass deposits in California were mined from two large open pits (Jackson and Christiansen 1993). X-ray fluorescence for rare earth oxide mining involved blast holes drilled at 3–4 m spacing (Castor and Hedrick 2006). Mount Weld deposits in Western Australia, mined by open pit method. Dry and wet mining methods also encountered. Dry mining is suitable for hard bands of rocks, shallow deposits and discontinuous ore bodies. Dredger typically used for submerged placer deposit extraction.
In situ mining: In Southern China, near surface and unconsolidated nature, low grade ion adsorption deposits involve simple mining and processing, reducing extraction cost (Kanazawa and Kamitani 2006). Deposits mined using open pit method, Jiangxi south rare earth Hi- Tech (SREC) developed technique, involves drilling holes, leach reagent, enable rare earth rich reagent to collect in tank containing ammonium carbonate as precipitating reagent. Ninety percent REE recovered by leaching, followed by oxalic acid precipitate as oxalates, filtration, and finally roasting to rare earth oxide (Roskill 2007).
Underground mining: depend on ore characteristic involving standard mining methods with labor intensive drilling and blast techniques. Room and pillar, one of underground mining method, involve ore blasted, transportation to shaft by underground rail system. Russia mined Loparite using underground and open pit method (Castor and Hedrick 2006). Elliot take mine in Canada extracted REE as a by-product in uranium production by underground room and pillar methods (Bourzac 2010; Eliopoulos et al. 2012).
By-product production: Mineral sand deposit in Brazil worked exclusively on Monazite for thorium content and REE also extracted by sand mining as a byproduct in titanium, zirconium, and tin extraction. Mountain pass in Western Australia operated for REE rarely mined as basic or primary product of mine (Gupta and Krishnamurthy 2005; Lynas Corporation 2013). Main steps involved are shown in Fig. 2.
Main steps in rare earth extraction, mining, and processing (Doris et al. 2011)
Most common chemical extraction method of rare earth oxides (REOs) separation is hydrometallurgy. Different methods employed for REOs production include solid-liquid extraction, liquid-liquid extraction, ion-exchange, super critical extraction, solid phase, electro wining, electro refining, and electro slag refining (Meyer and Bras 2011). Bastnasite extraction: Typical processes that increase REO percentage from 60 to 90 % include washing, leaching, filtering, calcining, or drying. For every trace metal, calcination is performed to convert trace metal such as cerium to +4 valences leaving others in +3 states, followed by acid digestion. High purity rare earth compounds (neodymium-praseodymium carbonate, lanthanum hydrate, cerium concentrate, samarium oxide, gadolinium oxide, terbium oxide, europium oxide) were obtained by running the resulting solution on multistage solvent extraction (Kulaksiz and Bau 2011a). Monazite/xenotine extraction: Rare earth hydroxides beneficent produced by concentrating ore digested with 70 % NaOH. RE chlorides recovered by leaching with HCl, processed using multistage solvent extraction to produce 95–99.995 % pure REOs. Tailing extraction: Mineral processing operations produce tailing waste, acts as potential source of REOs. Apatite from Pea Ridge produces REOs by selective acid extraction and physical separation techniques. US Bureau of Mines, recover 90 % REOs and 70 % lanthanide concentrate by gravimetric processes (USGS 2010; Fornadel et al. 2011). Reduction processes: Three primary methods such as decrease in anhydrous chlorides or fluorides, reduction of REOs, oxides-fluoride mixture, or fused salt electrolysis of RE chlorides, employed for the extraction of rare earth metals from compounds such as oxides or chlorides. Less common methods employed in the reduction include electrolysis, mercury amalgamate oxidation and reduction, gaseous diffusion, vacuum distillation, Si-octyl phenyloxy acetic acid treatment, and high performance centrifugal partition chromatography. Reductants react with oxidants (oxygen, sulfide, carbonates) in the furnace to separate and free the metal (Gupta and Krishnamurthy 2004).
Analytical methods
REE concentration in the environment is analyzed by using different advanced spectroscopic techniques such as atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), isotopic-dilution thermal ionization mass spectrometry (ID-TIMS), and neutron activation analysis (NAA). Preconcentration such as iron hydroxide precipitation, ion-exchange chromatography, or liquid-liquid extraction required for REEs as in unpolluted water system ranged from ppm to ppt (Lawrence and Greig 2006; Makreski et al. 2011; Li et al. 2013). A highly precise and sensitive technique, i.e., neutron activation analysis for qualitative and quantitative detection, was discovered by Hevesy and Levi in 1938. They found that samples analyzed for REEs become radioactive after exposure to a source of neutrons and can be detected at low concentrations. Neutron activation analysis provides detection limits up to 10−15 g/g (Krivan 1985; Li et al. 2013), whereas ICP-MS works in high-temperature plasma.
Speciation and solubility of rare earths
Speciation of REEs usually determines solubility and bioavailability of a substance. It depends on salinity, pH, and presence of anions (Liang et al. 2005; Ashraf et al. 2015). These parameters cause difference in REEs speciation in salt water compared to fresh water which contains 70–96 % of rare earth elements (REEs) as carbonate complexes, while fresh water is mostly impacted by humate complexes. At high pH compared to LREEs, strong complexes with carbonate ions are significantly made by HREEs, as with increase in atomic number, stability constant also increases, whereas at low pH, REE3+ and (REE)SO4 + are more common. When hydrolysis is carried out at high pH (10), products such as REE(OH)2 +, REE(OH)3(aq), and REE(OH)4 − are more common. Generally, complex formation and low solubility product (Ksp) make the solubility of REEs low. Ksp of rare earths phosphate can be as low as 10−25 mol2/I2 (Liu et al. 1997), while Ksp of RE carbonates and hydroxides at 25 °C is lowest as 10−30 mol5/I5 and 10−24 mol4/I4 respectively. Solubility depends on pH and temperature. Each factor reduces the concentration of dissolved rare earth element. In broad term, freely distributed ligands in the aquatic environment are considered carbonates, phosphates, and hydroxides, as these decrease concentration of rare earth elements (Delgado et al. 2012a; Foucault-Collet et al. 2013; Pfau et al. 2013).
Speciation analysis is widely acknowledged in environmental chemistry as importance tool for metals state in any medium. Chemical speciation encompasses functionally and operationally defined speciation. Functionally defined speciation determines speciation for plants in exchangeable form, while extractable forms of elements are determined by operationally defined speciation. Many geochemical fractions among the sequential extraction schemes developed by Tessier et al. (1979) and Kersten and Forstner (1986), respectively followed by five or six steps, were analyzed for metals in soil and sediments (Clough et al. 2012; Ashraf et al. 2015).
Speciation study in soil
Heavy metal-contaminated soil in Sfax, Tunisia, was used for speciation study using four steps BCR sequential extraction (Wali et al. 2014). Speciation could be helpful in developing effective management strategies to control metal pollution in affected area. First step involves extraction of exchangeable and soluble fractions, reducible fraction in the second step, while oxidizable fraction and residues were extracted in third and fourth step, respectively. Study indicates contamination of top soil than other deep layers. Zn was mostly bound with nonresidual fractions of the soil, while Pb was bound to nearly all fractions with 60 %. Other heavy metals (Cr, Cu, Ni, Fe) were also found in considerable amounts in all fractions of sequential extraction (Clough et al. 2012; Dołegowska and Migaszewski 2013).
Qingsong et al. (2013) studied the contaminated soil around the mining area to evaluate heavy metals by using four steps BCR sequential extraction procedure. Procedure 1 consists of five steps, i.e., exchangeable, carbonates, iron- manganese oxide bound, organic bound, and residuals, while the second procedure includes all steps except organic matter. In the third procedure, exchangeable, water and acid soluble, reducible, oxidizable, and residual fractions were studied. In the fourth procedure, soluble and exchangeable, Mn oxides, organic matter, crystalline Fe oxides, and residuals were analyzed. The results predicted that Fe and Zn mainly recovered in the recalcitrant soil fractions, while Cd in the exchangeable fraction. Copper was found highly bound with soil organic matter. Tessier and BCR procedures classify amorphous Fe fraction in Silveira associated with Fe-Mn oxide fractions, while the crystalline Fe oxide fraction as residual fraction in Sposito.
Speciation of rare earth elements (REEs) in lateritic profiles from Southern China was also conducted. Mobilization of rare earth elements (REEs) during weathering processes is also well recognized, but less data was found on REEs speciation in weathering profiles (Naji et al. 2011; Aiju et al. 2012). Data analysis describes fractionation during weathering. Light rare earth elements (LREEs) leached from top zone, extremely enriched in the middle of profile, whereas heavier rare earth elements (HREEs) are found more depleted from bottom to top. Strong cerium anomalies are found in the oxidized soil zone. Exchangeable fractions are the highest carrier of rare earth elements with 40–90 %, while 10–30 % are Fe-Mn-bound oxides and 35 % in oxidized soil zone. Upper zone contains 10–25 % organic-bound rare earths and 30 % in humus layer. Positive cerium anomaly in this zone was found with greater than 50 % exchangeable in clayey zone, while up to 70 % bound to Fe-Mn oxides and in same percent ratio of Ce/Ce+ found in organic fractions. Study concludes that in soil clay minerals, Fe-Mn oxides and organic materials act as main trap for rare earth elements and carrier of cerium anomalies.
Duan et al. (2002) determined REE in soil and sediments with NaOH-Na2O2 as flux, preconcentration technique for titanium and iron hydroxides [Ti(OH)4-Fe(OH)3] coprecipitation, and ICP-MS as concise for multielement solutions batch analysis. Accuracy of the results was recognized by Chinese soil (six samples) and sediments certified reference materials (GSS and GSD) with less than 10 % relative standard errors.
Results showed that in geological samples, tightly coexisting Ti with Nb, Zr, Ta, and Hf causes complete coprecipitation and recoveries of these metals become less with a decreased trend in basicity.
Liu et al. (2013) determined rare earth elements (REEs) in Chinese soil and clay reference material especially GBW03102 and GBW03102a by using mixture of HF and HNO3 acids in high temperatures and high pressures closed vessel digestion technique and detection of thirty seven (37) elements by ICP-MS. Results show precision lower than 5 % RSD, significantly different with reference values, reflecting the existence of >70-μm coarse grained fractions in the sample and formation of fluorides.
Zhang and Shan (2001) conquered rare earth elements (REEs) speciation in soil and their accumulation in wheat by using RE-based fertilizers. Winter wheat variety, Triticum aestivm L., was investigated in the current study. It was found that accumulation behavior of La, Pr, Ce, and Nd varies depending on the concentration of fertilizer. Three stages sequential extraction procedure adopted for the fractionation of fertilized and unfertilized soil into three distinct fractions: water soluble, exchangeable and carbonate bound (B1), Fe-Mn-bound oxide (B2), and organic sulfide bound (B3). Significant negative correlation was found between rare earth contents in roots and soil pH (r = −0.5787 to −0.8442 for La). Roots and shoots of wheat show considerable amounts of rare earth elements. A significant correlation was obtained between REEs in B1 fraction and in the roots. In fertilized soil, rare earth elements were present in B2 and B3, while small amount in B1. Results show correlation coefficient ranging from 0.6519 to 0.7410 when application of fertilizer was lower than 20 mg/kg of the soil.
Tao et al. (2005) environmentally studied the biogeochemical activities of REEs in wheat and soil under different soil plant system in China due to increased utilization of REEs for industrial and agricultural purposes by INAA and ICP-MS. Results indicate mean REE soil value 176.8 mg/kg with ΣLREE/ΣHREE mean ratio of 8.0. REE in wheat seed ranged between 10−11 and 10−8 g/g in rye grass roots significantly related to soil. REE contents in the spring wheat at maturing stage show ordered in roots > leaf > stem > crust.
Germund (2003) reviewed REEs in primary and secondary soil minerals, surface soil, their solubility, and transport and in plant systems with distribution and localization in different organs, growth rate, crop production, plant physiology and biochemical study, and soil-plant relationship and interactions. Study also focuses on some of the factors influencing adsorption and vertical distribution in soil profiles along with REE concentration. Due to weathering and leaching processes, REE concentrations in surface soil of humid climate were found lower than in parental material. Transfer ratio from soil to plant was observed low. Roots generally show high affinity than shoots, and their uptake capacity was highly associated with soil acidity. Low concentration of REE favors plant growth and productivity. Due to increased Analytical techniques in environmental sciences, use of REEs as fertilizers in East Asian agriculture soil and in both pedagogical and physiological processes contributes great interest for trace metals.
Zhang et al. (2001) made possible the use of RE oxides for soil erosion and aggregation studies as tracers. RE oxides were applied by examining the binding capabilities with soil and quick acid extraction procedures, as direct mixing of REE oxides not substantially alters the physicochemical properties of soil particles and aggregates. In the study, five REE oxides in powder form mixed with Miami Silt Loam soil leached with water to find the mobility of REES by simple acid leaching method. Samples analyzed on ICP-MS showed maximum coefficient variation of <10 % for all REEs. Results also indicate that direct application of REE oxides would be better comparative to other REEs.
Xinde et al. (2001) studied the REEs released from soil for their effect of redox potential and pH. Three pH values of 3.5, 5.5, and 7.5 along with redox potential of 400, 0, and −100 Mv were applied by allowing oxygen and nitrogen to flow into the soil suspension. Results showed rapid release of La, Ce, Gd, and Y, which correlated positively with Fe-Mn indicating Fe-Mn oxyhydroxides under low pH conditions and reduction, but Ce was remarkably influenced by redox potential. Low pH and redox potentials were found more favorable. In exchangeable fractions, contents of La, Ce, Gd and Y, and Fe-Mn fractions in solid phase release decrease as pH and redox potential decrease from soil solution.
Speciation study in water
When dealing with solutions like seawater, compounds in specific oxidation states in speciation analysis can be recognized. However, it becomes very difficult to characterize actual chemical form of elements. Rare earth metals detected and evaluated in chemical speciation are important from environmental point of view. Chemical speciation has been an important method for metals chemical form detection and has gained much attention in recent years. ICP-MS and GC-ICP-MS HPLC are known as best in all techniques. Two different types of techniques such as nonnuclear and nuclear analytical techniques are used in speciation. In comparison with non-NATs, nuclear activation analysis techniques have unique features such as good accuracy, high sensitivity, precision, nondestructiveness, and reduced matrix effect. Sometimes, in chemical speciation, nonnuclear analytical techniques (non-NATs) and nuclear analytical techniques such as molecular activation analysis (MAA), proton-induced X-ray emission technique (PIXE), Mossbauer, and spectrometry or synchronous radiation-based analytical techniques are applied but due to user’s difficult and expensive nuclear facilities specifically limited to certain laboratories of the world.
Table 1 describes the essential features of these techniques along with ICP-MS for speciation study.
In recent years, rare earth elements (REEs) are extensively used in industries such as fertilizer and feed industry, agricultural, forestry, and animal husbandry. Due to large-scale use, rare earth elements (REEs) are getting into the environment through many pathways, but their chemical speciation forms and biological effects have not been yet evaluated. Molecular activation analysis (MAA) technique is based on instrumental neutron activation analysis (INNA) that has been applied to study rare earth absorption, subcellular distribution, and rare earth element-binding macromolecules, e.g., DNA, proteins, polysaccharides in human beings, plants, and liver of rats.
Recent study was done on surface water speciation in ex mining area, in Malaysia by Ashraf et al. (2015). Metal detection for their speciation patterns was done by adsorptive stripping voltammeter (ASV), but metal particularly oxidation state was determined by using new leaching sequential extraction procedure involving five steps. Study indicates heavy metals such as Cu, Pb, Zn, Cr, As, and Sn bound with reducible fractions, while Pb and Sn were also found in organic fractions of sequential extraction.
Butnariu et al. (2015) studied sorption capacity of rare earth pollutants using organic natural sorbents and proposed proficient and cost-effective environmental purposes. For determining equilibrium sorption data, Langmuir, Freundlich, and Redlich-Peterson isotherms were used. The order for these isotherms obtained in experimental data based on their coefficient of determination values is as follows: Langmuir > Redlich-Peterson > Freundlich. Bone powder was tested for its sorption capacity prepared in laboratory. Some of the characteristics of bone powder measured include humidity, protein content (14 %), complex lipids (4.93 ± 0.01), Ca (13.4 %), and P (7.2 %) after proximate analysis (Butu et al. 2014). Data indicate higher adsorption capacity of bone powder in the removal process of Nd(III), Eu(III), and La(III) from aqueous solutions than Cs(I), Sr(II), and Tl(I).
Johannesson et al. (2004) predicted rare earth concentration and speciation analysis in organic-rich black waters of the Great Dismal Swamps in USA. REEs were found in high concentration in the lake with acidic DOC-rich inflow and outflow water. Middle REEs were in high concentration than LREEs and HREEs. Speciation analysis significantly shows dissolved REEs bound with organic matter, and to determine REE complexation with natural organic ligands, ocresolphthalexon (OCP) ligand was used in adsorptive catholic stripping voltammetry (ACSV). pH and DOC were also correlated to REEs and found DOC strongly related than pH. DOC was found to increase with increase in atomic number.
Mohammad et al. (1990) determined the trace lanthanides and yttrium in the seawater after preconcentration by solvent extraction and back extraction. Sample preparation needs only 30 min using two internal standards, indium and cadmium. Method determines parts per trillion levels of REEs and yttrium (Y). The standard deviation obtained for triplicate separation of 100–1000-mL samples of the same seawater was better than 5 % for all REE. Contamination below 1 % appears, accuracy calculated to be less than 2.5 %. Results compared with isotope dilution with mass spectrometry coupled with Fe co precipitation indicate good accuracy.
Renie and Janssen (2003)worked on geochemistry of REE in groundwater in the Netherlands. Samples taken from seven bore holes. Results show shale normalized patterns generally flat and REE probably of natural origin, behavior understood by speciation and statistical data. Complexation and precipitation reactions including dissolved organic compounds (DOC) and inorganic compounds are encountered for speciation. The REE speciation showed REE3+, REE(SO4)+, REE(CO3)+, and REE(DOC) being the major species. Variation in REE concentration in groundwater affected by pH approved by statistical data (multiple linear regressions) is found successful.
Gwendy et al. (1994) studied the development and application of new, sensitive method ICP-MS to identify REE in surface water. Sensitivity ranges from 0.2 to 1 ng/L. Method follows automated preconcentration using Dionex Met Pac CC-1 resin of iminodiacetate functionality. The 0.8 M HNO3 as eluant provides medium for analysis. Method applied on 135 samples indicates no contamination from sampling bottles, during filteration and acidification. High degree of correlation in element distribution pattern between lake and center lake sediments for Yb and Tb was observed. Chondrite normalized plots show consistent pattern with geological unit.
Hathorne et al. (2012)) worked on preconcentration of REE in Seawater by using 69 samples by using “seaFAST” system (Elemental Scientific Inc.). Preconcentration of REEs was done using a resin with ethylenediaminetriacetic acid and iminodiacetic acid functional groups. Usually, a small sample volume is used, but large data can be obtained for REEs with efficient separation by using ICP-MS.
Colin Neal (2007) studied the variations in REE concentration in the rivers of eastern England and borders with Scotland’s known in relation to dissolved fraction (<0.45 μm) and AAP. Yttrium and lanthanum were found in linear correlation. Lanthanum shows high concentration in the rivers such as Great Ouse, Thames, and Wear linked to pollutant sources. The Swale, one of tributaries of Ouse within catchment and contamination of flood plains, was connected with mineralization that shows high samarium concentration.
Leybourne et al. (1998) described detailed study of two undisturbed sulfide deposits (Zn-Pb) to explain the process of controlling oxidation and dissolution of sulfide minerals, precipitation of secondary minerals, and subsequent dispersion of metals in ground and surface waters of Restigouche and Halfmile Lake deposits, Bathurst Mining Camp, New Brunswick. Halfmile Lake waters show lower metal content. Depth and geometry of the massive sulfides and host lithologies for ground and surface waters show variation in their composition. The Restigouche groundwaters ranged up to 21,000 mg/L TDS (Na–Cl waters) and display heavier oxygen and hydrogen isotopic compositions than local surface waters. Groundwater REE patterns, flat to LREE enriched and similar to host lithologies, unlike the surface waters were LREE-depleted compared to shale.
Leybourn et al. (2003) studied the stream water and sediment geochemistry around volcanogenic massive sulfide (VMS) in the Bathurst Mining Camp, New Brunswick deposits in Canada following partial extraction process for metals and REEs by using ICP-MS. Stream sediment composition indicates the same behavior as surface water suggesting surface water for mineral exploration purpose. Ionic composition and hydrology of groundwater and surface water control magnitude of metals. A single partial extraction allows identification of most labile phases (adsorbed and bound to amorphous Mn and Fe oxyhydroxides) that yields greater anomaly contrast for some metals (e.g., Pb, Zn, Tl). At elevated metal contents, Zn, Pb, and Tl are primarily hydromorphically dispersed.
de Boer et al. (1996) determined the level of REE in Dutch drinking water samples by ICP-MS with respect to isotope selection, matrix effect, precision, spectral interferences, and detection limits. Samples were taken from surface water, groundwater, two rivers Rhine and Meuse, and a lake Ijsselmeer. Results show more REE concentration in raw and drinking water. Phreatic winning of moderate depths having low carbonate contents and high concentration of REE was found and at a pH of 5.44 and 6.13, REE totally dissolved.
Qiang et al. (2007) worked on the preconcentration of soluble REEs in seawater by newly synthesized alkyl phosphonic acid resin (APAS), determined by ICP-MS with detection limit from 1.43 pg/L of holmium to 12.7 pg/L of lanthanum. REEs recovered more than 97.9 % with 5 % precision of relative standard deviation (R.S.D., n = 6). For 200 sample volume of REEs, enrichment factor of 400 achieved by online preconcentration with APAR packed column (4.6 mm i.d. × 500 mm in length) eluted with 0.5 mL 0.1 mol/L HNO3 within 30 s.
Results are shown in Figs. 3 and 4:
Risks of REEs mining in mining areas without any proper safety and disposal. (Doris et al. 2011)
Mohammad et al. (1992) developed a new and reliable method for the separation of REEs from seawater sample by preconcentration with minimum time consumption and labor by using ICP-MS. Adsorption of bis(2-ethylhexyl) hydrogen phosphate (HDEPH) and (2-ethylhyexyl) dihydrogen phosphate on C8, due to complexing agent matrix elements pass through to the drain and REEs removed through acid eluant. Method permits large enrichment factor 200–1000-fold. Technique used eight-channel peristaltic pumps with 1-L sample at flow rate of 20 mL/min within 1 h. Contamination determined less than 1 %. Average precision is calculated to be 2.72 and 1.04 % respectively.
Kara et al. (2005) preconcentrated and analyzed the trace elements in natural waters and solid particles by using mini column packed with 2,6-diacetylpyridine functionalized Amberlite XAD-4 on inductively coupled plasma mass spectrometry (ICP-MS) or flame atomic absorption spectrometry (FAAS). Analysis showed good agreement with certified values. Metals eluted with 0.1 M HNO3 directly to detection system. Resin work best at 5.5 pH. After digestion with NaF, sample contains large concentration of iron. Detection limits obtained for trace elements for FI-ICP-MS systems are described as Ni = 0.30 μg/L, Pb = 0.43 μg/L, U = 0.067 μg/L and Zn = 0.20 μg/L, Cd = 0.33 μg/L, Co = 0.094 μg/L, Cu = 0.34 μg/L, Mn = 0.32 μg/L and for the FI-FAAS Cd = 22 μg/L, Co = 60 μg/L, Cu = 10 μg/L, and Ni = 4.8 μg/L.
Cennet et al. (2012) determined REE (La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu) in the seawater by ICP-MS with off-line preconcentration using micro column packed with 2,6-diacetyl pyridine functionalized amberlite XAD-4. Technique involves passing 2–10 mL of sample solution through column, residual matrix removal by ultrapure water and removal of adsorbed cations on the resin by 0.1 mol/L HNO3 with 10 ng/mL indium as internal standard. Limits of detection (3σ), without any preconcentration ranged from 2 to 10.3 ng/L (for Tm and Lu, respectively). Sorption capacity of resin determined by batch process for 4 h at pH 6 at 26 °C found between 47.3 and 136.7 μg/mol.
Yoshiyuki and Jing (1995) measured yttrium and all the lanthanides in seawater in mixing zone of Tokyo Bay waters and the Kuroshio by ICP-MS. Relative abundance of rare earth elements changes gradually and systematically. REEs inside the Bay removed by scavenging in the order of light REEs > middle REEs > heavy REEs. Positive anomaly at Gd and negative at Tb and Tm is indicated by shale normalized REE patterns, due to complexation with carbonate ions in the water. REEs in Tokyo Bay waters enriched up to 10–34 times and 6.6 times yttrium relative to Kuroshio surface water. Results also indicate yttrium best correlated with Ho, in agreement with previous observation in open ocean profile indicated by Zhang et al. 1994.
Speciation study in plants
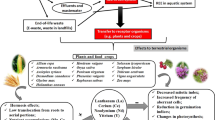
Scheme 1 shows chemical speciation study of rare earth elements (REEs) in plants. REEs were found more susceptible to liver while they go into the blood stream of human or animal body (Li et al. 2006; KulaksIz and Bau 2011b). Distribution patterns of all rare earth elements in subcellular fractions in liver cells of human and rats were found comparable with highest in microsomal fractions and lowest in systosolic fractions but in rare earth ions cultured rat cells, lanthanum, cerium, and gadolinium found mostly in nuclear fractions and cell membrane (Lawrence 2010). Molecular activation analysis technique also shows three lanthanum-binding proteins, three cerium-binding protein, and four samarium-binding proteins in supernatant fraction of human liver sample. It also found that water-soluble rare earth elements (REEs) in the soil easily assimilated in plant root firmly bound with cell wall while in mesophyll protoplast of Brassia napus, rare earth elements accumulated in chloroplast and show enhanced photosynthetic rate of crops because every 200 chlorophyll molecules contain single rare earth atom (Zhang et al. 2001). Dicronapteris dichotama, a highly rare earth enriched fern, show two rare earth element-binding proteins, four rare earth-binding polysaccharides, and one rare earth element-binding DNA in their leaves (Schuler et al. 2011).
Carpenter et al. (2015) recently investigated REE-based phosphate fertilizers that may increase the probability of environmental contamination by these metals. Phytotoxicity and uptake capacity of rare earths from contaminated soil on three native plants species and two crop species in separate dose-response experiments under growth chamber conditions were determined. REE uptake by plants was higher in the belowground parts than in the aboveground plant tissues. Different inhibition concentrations (IC25 and IC50) were measured because of their tendency to reduce plant biomass. For the native species, the majority of aboveground biomass IC25s (11 out of 18) ranged from 100 to 300 mg REE/kg of the dry soil. The crops were found greater than 400 mg REE/kg, with maximum value of 700 mg REE/kg. Results are described in Tables 2 and 3 and Fig. 5 as follows.
D. canadense was found with all REEs having major effect on root biomass ranging from 146.50 (Er) to 430.00 mg REE/kg (Sm). Although the dose at which the effects were prominent varied greatly between the REEs ranging from 40.95 mg/kg for Er to 819.00 mg/kg for Nd, A. syriaca show no effects observed in Nd or Dy, from 146.50 (Er) to 819.00 mg REE/kg (Nd), whereas P. virgatum also show no effects for Sm, Tb, or Dy, and from 152.50 (Tb) to 819.00 mg REE/kg (Nd) for S. lycopersicum, with no effects observed in Dy.
Cai (2012) studied the contents of heavy metals in lotus seeds and soil from REE-mining areas in China by using ICP-MS. The results showed that the contents of the heavy metals Cr, Mn, Cu, Zn, As, Cd, Hg, and Pb in lotus seeds were 0.25, 86.94, 8.32, 19.76, 0.13, 0.08, 0.01, and 0.20 lg/g in lotus seeds, respectively, and corresponding contents in soil were 27.68, 168.71, 20.23, 47.16, 1.83, 0.13, 0.04, and 23.15 lg/g. Data show the contents of heavy metals in lotus seeds from REE-mining area not higher than reference area and meet national food safety standards of China.
Tobacco and peach tree species and pasture grasses were subjected to the application of REEs (20 μmol/L La or Ce) on pollens which demonstrate pollen tube germination and growth. Previous study suggests that REEs affect into three groups: one being the beneficial other inhibitory and last as toxic. In China and other parts of the world, REEs are found to be more important as fertilizers. Nongle (happy farmer), a complex soluble chloride form of RE compounds, was used as the first fertilizer in field experiments. Changle (happiness forever) is registered as commercial fertilizer. REE-based fertilizers contain RE oxides, nitrates, chlorides, and ammonium hydrogen carbonate. In China, about 100 factories producing REE-based fertilizers for more than 30 years with application were recommended at primary growth stage (Industry Bulletin 2012).
Feng et al. (2001) studied the variations of REE patterns in soil-grown plants. Five plant species (Taxodium japonicum, Populus sieboldii, Sasa nipponica, Thea sinensis, and Vicia villosa) and soil selected to observe variations in REE uptake and to elucidate source in soil grown plants were analyzed on ICP-MS. Plant body is categorized into root, stem, and leaf and soil into water soluble, HF soluble, and HCl and HNO3 soluble. REE patterns independent of species and locations, W-shaped variations (W-type tetrad effect), and abundance depletion of Ce were found in all plant species. Secondary root system shows similar patterns to soil silicate fraction not to soil soluble fraction except tetrad effect and Ce anomaly. Results showed REE uptake by plants from any part in soil, and sasa inponica and visia villosa take free REEs and Si directly from silicates in soil. In HREE region, conspicuous tetrad effect was observed than in LREE region.
Ke et al. (2007) studied high precision analysis on annual variations from 1982 to 1999 of rare earth elements, heavy metals, and lead isotopes in mangrove, Rhizophora apiculata tree rings by ICP-MS on digested ash. Study indicates potential use of mangroves’ as monitors of historical environmental changes. Majority of elements show a decline trend in concentration with growth, positively correlated with ring width and negatively with inferred water use efficiency, punctuated by high concentration spike, suggesting a physiological control over metal uptake in the species. Shale normalized REE patterns seem flat and consistent across the growth period. The annual concentration of total REEs varies from 0.024 to 0.173 ppb, with an average of 0.066 ± 0.035 ppb. Some results of the study are shown as in Figs 6, 7, and 8.
Susanne et al. (2000) determined trace elements along with platinum in tree bark by ICP-MS. Tree barks near motor ways and heavy industrial area are utilized as particulate receivers for air borne particles. Large quantities of platinum released from automobiles require highly sensitive methods for the environmental and biological ultratrace analysis. Sample digested with HNO3 and HCl. Platinum in tree bark ranged from 0.07 to 5.4 ng/g. Detection limit in the tree bark observed was 0.03 ng/g on double focusing ICP-MS and 0.2 ng/g on Quadrupole ICP-QMS, respectively. REEs are usually measured from nanograms per gram to as low as low microgram per gram concentration. Higher REE concentration was found for Ce (13 μg/g), Nd (7 μg/g), and La (6.5 μg/g) in the tree barks near coal-fired power stations.
Petre et al. (2011) determined simple and efficient method for the detection of rare earth elements abundance, distribution, and geochemical behavior in minerals by Raman spectroscopy. Method provides lower detection limit for REE compared to modern analytical techniques, appears convenient, rapid, straight forward and inexpensive, could be practiced in the laboratory with FT-Raman instrumentation. Results obtained for rare earth elements are best shown as in Fig. 9a, b.
Speciation study in sediments
In aquatic environments, large amount of rare earths are found sediment bound or in suspended form. Rare earth elements have high affinity to sediments (Hursthouse 2001; Li et al. 2013). Distribution coefficient of rare earth elements in suspended matter and river water is about 31/kg described by Maas and Botterweg (1993). Oceans have value around 4 with exception for Ce which is about 5 due to low solubility main form of Cerium, i.e., CeO2 in which it is present in Ce4+ state, far away from normal trivalent oxidation states of other rare earth elements. Log Kp values (sediment to pore water) for rare earth elements are much higher than other heavy metals. Variables like pH, salinity, organic carbon content, composition of suspended matter, and Fe-Mn-bound hydroxides constantly change the equilibrium among sediment, pore water, and surface water due to tidal movements causing change in rare earth concentration in each partition. Rare earth element concentration varies in the ecosystem compared to other metals. Concentration of La, Ce, and Nd tend to be higher than lead, while Tm and Lu (less abundant REEs) have high concentration than cadmium in earth crust. Generally, odd numbered rare earth elements have lower concentration than even numbered elements. Cerium is found highest in concentration, while Tm and Lu are about two orders of magnitude lower than cerium. Main anthropogenic emission of rare earth elements is to the surface water, while most lanthanides accumulated in sediments. In the Netherlands, study indicates rare earths elements deposition associated with phosphatic fertilizers, and discharge from industries resulted in contamination of sediments in Rhine R estuary with 170 mg/kg Ce, 80 mg/kg Nd, 30 mg/kg Pr, and 20 mg/kg Sm (Sneller et al. 2000). In Australia, lanthanum modified clay phoslock for phosphate removal from wastewater shows 400 μg/L La.
Fagbote and Lanipekun (2010) did speciation study on the sediments from Agbabu Bitumen deposit area, Nigeria, on heavy metals. Sequential extraction procedure consisting of five steps was adopted during dry and wet season. By comparing the results obtained from two seasons (dry season and rainy season), Cr was found in fraction 1 with 30 and 35.82 % in dry and rainy seasons, respectively. Mn was also found high (23.73 %) in this fraction. In dry season and rainy season, Zn was high in fraction 3 in 30.97 and 36.71 %, while Cu was found high in fraction 4 with 45.14 % in dry season and 29.28 % in rainy season. Other metals were found bound to fraction 5. Metals having high abundance in fraction 5 indicate lithogenic origin of metals, low mobility, low degree of pollution, and bioavailability. Results are also indicated in Fig. 10.
Wenfeng et al. (2008) studied the geochemistry of REE in aquatic influenced and organic solvent extracts coal from Antaibo mining district in China. Twenty-six samples were collected, analyzed by solid and organic solvent extracts on inductively coupled plasma atomic emission spectroscopy (ICP-AES). Different distribution patterns observed shale like, LRRE rich, HREE rich, etc. Detrimental minerals mainly control REEs in the coal, but seawater has considerable effect on it.
Kumar et al. (2014) deliberated the concentration of rare earth elements (REEs) in the surface mangrove sediments collected from ten locations throughout the west coast Malaysia by instrumental neutron activation analysis (INAA). Samples were dried, crushed to powder, and weighted 150 mg for short irradiation and 200 mg for long irradiation time. REEs in the sample study were Dy, Sm, Eu, Yb, Ln, Tb, La, and Ce. Level of concentration of all REE varies in the range of 0.35–117.4 mg/kg by irradiation with neutron flux of 4 × 1012/cm2/S1 at MINT TRIGA Mark II research reactor at 750 kW by pneumatic transport facility. Results showed 0.75–6.75 enrichment factor and high LREE abundance than HREE due to heavy mineral deposits in the current study area.
Mathew and Karen (2008) determined rare earth elements and yttrium (REE + Y) in 500 stream water and sediment samples by total digestion and leaching with 0.25 M hydroxylamine hydrochloride in 0.05 M HCl and Fe-Mn oxyhydroxides phases with fractionation, speciation, and controls over REE + Y patterns in surface environment. REE partitioned into dissolved (<0.45 μm), labile (hydroxylamine), and detrimental sediment fractions in particular with respect to Ce and Eu anomalies in oxygenated surface environments. Partial extraction recovered 20 % Fe in total sediments, 80 % of Mn, and 21–29 % of REEs. Two main sources of REE + Y in stream sediments found the hostile lithologies (mechanical dispersion) and hydromorphically transported (labile fractions) by comparison between REEs in water, partial extraction, and total sediment analysis. Results suggest sediment organic matter and δ-MnO2 (FeOOH) likely predominant sinks for Ce and to lesser extent to other REE in stream sediments. Results also indicate REEs normalized to the North America Shale Composite, showing strongly negative Ce anomalies ([Ce/Ce*] NASC ranged from 0.02 to 1.25, average = 0.277, n = 354) and positive Eu anomalies ([Eu/Eu*] NASC ranged from 0.295 to 1.77, average = 0.764, n = 84). Sediment have flatter REE + Y patterns relative to NASC ([La/Sm] NASC ranging from 0.352 to 1.12, average = 0.778, n = 451) and are slightly middle REE-enriched ([Gd/Yb] NASC ranged from 0.55 to 3.75, average = 1.42).
Germain et al. (2009) determined the rare earths elements, Sc, Y, Zr, Ba, Hf, and Th in geological samples by ICP-MS after Tm spike addition and alkaline fusion (NaOH-Na2O2) and coprecipitation on iron hydroxides. Different rock-type samples consist of basalt, ultramafic rock, sediment, soil, granite along with low trace element abundances (sub ng/g). Nine different geological samples were tested. Precision rate was found better than 10 % observed for most samples and 5 % below for the rare earth elements.
Tsuyoshi et al. (2003) determined REEs in the rock sample by an improved high-performance ion chromatography. Technique provides cheaper means of obtaining high-quality results of all the 14 REE in sample as small as <50 mg and showed potential as alternative to ICP-MS. Rare earth elements (REEs) analyzed without any interference from yttrium and other transition metals by using high-resolution HPLC column. Japanese geological survey of 12 reference samples with variable element composition analyzed for REE showed reliable results in agreement with recommended values obtained by ICP-MS. REEs in the rock samples were measured by HPIC with eluant used as α-hydroxyisobutyric acid and a post-column Asrenazo III.
Adam et al. (2003) studied the isotope dilution MC-ICP-MS rare earth elements concentration of 11 international geochemical reference materials NIST SRM 610, SRM 612, SRM 614, BHVO-2G, BHVO-2, BCR-2G, JB-2, WS-E, W-2, AGV-1, and AGV-2. Rock powder and synthetic silicate glass material were analyzed in the study. Data gives abundance of mono-isotopic Pr, allowing precise check on anomalous redox related behavior of Ce. For NIST SRM 610, USGS BHVO-2, AGV-1, and AGV-2, measured REEs abundance ranged typically <2 %. Results for some glass materials and powders recommend concentration or dilution of REE contents during glass manufacture and little fractionation. Materials analyzed in replicate provide data better than 1 % as reproducibility.
Bioavailability of REE
Bioavailability of REE in soil
REE is widely distributed in the biosphere. REEs can be accumulated in different areas of the environment following anthropogenic inputs because of the low mobility of these elements. Life processes (physiological and biochemical) of plants, soil, and living organisms are effected by rare earth elements (Liu et al. 2011; Laveuf et al. 2008; Balabanova1et al. 2013; Rollinson 2014; Balabanova et al. 2015). There is a scarcity of information on REE bioavailability in soil. REE content in soil range from 30 to 700 mg/kg (Bohn et al. 1985) moves through roots and aboveground organs in plants. Cerium, as the most dominant of the REEs, shows a certain variation compared with data from similar investigations; for example, in Australian soil, Ce ranges from 21.0 to 120.3 mg/kg in Japanese soil and from 2.46 to 116 mg/kg, and in Swiss forest soil, it ranges from 10.4 to 100.8 mg/kg. The contents of La in E. citriodora planted in La-treated soil were significantly higher than those grown in soil without addition of La. This implies that the plant could take up La. Rare earth elements (REEs) distribution in automorphic and alluvial soil (top and subsoil samples) in the environs of Bregalnica River was studied by Balabanova et al. 2015. The distribution of the LREEs (La, Ce, Pr, Nd, Pm, Sm, Eu, and Gd) is predominantly related to the quaternary terraces and the Paleogene flysch. The element contents were determined using inductively coupled plasma mass spectrometry. The mean value of the sum total of rare earth elements (ΣREEs) was determined to amount to 79.3 mg/kg. The content of the light rare earth elements (LREEs) in the whole investigated area ranges from 8.6 to 225 mg/kg, while the content of the heavy rare earth elements (HREEs) ranges from 0.92 to 33.7 mg/kg. Some of the results are described in Tables 4 and 5 where the basic statistics for the REEs contents in the topsoil layer are presented (Balabanova et al. 2015).
The distribution of this group of REEs shows significant differences in their contents in automorphic and alluvial soils in areas with different geological bases shown in Table 5.
From the above table, it is indicated that the lanthanum contents vary in the range of 1.6–42 mg/kg, with median values of 19 mg/kg in the Kamenica–Zletovska Rivers Quarter terraces. Lanthanum distribution is specifically correlated with the older volcanic formations of Paleogene flysch (with a median of 15 mg/kg). Cerium contents vary from 3.6 to 65 mg/kg in the topsoil sample, with no significant variations between the contents in the topsoil versus the subsoil layer. The HREEs (Tb-Dy-Ho-Er-Tm-Yb-Lu) are deposited as a geochemical association predominantly in automorphic soil types on the terraces in the Bregalnica Basin (Balabanova et al. 2013; Rollinson 2014; Balabanova1et al. 2015).
Bioavailability of REEs in the soil depends in exchangeable fractions that range from trace to 24 mg/kg and water soluble content from 10 to 20 mg/kg (Zhu and Xi 1992; Zhu et al. 1997). The contents of REE, their distribution, and transformation in soil strongly affected by physicochemical properties of the soil (Liang et al. 2005) including soil cation exchange capacity (Li et al. 2001; Alonso et al. 2004), pH, organic matter, and organic acids. At a higher pH level, the immobilization by soil was found more rapid, and insoluble compounds would be formed when REEs reacted with hydroxides or oxides. However, at low pH, they dissolved and so released the bound REEs (Shan et al. 2002; Ghaderi et al. 2014).
In extracted soil, REE fractions are water soluble fractions, carbonate bound fractions, the exchangeable fractions, the sulfide bound fractions, the Fe-Mn oxide bound and organic matter, and residual fractions (Wang et al. 2001c; Wali et al. 2014). Higher concentration of rare earths in the soil may cause changes in the ecological system of microorganisms. REM concentration in soil solution mostly controlled by adsorption and desorption process acts as indicator of bioavailability of rare earths (Wang et al. 2003a). Adsorption capacity varies with clay type and amount of amorphous iron and manganese oxides (Wen et al. 2001). Fast reactions of REE with soil followed by sorption but slow desorption rate results in low bioavailability with time and reduced uptake by plants.
Bioavailability of REEs in plants
Mobility differs in different parts. The total REE concentration included not only the portion available to plants but also those strongly bound within crystal structure of soil. EDTA enhances the REEs uptake. Degree of translocation of REE in plants is 20 %, mostly present in roots, in intracellular and extracellular parts forming chelates with the components of metabolism (Tai et al. 2010). REEs in plants stimulate uptake of certain elements and act like calcium by inhibiting calcium uptake. REE also effect enzyme activity, production, and intensity of photosynthesis, content of phytohormones, water regime of plants, and water deficiency resistance, and put favorable effects on seed germination (Liu et al. 2013). Some crops like sugar cane, sugar beet, and rice are favorably affected by REE. Rare earth elements in the grass roots depend on soil. Lots of research on biogeochemical behaviors (Zhang and Shan 2001) and effect of REEs on agricultural ecosystems divided into four periods include study on the efficiency of REE-based fertilizers, geochemical behavior of REEs in ecosystem, practices on employing REE-based fertilizers, and physiological process of enhanced yield. REE-based fertilizers applied on tea plant for a safety interval of 25 days between spraying and picking suggest REE bound with polysaccharides which decreases with time (Wang et al. 2003a). Rare earth-based fertilizers show increased concentration of REE in roots, stem, and leaves (Wen et al. 2001). REE content in wheat seed ranged between 10−11 and 10−8g/g (Liang et al. 2005). REE-based fertilizers increased the yield and quality of crops mostly used in China since the 1990’s. Every year, about 50–100 million tons of REE-based salvolatile and phosphate fertilizers enter the agriculture system causing adverse health effects and detrimental environmental issues through bioaccumulation through the food chain (Xiong et al. 2001). A lower pH favors the uptake of REEs by plants. Rhizosphere has a lower pH than the soil far away from plant roots as root secretes acidic substances such as organic acids to facilitate the metal uptake. The result of the present study shows that the final pH was lower than the preset pH which may be explained by the secretion. This would help the dissolution of metals, so more REEs appeared as free ions for plant assimilation. Organic matter in soil plays an important role in providing REEs to plants. Dissolved organic matter had the highest affinity to REEs and was easily assimilated by plants.
A study conducted by Tyler (2004) in a forest in Germany comprises different varieties of plant materials, and concentration of all REEs was also reported. Commercially grown cabbage (Brassica oleracea var. capitata) shows very low concentration with transfer factor 0.04–0.09, and also grass Agrostis capillaries grown in soil from the A-horizon of an acid Cambisol was also found with low concentrations. However, roots of plants such as maize and mung bean indicate 20–150 times high La if grown in culture media (Tyler and Olsson 2001b), while root tips of rice and pea plants significantly show La and Yb in the xylem and endoderm (Tyler 2004). Pteridophytes (ferns) are also well-known as RE accumulators. Strong positive concentration anomalies of La and Ce were reported in at least nine species of the genera Dryopteris, Asplenium, Adiantum, and Dicranopteris in a Japanese study comprising 96 species of ferns (Ozaki et al. 2000). Norwegian mosses containing La and Y indicate their main origin from windblown minerals particles with less contribution from anthropogenic pollution. Leaf mesophyll tissue contained 10–40 μg/g dry weight of La and 3–30 μg/g of Ce in the accumulators. Eichhornia crassipes root hairs adsorb Eu3+ on their root hairs surface. This is also supported by the fact that carboxyl acid groups bind the Eu3+ on root hair surface (Kelley et al. 2000). Pseudomonas aeruginosa having carboxylic and phosphate groups seem active in binding Eu3+ on extracellular sites (Tyler 2004).
Bioavailability of REE in aquatic environment
A key way for contaminants such as pharmaceuticals, personal care products, steroids, and hormones to enter aquatic ecosystems is considered sewage treatment plants (STP). However, it is important to identify technologies that can minimize the amount of these contaminants released. Previous studies in chemical sediments have provided insights into the composition of ancient seawater and the evolution of the environmental system based on the distribution of rare earth elements (REEs). In aquatic environments, total REE concentration provides low interpretation of REE potential interaction with biotic and abiotic components (Alexander et al. 2009; Viehmann et al. 2014).
In organisms, bioaccumulation of REE depends on its bioavailability. The physicochemical characteristics of organisms influence the bioavailability of contaminant. Sediments are considered as major pollutants and water as carrier and transporter. Fate of REE in aquatic environments studied on different kinds of fishes (fish, daphnia, duckweed, shellfish) shows higher level of light REEs than medium and high REEs. Phytoplankton shows high-level bioaccumulation of REE, suggesting that REEs have been released in aquatic environment, phytoplankton proliferation, eutrophication, and origination of algal flowers causing acute or chronic changes in aquatic ecosystems. Most of the available forms to plants are water soluble and exchangeable forms (Zhang and Shan 2001; Cao et al. 2000; Wen et al. 2001; Xu et al. 2001). A precise correlation was observed between bioaccumulation values of rare earth elements (REEs) in plants and the enzyme activity of glutamic oxaloacetic transaminase (GOT) that gives useful information on the bioavailability and accumulation of rare earth elements (REEs) (Wen et al. 2002).
Twelve-year study performed using foliar dressing of corn with 15 mg/m2 of RE indicates no increase in REE concentration in treated areas rather than other parts (Wyttenbach et al. 1998a). REE concentration in plants reported to be very low varies with plant species and growing conditions (Feng et al. 2001). For ordinarily plants, REE concentration range in nanograms per gram. Higher REE concentration was found in rice than corn due to higher accumulation ability, but both plants show high concentration in roots. Plants growing on mineralized grounds were reported to have high REE content. Beet and apple act as biological stores of REEs. Plants like ferns and hickory trees are capable of accumulating REE, called hyper accumulators (Wei et al. 2001; Xu et al. 2002).
By determining gadolinium content (more in top than in roots), safety level of agricultural application of REE was estimated. REEs accumulate in the aquatic environment due to increased industrial and agricultural application. REE enters the groundwater by two means (Keasler and Loveland 1982): already present in soil dissolve in groundwater and reaching onto soil surface and migrate through soil layer to groundwater. An inverse correlation between concentration of RE and river water pH was also reported (Moermond et al. 2001; Astrom and Corin 2003). Alkalinity negatively correlates with lanthanide uptake that indicates reduced bioavailability. REEs concentrate in the surface water up to 1 μg/L. In river water, high concentration of REE was found due to increased solution complexation with ligands (F−, CO3 −2, OH−, HPO4 −2). Concentration of REE by a factor of seven in drinking water shows possible health risk to humans. Aquatic plants also show susceptibility to REEs like general plants. Leaves of the plants exposed to atmospheric contaminants were found able to take REE. It may conclude that high concentration of REE in substrate water impairs aquatic flora. REE in substrate water at 0.4 ppm concentration shows accumulation in the plants 8.8 times higher especially leaves concentration ratio of 13.9 (Wyttenbach and Tobler 2002).
Bioaccumulation of REE in humans
In China, REE-accumulated plants enter the human food chain through different pathways causing danger to public health (Naczynski et al. 2013). Small concentration ratio (CR) shows low transfer of REE from soil to plants. A field study reports mean concentration ratio (×10−4) for edible vegetables of 22.5 for lanthanum, 16.8 for neodymium, and 9 for cerium (Zhang et al. 2000a). Different vegetable food stuffs show REE concentration ranging from 0.5 to 2 ppm. For humans, sample’s acceptable daily intake (ADI) of rare earth nitrates is 0.2–2 mg/kg (Hutchison and Albaaj 2005), a safety standard range of 12–120 mg/person/day (Zhu et al. 2002). Low level of REE in human tissues and fluids supports low transfer level of REE through food chain. Workers in REE mining area show high concentration in hairs, although 0.6 mg/mg3 concentration reported in atmosphere. Values defined in former Soviet Union account only one fifth of the maximum acceptable concentration of 5 mg/m3 (Hutchison and Albaaj 2005). In areas with high REE concentration, humans show high deviation of blood parameters including albumin, β-globulin, serum triglycerides, and decrease in total protein, glutamic pyrovic transaminase, and increase in cholesterol. Study indicates irreversible blood changes in men than in women (Krivans 1985).
In Jiangxi Province of China, rare earths mining activities are highly done and show strong correlation between the hairs of the mothers and their young children. Pair-comparison analysis for the means showed that the average hair level of five kinds of REEs in the young children was two times high as their mothers. Results indicate children living in nearby area found with maximum mean La concentration of 2202.9 ng/g that decreases with increase in distance from the mining sites. The levels of REEs in the hairs can be used as a biomarker to reflect body’s level of exposure to REEs (Pang et al. 2002).
Bioavailability of REE in aquatic microcosm
The concentration of REEs in aquatic biota depends on uptake, elimination kinetic, and biologically active fraction, and these fractions suggest that at organism level, physiological processes can also partly explain differences between species (Delgado et al. 2012b.
Xingye et al. (1999) studied the distribution and bioavailability of REE in static laboratory aquatic microcosm by ICP-AES. Results indicate adsorption of REEs by sediment and desorption in water. Different aquatic species like Duckweed represented phytoplankton. Shellfish represented benthic species, gold fish represented fishes, and daphnia represented crustacean in aquatic environment also studied for REE. Bioconcentration order in the species is as follows: duckweed > daphnia > shellfish > goldfish. In most of previous literature, La and Ce were found in aquatic ecotoxicity, while others describe in relation with fish, algae, and arthropods (Veronica et al. 2014). For Potamogeton pectinatus, BCF values calculated in the same way were also similar across REEs but varied among sampling locations. Furthermore, a decrease in BCFs with increasing atomic number was observed when they were calculated as the ratio between concentrations in biota vs. pore waters (Veronica et al. 2014). La in different fish organs found with decreased bioconcentration in the order: internal organs > gill > skeleton > muscle in Cyprinus carpio, whereas muscles of flying squid (Sthenoteuthis oualaniensis) accumulate high REEs compared to liver which show decrease in bioaccumulation with increase in atomic number (the Oddo-Harkins law).
Mussels and their shells have increasingly gained importance as bioarchives of proxies that record physicochemical changes in their marine or freshwater habitat (Yang et al. 2013; Merschel and Bau 2015). A new and more efficient protocol for sample preparation and determination of REE concentrations in bivalve shells was established by Ponnurangam et al. (2015). The method proves quicker and cleaner and includes sample treatment with NaOCl followed by REE separation and preconcentration. Trace elements such as the rare earths and yttrium (REY) have been shown to be useful indicators of environmental change (Bau et al. 2010; Viehmann et al. 2014). The Mytilus edulis shells from the North Sea have Nd concentrations of 8.0 μg/kg (average for ODAS), 6.1 μg/kg (Jade), and 8.7 μg/kg (Roter Sand), respectively (Zuykov et al. 2013).
Verplanck et al. (2010) studied the behavior of gadolinium as tracer and contrasting agent in magnetic resonance imaging (MRI). Influent and effluent waters display large enrichments in Gd compared to other REEs. The excess concentration of Gd appears to retain in the liquid phase throughout the STP operations.
Role of metal speciation in risk insight and remediation
Remediation of rare earths contaminated site is a composite dilemma due to different oxidation states, organic and inorganic complexes, and their toxicity that depends largely on chemical forms. Rare earth removal from contaminated site is achieved by ensuring that said conditions can be sustained for a specific time (Morgan 2008). In soil, sediments and water samples removal of trace elements need to consider all the aspects of metallic contaminants transfer, speciation, and bioaccumulation (Tangahu et al. 2011).
The importance of metal speciation and bioavailability research to the development of pollution law and control techniques is increasingly evident (USEPA 2008a). These techniques have been appraised to be among the prerequisite approach to determine fast and cheap methods for remediating of REEs from different contaminants. However, remediation techniques and procedures are becoming increasingly more costly. Speciation helps in developing effective and economic remediation strategies and governs its fate, mobility, toxicity, and bioavailability in sediment and water. Rare earth element (REE) detection and concentration in geological samples have no longer been a curiosity but a substantial deal of interest for researchers, industrialists, governmental agencies, and legislative bodies for environmental pollution/ contamination monitoring and management.
When speciation is applied, we get information and interpretation which is not less than a powerful mean for considering any remediation technique. Physiological and toxicological effects and bioavailability of trace metal contaminants depend on actual species present rather than total concentration. Organometallic species of some metals are considered more toxic than organics (Tangahu et al. 2011; Kingsnorth 2012). In a nutshell, chemical speciation and bioavailability have played pivoted role in risk and hazard estimation, their removal techniques, and very time-consuming management of REE-contaminated areas.
Environmental impacts of REEs
According to EPA, rare earth elements processing waste dumped in stream water possess hazardous potential, classified as waste solvent due to ignitability, zinc waste with mercury contamination, spent lead filter cake due to toxicity, and solvent extraction crud Eliseeva and Bunzli (2011). During mining and processing of REE, major environmental risk related with the behavior and removal of tailings, due to wastewater, process chemicals, and high surface area particles. REE affected areas, if not properly managed and controlled when exposed to weathering, contaminate air, soil, and water. The solids, radionuclides, radons, and ore-associated metals act as pollutants in areas affected with REEs. Fugitive dust contaminates air and soil in the surroundings. Precipitate events and dam overtopping transfer the pollutants from catchment to surroundings soil and surface water bodies through surface runoff water. A serious long-term environmental damage occurred when dam fails due to poor construction or from catastrophic events, termed as worst case scenario. Poor operation, design, handling, management of REEs mine, and concerned pollution control system collectively decrease the danger of REEs contamination due to mining activities. Environmental components of sustainability are discussed in REEs operating techniques, both positively in terms of energy and negatively as waste (Yang et al. 2013; Ichihara and Harding 1995); however difficult to trace environmental aspects of REE compared to others, situation becomes unclear and limited. In China, environmental degradation due to leaching has become a significant issue (Cui et al. 2009). Some industrial, hazardous waste, when disposed off causes unrecovered REE. China as high in REE production causes serious environmental damage in areas with mining and processing, if in agreement with limited environmental regulations. Bayan Obo mine after its 40 years of operation radioactively contaminates soil, water, and vegetation. Chinese society of RE stated, every tons of REE produced generate 8.5 kg of fluorine and 13 kg of dust along with 9600–12,000 m3 of waste gases with dust concentrate, SO2, 75 m3 acidic wastewater, HF and 1 t of radioactive waste residue. Saponification process produces harmful wastewater. By estimation, up until 2005, process generates 20–25,000 t of wastewater and 300–5000 mg/L total ammonia nitrogen concentration (Eliseeva and Bunzli 2011).
Radioactive elements like uranium and thorium associated with RE deposits pose key issue with processing and disposing of waste. In the USA, Molycorp mountain pass site observed for wastewater and tailings as primary environmental pollution source. To reduce its effects, dams used to dispose tailings cause increase in TDS and neutralization of HCl in water with NaOH. TDS range 10,000 mg/L with low concentration barium, boron, strontium, and radiological constituents. Metals, nutrients, and radiological constituents in wastewater and tailings show negative impact on groundwater quality. From a social sustainability perspective point of view, ex-mining sites of REE were adequately rehabilitated for other uses like Lynas corporation plant in Malaysia (Government of Japan 2013). REE development puts social contribution toward green economy. After great centralization of supply in China, efforts made on the development of optional resources—predictable resources like USA and Australia—reduced China dominance of production of REEs to 86 % in 2012 and unconventional resources like coal, coal ash, and recycling and deep sea deposits that will be examined by Japan until 2018 (Calvert 2003; Richter and Schermanz. 2006). Risks of REEs mining without any sufficient environmental protection schemes are shown in Fig. 11.
Conclusion
Rare earth elements will continue to be of considerable interest in future. Geological and mineralogical research must continue for exploring REE ore deposits and extraction, important to ensure funding of research from exploration, mining, manufacturing, recycle, reuse, and disposal and together essential to maintain reliable and comprehensive information on REE geology, reserves, resources, production, trade, and consumption. This review briefly overlooks on different areas of rare earths elements (REEs) showing any superior research about sustainability across many aspects, leaving significant gap in knowledge to future examination. Different techniques and methods are available for finding REE in soil, sediment, and water samples. In mining processes, environment concerns must be paid, as all REE deposits contain radioactive elements especially Thorium. Different methods of speciation in different samples have been discussed in detail covering previous research until recent times. Bioavailability of REE is also found in consideration. There is a need to remediate hazards of REE from our ecosystem.
US Geological survey (USGS 2010) estimates a sum of all RE oxides to 99,000,000 t quite high to estimated worlds production of 124,000 t in 2009. The major global reserves of REEs are spread in the USA, China, Australia, India, Canada, South Africa, Malawi, states from former Soviet Union, and other countries. Primarily, all deposits were found to contain more LREE than HREE. Up to date, no data on overall reserves of HREE is available, but large deposits of HREE are found in China (97 % REEs), whereas mall amount is produced in Malaysia, Brazil, India, and Russia. Due to high demand, many projects are aimed at opening of mines. Most advanced by Molycorp Minerals is the Mountain pass mine in California. In USA, complete REE supply chain is currently lacking and must be developed nationally or in conjunction with other allies. China being the highest producer of REE still needs educated labor, technicians, scientists, and engineers. Generally, human capital investment in expertise and technical capacity must be maintained and improved to reduce the gap between China and other countries. Green technologies currently use REE in magnets, energy efficient lightening, industrial catalysts, and batteries, for e-mobility, automotive catalysts, metal alloys, ceramics, polishing, electronics, and glass additives. The global demand estimated in 2014 increases up to 170,000–200,000 t. High application of REE leads to high environmental risks. Main risk concerns with tailings are related to minute particles, wastewater, and chemical flotation which cause increase in concentration of mine ore. Tailing exposed to risks like overtopping due to storm water, seismic events, or poor construction. Recycling considered much complex process, with no large-scale recycling operation. If no any reuse process is adopted, then physical and chemical treatment must be done. Advantage of REE recycling includes utilization of resources, release from external resources, and environmental benefits. To carry on with REEs, still many obstacles are needed to cross. Time span for mining operation takes 6–10 years. Increasing demand for REE production leads to increase in prices.
References
Adam JR, Benjamin J, David WP, Tod EW, Joel AB (2003) Isotope dilution MC-ICP-MS isotope dilution MC-ICP-MS rare earth element analysis of geo-chemical reference materials NIST SRM 610, SRM 612, SRM 614, BHVO-2G, BHVO-2, BCR-2G, JB-2, WS-E, W-2, AGV-1, and AGV-2. Geo standard and Geo Analytical Research 28:417–429
Aiju L, Yanchu G, Honghai W, Gao Peiling G (2012) An assessment of heavy metals contamination in Xiaofu River sediments through chemical speciation study. Int J Earth Sci 5(5):1235–1240
Alexander BW, Bau M, Andersson P (2009) Neodymium isotopes in Archean seawater and implications for the marine Nd cycle in Earth’s early oceans, earth planet. Sc Lett 283:144–155
Alonso ES, Callejon MA, Jimenez JC (2004) Speciation as a screening tool for the determination of heavy metal surface water pollution in the Guadiamar river basin. Chemosphere 56:561–570
Ashraf AM, Maah JM, Yusoff I, Ghararibreza M (2015) Speciation of heavy metals in the surface waters of a former tin mining catchment. Taylor & Francis 24:1–12
Astrom M, Corin N (2003) Distribution of rare earth elements in anionic, cationic and particulate fractions in boreal humus-rich streams affected by acid sulphate soils. Water Res 37(2):273–280
Balabanova B, Stafilov T, Sajn R (2015) Lithological distribution of rare earth elements in automorphic and alluvial soils in the Bregalnica river basin. Maced J Chem Chem Eng 34:201–212
Balabanova B, Stafilov T, Šajn R, Bačeva K (2013) Spatial distribution and characterization of some toxic metals and lithogenic elements in topsoil and subsoil from copper mine environs, int. J Environ Protect 3:1–9
Bau M, Balan S, Schmidt K, Koschinsky A (2010) Rare earth elements in mussel shells of the Mytilidae Family as tracers for hidden and fossil high-temperature hydrothermal systems, earth planet. Sc Lett 299:310–316
Bohn RL, Mc Neal BL, Conner OGA (1985) Soil chemistry, 2nd edition. York, John Wiley & Sons New
Bonev N, Dilek Y, Hanchar JM, Bogdanov K, Klain L (2012) Nd-Sr-Pb isotopic composition and mantle sources of triassic rift units in the Serbo-Macedonian and the western Rhodope massifs (Bulgaria–Greece). Geol Mag 149:146–152
Borovicka J, Kubrova J, Rohovec R, Randa Z, Dunn CE (2011) Uranium, thorium and rare earth elements in macrofungi: what are the genuine concentrations? Biometals 24:837–845
Borrego J, Carro N, Lopez-Gonzalez N, de la Rosa J, Grande JA, Gomez T, de la Torre ML (2012) Effect of acid mine drainage on dissolved rare earth elements geochemistry along a fluvial–estuarine system: the Tinto-Odiel estuary (S.W. Spain). Hydrol Res 43:262–274
Bourzac K (2010) Can the US rare earth industry rebound. Technology Review, Boston: MIT
Bulletin I (2012) Chinese Ministry of Commerce Announces Rare-Earth Export-Quota Allocations for. http://avalonraremetals.com A review J Plant Nutr 27:183–220
Butnariu M, Negrea P, Lupa L, Ciopec M, Negrea A, Pentea M, Sarac I, Samfira I (2015) Remediation of rare earth element pollutants by sorption process using organic natural sorbents. Int. J. Environ. Res. Public Health 12:11278–11287
Butu M, Rodino S, Pentea M, Negrea A, Petrache P, Butnariu M (2014) IR spectroscopy of the flour from bones of European hare. Dig J Nanometer Biostruct 9:1317–1322
Cai D (2012) Contents of heavy metals in lotus seed from REEs mining area. Journal of Saudi Chemical Society 16:175–176
Calvert JB (2003) Rare Earths. World Wide Web. http://www.du.edu/jcalvert/phys/rare
Cao X, Wang X, Zhao G (2000) Assessment of the bioavailability of rare earth elements in soils by chemical fractionation and multiple regression analysis. Chemosphere 40(1):23–28
Carpenter D, Boutin C, Allison JE, Parsons JL, Ellis DM (2015) Uptake and effects of six rare earth elements (REEs) on selected native and crop species growing in contaminated soils. PLoS One 10(6)
Castor, S. B., & Hedrik, J. B. (2006). Em Industrial Minerals and Rocks: Commodities, Markets, and Uses.
Cennet K, Derya K, Andrew F (2012) Determination of rare earth elements in seawater by inductively coupled plasma mass spectrometry with off-line column preconcentration using 2,6-diacetylpyridine functionalized Amberlite XAD-4. Anal Chim Acta 689:184–189
Chakhmouradian AR, Wall F (2012) Rare earth elements: minerals, mines, magnets (and more). Elements 8(5):333–340
Clough R, Lindsay R, Drennar H, Harrington CF, Hill SJ, Tyson JF (2012) Atomic spectrometry update: elemental speciation. J Anal Atomic Spectr 27:1185–1224
Connelly NG (2005) Nomenclature of inorganic chemistry: IUPAC recommendations. The red book Royal Society of Chemistry, Cambridge
Cui YC, Liu JH, Ren XW, Shi XF (2009) Geochemistry of rare earth elements in cobalt-rich crusts from the mid-Pacific M seamount. J Rare Earths 27:169–176
de Boer MA, Lammertsma K (2013) Scarcity of rare earth elements. Chem Sus Chem 6:2045–2055
de Boer JLM, Verweij W, van der Velde-Koerts T, Mennes W (1996) Levels of rare earth elements in Dutch drinking water and its sources. Determination by inductively coupled plasma mass spectrometry and toxicological implications. A pilot study. Water Res 30(1):190–198
Delgado J, Perez-Lopez R, Galvan L, Nieto JM, Boski T (2012a) Enrichment of rare earth element as environmental tracers of contamination by acid mine drainage in salt marshes: a new perspective. Mar Pollut Bull 64:1799–1808
Delgado J, Perez-Lopez R, Galvan L, Nieto JM, Boski T (2012b) Enrichment of rare earth element as environmental tracers of contamination by acid mine drainage in salt marshes: a new perspective. Mar Pollut Bull 64:1799–1808
Dołegowska S, Migaszewski ZM (2013) Anomalous concentrations of rare earth elements in the moss–soil system from south-Central Poland. Environ Pollut 178:33–40
Doris S, Mattias B, Ran L, Georgr SD, Cornelia M (2011) Study on Rare Earths and their Recycling. Oko- Institut eV institute for applied Ecology
Duan T, Chen H, Zeng X (2002) Determination of rare and rare earth elements in soils and sediments by ICP-MS using Ti(OH)4–Fe(OH)3 co-precipitation preconcentration. J Anal At Spectrom 17:410–413
Ebrahim R, Khadijeh S, Saion B, Wood EAK, Reza AM (2009) Rare earth elements distribution in marine sediments of Malaysia coasts. J Rare Earths 27(6):1066
Eliopoulos DG, Economou-Eliopoulos M, Apostolikas A, Golightly JP (2012) Geochemical features of nickel-laterite deposits from the Balkan peninsula and Gordes Turkey: the genetic and environmental significance of arsenic. Ore Geol Rev 48:413–427
Eliseeva SV, Bunzli JCG (2011) Rare earths: jewels for functional materials of the future. New J Chem 35:1165–1176
Fagbote EO, Lanipekun EO (2010) Speciation of heavy metals in sediment of Agbabu bitumen deposit area Nigeria. J Appl Sci Environ Manage 14(4):47–51
Feng FF, Tasuku A, Sadayo Y, Masaya I (2001) The variation of REE (rare earth elements) patterns in soil-grown plants: a new proxy for the source of rare earth elements and silicon in plants. Plant Soil 235:53–64
Fornadel AP, Spry PG, Melfos V, Vavelidis M, Voudouris P (2011) Is the Palea Kavala Bi-Te-Pb-Sb ± Au district, northeastern Greece, an intrusion-related system? Ore Geol Rev 39:119–133
Foucault-Collet A, Gogick KA, White KA, Villette S, Pallier A, Collet G, Kieda C, Li T, Geib SJ, Rosi NL, et al. (2013) Lanthanide near infrared imaging in living cells with Yb3+ nano metal organic frameworks. Proc Natl Acad Sci 110:17199–17204
Geoscience Australia (2012) Australia’s Identified Mineral Resources. Commonwealth of Australia Canberra
Germain B, Jean AB, Joel E, Mathieu B, Claire B, Sidonie R (2009) Determination of rare earth elements, Sc, Y, Zr, Ba, Hf and Th in geological samples by ICP-MS after Tm addition and alkaline fusion. Geostandards and Geoanalytical Research 33(1):51–62
Germund T (2003) Rare earth elements in soil and plant systems – a review. Plant Soil 267:191–206
Ghaderi AA, Abduli MA, Karbassi AR, Nasrabadi T, Khajeh M (2014) Evaluating the effects of fertilizers on bioavailable metallic pollution of soils, case study of Sistan farms Iran. International Journal of Environmental Research 6(2):565–570
Government of Japan (2013) Basic plan on ocean policy. Headquarters for Ocean Policy Government of Japan, Tokyo
Gupta CK, Krishnamurthy N (2004) Extractive Metallurgy of Rare Earths. CRC Press Tylor and Francis
Gupta CK, Krishnamurthy N (2005) Extractive Metallurgy of Rare Earths. CRC Press, Tylor and Francis p508
Haque N, Hughes A, Lim S, Vernon C (2014) Rare earth elements: overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 3:614–635
Hathorne EC, Haley B, Stichel T, Grasse P, Zieringer M, Frank M (2012) Online preconcentration ICPMS analysis of rare earth elements in seawater. Geochemistry, Geophysics, Geosystems 13(1). doi:10.1029/2011GC003907
Haxel GB, Hedrick JB, Orris GJ (2014) Rare earth elements critical resources for high technology. USGS Fact Sheet:087–002
Heber A (2013) Solving a sticky situation: mineral processing is turning green to extract rare earths at cheaper rates. Australian Mining 60
Hurst C (2010) China’s Rare Earth Elements Industry: What Can the West Learn?. Institute for the Analysis of Global Security Washington DC.
Hursthouse AS (2001) The relevance of speciation in the remediation of soil and sediments contaminated by metallic elements an overview and examples from Central Scotland, UK. J Environ Monit 3:49–60
Hutchison AJ, Albaaj F (2005) Lanthanum carbonate for the treatment of hyperphosphataemia in renal failure and dialysis patients. Expert Opin Pharmacother 6(2):319–328
Ichihara M, Harding A (1995) Human rights, the environment and radioactive waste: a study of the Asian rare earth case in Malaysia. Rev Eur Community Int Environ Law 4:1–14
Jackson WD, Christiansen G (1993) International strategic minerals inventory summary report; rare-earth oxides (No. 930-N). US Dept. of the Interior, Geological Survey
Johannesson KH, Tang J, Daniels JM, Bounds WJ, Burdige DJ (2004) Rare earth element concentrations and speciation in organic-rich blackwaters of the great dismal swamp, Virginia, USA. Chem Geol 209:271–294
Kanazawa Y, Kamitani M (2006) Rare earth mineral and resources in the world. J Alloys Compd:408–412
Kara D, Fisher A, Hill SJ (2005) Preconcentration and determination of trace elements with 2, 6-diacetylpyridine functionalized Amberlite XAD-4 by flow injection and atomic spectroscopy. Analyst 130(11):1518–1523
Ke FY, Balz SK, Michael GL, Alan G, Jian-Xin Z (2007) High-precision analysis on annual variations of heavy metals, lead isotopes and rare earth elements in mangrove tree rings by inductively coupled plasma mass spectrometry. Nuclear Instruments and Methods in Physics Research B-beam Interactions With Materials And Atoms 255:399–408
Keasler KM, Loveland WD (1982) Rare earth elemental concentrations in some Pacific northwest rivers. Earth Planet Sci Lett 61:68–72
Keith R, Long BS, Van G, Nora KF, Daniel C (2010) The Principal Rare Earth Elements Deposits of the United States. A Summary of Domestic Deposits and a Global Perspective U.S. Geological Survey Reston Virginia, Scientific Investigations Report 2010-5220, p 1–96
Kelley C, Curtis AJ, Uno JK, Berman CL (2000) Spectroscopic studies of the interaction of Eu (III) with roots of water hyacinth. Water Air Soil Poll 119:171–176
Kersten M, Forstner U (1986) Water Sci Technol 18:121–130
Kingsnorth D (2012) The rare earths industry: a delicate balancing act technology metals summit, Centre for Research in energy and minerals economics (Curtin University. Industrial Minerals Company of Australia Pty Ltd, Western Australia)
Krivan V (1985) Analytiker Taschenbuch, Frenius W, Gunzler Huber W, Luderwald I, Tolg G, Wisser H. Eds Springer Verlag Berlin, pp. 35
Kulaksiz S, Bau M (2011a) Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Applied Geochemistry 26(11) :1877–1885
KulaksIz S, Bau M (2011b) Rare earth elements in the Rhine River, Germany: first case of anthropogenic lanthanum as a dissolved microcontaminant in the hydrosphere. Environ Int 37(5):973–979
Kumar K, Saion E, Halimah MK, Yap CK, Hamzah S (2014) Rare earth element (REE) in surface mangrove sediment by instrumental neutron activation analysis. J Radioanal Nucl Chem 301:667–676
Kynicky J, Smith MP, Xu C (2012) Diversity of rare earth deposits: the key example of China. Elements 8(5):361–367
Laveuf C, Cornu S, Juillot F (2008) Rare earth elements as tracers of pedogenetic processes. Compt Rendus Geosci 340:523–532
Lawrence MG (2010) Detection of anthropogenic gadolinium in the Brisbane River plume in Moreton Bay, Queensland. Australia Mar Pollut Bull 60(7):1113–1116
Lawrence MG, Greig A (2006) Direct quantification of rare earth element concentrations in natural waters by ICP-MS. Appl Geochem 21:839–848
Leybourne MI, Goodfellow WD, Boyle DR (1998) Hydrogeochemical, isotopic, and rare earth element evidence for contrasting water–rock interactions at two undisturbed Zn–Pb massive sulphide deposits, Bathurst mining camp, N.B. Canada Journal of Geochemical Exploration 64:237–261
Li X, Chen X, Chen Z, Zhang Y (2013) A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 93:1240–1246
Li D, Huang S, Wang W, Peng A (2001) Study on the kinetics of cerium (III) adsorption-desorption on different soils of China. Chemosphere 44(4):663–669
Li G, Jiang J, et al. (2006) Effects of rare earth elements on soil fauna community structure and their ecotoxicity to Holotrichia Parallela. Ying Yong Sheng Tai Xue Bao 17(1):159–162
Liang T, Zhang S, Wang L, Kung HT, Wang Y, Hu A, Ding S (2005) Environmental biogeochemical behaviors of rare earth elements in soil-plant systems. Environ Geochem Health 27(4):301–311
Liao CS, Wu S, Cheng FX, Wang SL, Liu Y, Zhang B, Yan CH (2013) Clean separation technologies of rare earth resources in China. J Rare Earths 31:331–336
Liu Y, Diwu C, Zhao Y, Liu X, Yuan H, Wang J (2013) Determination of trace and rare-earth elements in Chinese soil and clay reference materials by ICP-MS. Chin J Geochem 33:095––102
Liu Y, Peng Z, et al. (2011) Interannual variation of rare earth element abundances in corals from northern coast of the South China Sea and its relation with sea-level change and human activities. Mar Environ Res 71(1):62–69
Liu S, Wang L, Zhang S (1997) Effect of long-term foliage-dressing rare earth elements on their distribution, accumulation and transportation in soil-spring wheat system. Chin J Appl Ecol 8(1):55–58
Lynas Corporation (2013) Investor Presentation 1 August 2013. Available online:http://www.lynascorp.com/Pages/home.aspx (accessed on 16 Sept 2014). Lynas Malaysia Sdn Bhd. Jalan Gebeng 3, 26080 Kuantan, Pahang, Malaysia
Maas JL, Botterweg J (1993). Milieubezwaarlijkheid van zeldzame aardmetalen (lanthaniden, yttrium, scandium) in oppervlaktewater. Report No. RIZAnota 93.018
Makreski P, Jovanovski G, Runcevski T, Jacimovic R (2011) Simple and efficient method for detection of traces of rare earth elements in minerals by Raman spectroscopy instrumentation. Maced J Chem Chem Eng 30(2):241–250
Melfos V, Voudouris P (2012) Geological, mineralogical and geochemical aspects for critical and rare metals in Greece. Minerals 2:300–317
Merschel G, Bau M (2015) Rare earth elements in the aragonitic shell of freshwater mussel Corbicula fluminea and the bioavailability of anthropogenic lanthanum, samarium and gadolinium in river water. Sci. Total Environ 533:91–101
Meyer L, Bras B (2011) Rare earth metal recycling. 2011 I.E. International Symposium on Sustainable Systems and Technology (ISSST), pp 1–6
Moermond CT, Tijink J, van Wezel AP, Koelmans AA (2001) Distribution, speciation and bioavailability of lanthanides in the Rhine-Meuse estaury, the Netherlands. Environ Toxicol Chem 20(9):1916–1926
Mohammad B, Shabani TA, Akimasa M (1992) Preconcentration of trace rare-earth elements in seawater by complexation with bis(2-ethylhexyl) hydrogen phosphate and 2-ethylhexyl dihydrogen phosphate adsorbed on a C,8 cartridge and determination by inductively coupled plasma mass spectrometry anal. Chem 1002(64):737–743
Mohammad B, Shabani TA, Hiroshi S Akimasa M (1990) rDetermination of Trace Lanthanides and Yttrium in Seawater by Inductively Coupled Plasma Mass Spectrometry after Preconcentration with Solvent Extraction and Back-Extraction. Analytical Chemistry 62(24):3964–3968
Morgan P (2008) Contaminated land remediation technology. Land remediation yearbook Retrieved from www. eic-yearbook.co.uk p 39–42
Naczynski DJ, Tan MC, Zevon M, Wall B, Kohl J, Kulesa A, Chen S, Roth CM, Riman RE, Moghe PV (2013) Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat Commun 4:2190–2199
Neal C (2007) Rare earth element concentrations in dissolved and acid available particulate forms for eastern UK rivers. Hydrol Earth Syst Sa 11(1):313–327
Ozaki T, Enomoto S, Minai Y, Ambe S, Makide Y (2000) A survey of trace elements in pteridophytes. Biol Trace Elem Res 74:259–273
Ozdes D, Diran C, Senturk BH (2011) Adsorptive removal of Cd (II) and Pb (II) ions from aqueous solutions by using Turkish illitic clay. J Environ Manag 92(12):3082–3090
Pang X, Wang DH, Xing XY, Peng A, Zhang FS, Li CJ (2002) Effect of La3+ on the activities of antioxidant enzymes in wheat seedlings under lead stress in solution culture. Chemosphere 47:1033–1039
PePiper G, Piper DJ (2002) The igneous rocks of Greece. Berlin Germany, Gebroder Borntraeger
Pfau C, Pablick C, Gray SK, Johnson JA, Johnson CE, Schweizer S (2013) Mossbauer spectroscopy of europium-doped fluorochlorozirconate glasses and glass ceramics: optimization of storage phosphors in computed radiography. J Phys Condens Matter. doi:10.1088/0953-8984/25/20/205402
Ponnurangam A, Bau M, Brenner M, Koschinsky A (2015) Mussel shells of Mytilus edulis as bioarchives of the rare earth elements and yttrium distribution in seawater and the potential impact of pH and temperature on the partitioning behaviour. Biogeosciences Discuss 12:14911–14939
Qiang F, Limin Y, Qiuquan W (2007) On-line preconcentration with a novel alkyl phosphinic acid extraction resin coupled with inductively coupled plasma mass spectrometry for determination of trace rare earth elements in seawater. Talanta 72:1248–1254
Qingsong HE, Yue R, Ibrahim M, Maha A, Waseem H, Fangui Z (2013) Assessment of trace and heavy metal distribution by four sequential extraction procedures in a contaminated soil. Soil & Water Res 8(2):71–76
Renie PT, Janssen WV (2003) Geochemistry of some rare earth elements in groundwater, Vierlingsbeek the Netherlands. Water Res 37:1320–1350
Richter H, Schermanz K (2006) Seltene Erden, in: Dittmayer R, Keim W, Kreysa G, Oberholz A (Eds.) Chemische Technik - Prozesse und Produkte Band 6b Metalle. Wiley-VCH Verlag Weinheim 5:147–208
Rollinson HR (2014) Using geochemical data: evaluation, presentation, interpretation, Taylor & Francis, London
Roskill information services Ltd. (2007) The economics of rare earths and Ytterium, 13th edition. Roskill, London, p 275
Schuler D, Buchert M, et al (2011) Study on rare earths and their recycling. Oko-Institut e.V., Darmstadt, Germany
Shan X, Lian J, Wen B (2002) Effects of organic acids on adsorption and desorption of rare earth elements. Chemosphere 47:701–710
Skarpelis N (2002) Geodynamics and evolution of the miocene mineralization in the Cycladic-Pelagonian belt Hellenides. Bull Geol Soc Greece 34:2191–2206
Sprecher B, Xiao Y, Walton A, Speight J, Harris R, Kleijn R, Visser G, Kramer GJ (2014) Life cycle inventory of the production of rare earths and the subsequent production of NdFeB rare earth permanent magnets. Environ Sci Technol 48:3951–3958
Sun Y, Liu DL, Yu ZQ, Zhang Q, Bai J, Sun DY (2003) An apoplastic mechanism for short term effects of rare earth elements at lower concentrations. Plant, Cell and Environment 26(6):887–896
Susanne BJ, Bellis D, Staton I, McLeod CW, Dombovari J, Becker S (2000) Determination of trace elements including platinum in tree bark by ICP mass spectrometry. Fresenius J Anal Chem 368:490–495
Tai P, Zhao Q, et al. (2010) Biological toxicity of lanthanide elements on algae. Chemosphere 80(9):1031–1035
Tangahu BV, Abdullah SRS, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (as, Pb and Hg) uptake by plants through phytoremediation. Int J Chem Eng p:1–31
Tao L, Shen Z, Lijun W, Hsiang-Te K, Yuqi W, Aitang H (2005) Shiming D. Environmental biogeochemical behaviors of rare earth elements in soil–plant systems Environmental Geochemistry and Health 27:301–311
Tessier A, Campbell PGC, Bisson M (1979) Anal Chem 51:845–851
Tsirambides A, Filippidis A (2012) Exploration key to growing Greek industry. Ind Miner:44–47
Tsuyoshi I, Kenji S, Kazuya N (2003) Determination of rare-earth elements in rock samples by an improved high-performance ion chromatography. Geochem J 37:671–680
Tyler G (2004) Rare earth elements in soil and plant systems - a review. Plant Soil 267(1–2):191–206
Tyler G, Olsson T (2001b) Plant uptake of major and minor elements as influenced by soil acidity and liming. Plant Soil 230:307–321
US Geological Survey (2010) Mineral commodities summary 2010. US Department of the Interior January 26. Retrieved from http://minerals.usgs.gov/minerals/pubs/mcs/2010/mcs2010.pdf. Mineral Commodities Survey Report
USGS (2010) Rare Earth Elements in U.S. Not So Rare: Significant Deposits Found in 14 States http://www.usgs.gov/newsroom/article. asp?ID = 2642& from = rss_home). U.S. Department of the Interior. Full Report: The Principal Rare Earth Elements Deposits of the United States—A Summary of Domestic Deposits and a Global Perspective (http://pubs.usgs.gov/sir/2010/5220/). Rare earths statistics and information
USGS (2013) USGS Minerals Information. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/rare_earths (accessed on 11 Sept 2013)
Veronica G, Davide ALV, Corinne L, Laure G (2014) Environmental fate and ecotoxicity of lanthanides: are they a uniform group beyond chemistry? Environ Int 71:148–157
Verplanck PL, Furlong ET, Gray JL, Phillips P, Wolf R, Esposito K (2010) Evaluating the behavior of gadolinium and other rare earth elements through large metropolitan sewage treatment plants. Environ Sci Technol 44:3876–3882
Viehmann S, Homann JE, Munker C, Bau M (2014) Decoupled Hf-Nd isotopes in Neoarchean seawater reveal weathering of emerged continents. Geology 42:115–118
Wali A, Colinet G, Ksibi M (2014) Speciation of heavy metals by modified BCR sequential extraction in soils contaminated by Phosphogypsum in Sfax, Tunisia. Environmental Research, Engineering and Management 4(70):14–26
Wang Z, Shan X, Zhang S (2001c) Comparison of speciation and bioavailability of rare earth elements between wet rhizosphere soil and air-dried bulk soil. Anal Chim Acta 441:147–156
Wang D, Wang C, Ye S, Qi H, Zhao G (2003a) Effects of spraying rare earths on contents of rare earth elements and effective components in tea. J Agric Food Chem 51(23):6731–6135
Weber RJ, Reisman DJ (2012) Rare earth elements: A review of production, processing, recycling, and associated environmental issues. US EPA Region.
Wei Z, Yin M, Zhang X, Hong F, Li B, Tao Y, Zhao G, Yan C (2001) Rare earth elements in naturally grown fern Dicranopteris linearis in relation to their variation in soils in South-Jiangxi region (southern China). Environ Pollut 114(3):345–355
Wen B, Shan X, Lin J, Tang G, Bai N, Yuan D (2002) Desorption kinetics of yttrium, lanthanum and cerium from soils. Soil Sci Soc Am J 66(4):1198–1206
Wen B, Yuan D, Shan X, Li F, Zhang S (2001) The influence of rare earth element fertilizer application on the distribution and bioaccumulation of rare earth elements in plants under field conditions. Chem Speciat Bioavailab 13:39–48
Wenfeng W, Yong Q, Shuxun S, Yanming Z, Chaoyong W, Dominik JW (2008) Geochemistry of rare earth elements in a marine influenced coal and its organic solvent extracts from the Antaibao mining district. Shanxi, China International Journal of Coal Geology 76:309–317
Weng ZH, Jowitt SM, Mudd GM, Haque N (2013) Assessing rare earth element mineral deposit types and links to environmental impacts. Appl Earth Sci Trans Inst Min Metall B 122:83–96
Williams-Jones AE, Migdisov AA, Samson IM (2012) Hydrothermal mobilisation of the rare earth elements: a tale of ceria and Yttria. Elements 8(5):355–360
Wood AKH, Ahmed Z, Shazili NA, Yaakob R, Carpenter R (2004) Metal digenesis and transport in coastal sediment around Penang Island, Malaysia. Journal of Nuclear and Related Technologies 1(1):1–30
Wyttenbach et al. (1998) Rare earth elements in soil and in soil grown plants. Plant Soil 199:267–273
Wyttenbach A, Tobler L (2002) Soil contamination in plant samples and in botanical reference materials. J Radioanal Nucl Chem 254(1):165–174
Xinde C, Ying C, Xiaorong W (2001) Xihia D. Effects of Redox potential and pH values on the release of rare earth elements from soil Chemosphere 44:655–661
Xingye Y, Daqiang Y, Hao S, Xiaorong W, Lemei D, Yijun C, Mi C (1999) Distribution and bioavailability of rare earth elements in aquatic microcosm. Chemosphere 39(14):2443–2450
Xiong BK, Zhang SR, Guo BS, Zheng W (2001) Reviews of the use of REEs-based fertilizer in the past of three decades. Symposium of the use of REEs in agricultural of China. Baotou, P.R. China
Xu Z, Li D, Yang J, Peng A (2001) Effect of phosphate on the exchangeable form and the bioavailability of exogenous neodymium in soil. Huan Jing Ke Xue 22(3):66–69
Xu X, Zhu W, Wang Z, Witkamp G (2002) Distribution of rare earths and heavy metals in field-grown maize after application of rare earth-containing fertilizer. Sci Total Environ 293:97–105
Yang XJ, Lin AJ, Li XL, Wu YD, Zhou WB, Chen ZH (2013) Chinaʼs ion-adsorption rare earth resources, mining consequences and preservation. Environ, Dev
Yoshiyuki N, Jing Z (1995) The rare earth elements and Yttrium in the coastal/offshore mixing zone of Tokyo Bay waters and the Kuroshio. In: Sakai H, Nozaki Y (eds) Biogeochemical processes and Ocean flux in the Western Pacific. Terra Scienetific Publishing Company (TERRAPUB) Tokyo, Japan, p 171–184
Zawisza B, Pytlakowska K, Feist N, Polowniak M, Kita A, Sitko R (2011) Determination of rare earth elements by spectroscopic techniques: a review. J Anal At Spectrom 26:2373–2390
Zdzislaw MM, Agnieszka G (2015) The characteristics, occurrence, and geochemical behavior of rare earth elements in the environment: a review. Crit Rev Environ Sci Technol 45:429–471
Zhang SZ, Shan XQ (2001) Speciation of rare earth elements in soil and accumulation by wheat with rare earth fertilizer application. Environ Pollut 112:395–405
Zhang H, Feng J, Zhu W, Liu C, Xu S, Shao P, Wu D, Yang W, Gu J (2000a) Chronic toxicity of rare earth elements on human beings: implications of blood biochemical indexes in REE high regions, South Jiangxi. Biol Trace Elem Res 73(1):1
Zhang XC, Friedrich JM, Nearing MA, Norton LD (2001) Potential use of rare earth oxides as tracers for soil erosion and aggregation studies. Soil Sci Soc Am J 65
Zhu JG, Xi GX (1992) Study on the chemical form of REEs in soil by sequential extraction. Soil 24(4):215–218
Zhu JG, Chu HY, Xie ZB, Yagi K (2002) Effects of lanthanum on nitrification and ammonification in three Chinese soils. Nutr Cycl Agroecosys 63:309–314
Zhu JG, Zhang YL, Yamasaki S, Tsumura (1997) Water soluble rare earth elements in some soils of China. In proceedings of 4th international conference on biogeochemistry of trace elements, Berkely CA, 23–26 June 1997, p 303–310
Zuykov M, Pelletier E, Harper DAT (2013) Bivalve mollusks in metal pollution studies: from bioaccumulation to biomonitoring. Chemosphere 93:201–208
Acknowledgments
This research is supported by High Impact Research MoE Grant UM.C/625/1/HIR/MoE/SC/04 from the Ministry of Education Malaysia. Thanks also for the support by UMRG (RG257-13AFR) and FRGS (FP038-2013B).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the paper.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Khan, A.M., Bakar, N.K.A., Bakar, A.F.A. et al. Chemical speciation and bioavailability of rare earth elements (REEs) in the ecosystem: a review. Environ Sci Pollut Res 24, 22764–22789 (2017). https://doi.org/10.1007/s11356-016-7427-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7427-1