Abstract
Glyphosate is a worldwide-used herbicide occurring in many monitoring campaigns. Efficient technologies are currently unavailable for glyphosate removal from waters. In this work, a SBA-15 mesoporous silica-based material (Fe-NH2-SBA-15) was synthesized and studied for the adsorption of glyphosate from waters. In order to promote specific interactions between the sorbent and glyphosate via phosphoric group, iron oxide nanoparticles were encapsulated and a surface functionalization with (3-aminopropyl)triethoxysilane was accomplished. The adsorption of glyphosate on Fe-NH2-SBA-15 was investigated as a function of (i) pH, (ii) ionic strength (I), and (iii) adsorbate to adsorbent ratio (C), using a two-level, three-factor experimental design. The experimental design allowed for understanding the effect of the abovementioned variables and for proposing experimental conditions for quantitative removal (pH = 2.1, I = 1⋅10−2 M and C = 0.35) under both batch and dynamic conditions. Interaction mechanism between glyphosate and Fe-NH2-SBA-15 sorbent was elucidated by studying the adsorption behavior of sorbents derived from the intermediate stages of synthesis and by desorption tests. Fe-NH2-SBA-15 sorbent can be quantitatively regenerated by 12.5 mM NaOH, and can be reused at least for five adsorption/desorption cycles. Quantitative removal of glyphosate from inlet and effluent wastewaters from a wastewater treatment plant is shown.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glyphosate (i.e., N-(phosphonomethyl)glycine) is a broad-spectrum post-emergence herbicide used in agriculture and for the conservation of green spaces, such as parks and gardens. Glyphosate inhibits the enzyme 5-enolpyruvyl 3-phospate synthase, essential for the biosynthesis of aromatic amino acids and other aromatic compounds in algae, higher plants, bacteria, fungi, and apicomplexian parasites (Duke and Powles 2008).
In recent years, glyphosate use was associated with genetically modified plants, like corn and soya that have built-in resistance to this herbicide. Since Monsanto’s release of glyphosate in 1974, its production increased up to 718,600 tons in 2012 (Yuan 2014) with growing demand in emerging countries. The repeated glyphosate use without diversified weed management has increased the selection of resistant species, and weed tolerance to chemicals (Powles 2008), resulting in a continuous increase in the quantities of glyphosate used in cultivations (Benbrook 2016).
Glyphosate is moderately persistent in soil, with average half-life of 47 days (Vencill 2002), due to resistance to chemical degradation and to sunlight. Moreover, glyphosate is relatively immobile as a result of its strong adsorption to soil particles, through the phosphonic acid moiety (Sprankle et al. 1975), so minimizing the risk of groundwater contamination. Nevertheless, it can enter surface and subsurface waters by direct use near aquatic environment or by runoff or leaching from land applications (World Health Organization 2005). Glyphosate is highly soluble in water (octanol/water partition coefficient, logP = −3.1 (Chemicalize 2016)), stable in water, and resistant to photodegradation in buffered solution under natural sunlight. The median half-life of glyphosate in water varies from a few to 91 days (Tomlin 2006). Occurrence of glyphosate in surface water has been proved at concentration ranging from tens (Struger et al. 2008; Villeneuve et al. 2011) to hundreds (Coupe et al. 2012) μg/L by several monitoring campaigns. According to monitoring data obtained in 13 European countries in the 1993–2009 period, glyphosate was found in 29 % of the samples analyzed (Horth 2010). As regards Italy, glyphosate has been analyzed only in one northern region, where in 2012 it was observed in 31.8 % of the monitored surface waters, exceeding environmental quality standards (ISPRA 2014), according to the DIRECTIVE 2008/105/EC (European Parliament and the Council of the European Union 2008). It should also be mentioned that few studies suggested an apparent contribution of wastewater treatment plants (WWTPs) to the presence of glyphosate in stream effluents (Kolpin et al. 2006).
Glyphosate toxic and endocrine disrupting properties are nowadays well documented (Gasnier et al. 2009). Additionally, carcinogenicity of glyphosate (2A classification) was recently assessed in March 2015, based on evidence of carcinogenicity in humans and laboratory animals (Guyton et al. 2015). In November 2015, in contrast to the IARC evaluation, the European Food Safety Authority (EFSA) concluded that glyphosate is unlikely to pose a carcinogenic hazard to humans (European Food Safety Authority (EFSA) 2015). Policy makers are currently working on decisions about permissions on glyphosate use (Sarich 2015; The Healthy Home Economist 2014).
The presence of glyphosate in surface waters poses a problem for utilities using this source of water for drinking water production. The removal of glyphosate is greatly dependent upon the method employed during drinking water treatment (Jönsson et al. 2013). Conventional treatment stages like coagulation, sedimentation, and sand filtration were not effective in the removal of glyphosate from surface water (7 % removal), whereas removal increased to 56 % when freshwater is additionally treated with ClO2 in combination with conventional treatments (EPA (Environmental Protection Agency) 2015). Membrane (Carneiro et al. 2015) and adsorption processes are potential approaches for the removal of glyphosate from freshwater that require further investigations. Although granular activated carbon showed excellent adsorption performances on distilled water, reduced removal efficiencies were observed depending on water characteristics (Speth 1993). Advanced oxidation processes, based on photo-induced degradation of glyphosate, were investigated. As an example, a method for the degradation in water employing H2O2 and UV-C irradiation was developed (Manassero et al. 2010), but with conversion yields lower than 70 %, despite the high energetic (and extremely expensive) source of light. Although complete degradation of glyphosate by TiO2 under UV light was achieved by Assalin et al. (Assalin et al. 2009), the authors did not evaluate the performance of the method in real matrixes, which are usually affected by the presence of competitive species. The same authors highlighted the efficacy of ozonation process, which nevertheless is characterized by significant management costs.
Based on these considerations, the development of innovative adsorbents with high adsorption capacities is a challenge for the efficient removal of glyphosate. Mesoporous silica-based materials, like SBA-15, are considered possible sorbents for removal of a wide range of contaminants due to their high surface area, large and uniform pore size, and tuneable pore structure (Wu and Zhao 2011) and can represent cost-effective and environmentally acceptable solutions for water purification processes (Cooper and Burch 1999; Vunain and Meijboom 2014). Additionally, proper tailoring of the surface is possible due to the presence of a rich population of silanol groups (Shenderovich et al. 2003), which allows the grafting of functional groups selective towards target analytes.
Glyphosate is a polyprotic acid with pKa1 = 0.78, pKa2 = 2.29, pKa3 = 5.96, and pKa4 = 10.9 (Chemical Abstracts Service 2015), which forms zwitterion and mono/divalent anions in the pH range 2–8, see Fig. S1 of the Supplementary Data. Therefore, the best expected modification of the sorbent is with functional groups which can provide cationic fixed sites, e.g., aminopropyl groups. These species have been reported to enhance the sorption of anionic pollutants (Zhang et al. 2014) and metals (Chen et al. 2015).
Furthermore, the high adsorption of glyphosate in soils rich in aluminum and iron oxides (Borggaard and Gimsing 2008) is well known. The affinity of phosphate and phosphonic moieties towards iron containing sorbents is also well established. In fact, Krenkova and Foret demonstrated the enrichment of phosphopeptides (Krenkova and Foret 2011) using an iron oxide coated monolithic column. Moreover, impregnation of an anion-exchange resin with iron oxide nanoparticles provided interaction sites for adsorption of glyphosate (Jia et al. 2011).
Based on the abovementioned considerations, in this work, we prepared a SBA15/iron oxide system functionalized with amino groups (labeled as Fe-NH2-SBA-15) with the aim to provide a suitable material for glyphosate adsorption and to investigate the effect of the main experimental conditions on the removal of glyphosate from waters. In pursuance of these objectives, after physicochemical characterization of Fe-NH2-SBA-15, adsorption was studied using a two-level, three-factor experimental design, which allowed to set experimental conditions for quantitative removal of glyphosate.
Recoveries of glyphosate were evaluated by suppressed ion chromatography (IC), through a chromatographic method specifically optimized to ensure separation among glyphosate and inorganic anions commonly present in waters (e.g., Cl−, NO3 −, SO4 2− and PO4 3−).
Desorption of glyphosate from Fe-NH2-SBA-15 was also studied, showing a complete recovery of glyphosate and the possibility to reuse the sorbent for at least five repeated cycles. The optimized protocol for adsorption of glyphosate was tested on drinking water and wastewater, demonstrating the possibility to use the material also with water matrices with high organic load. The performance of Fe-NH2-SBA-15 was also assessed under dynamic conditions through column tests.
Materials and methods
Materials
All reagents were of analytical grade. SBA-15, iron (III) nitrate nonahydrate (98 %), (3-aminopropyl) triethoxysilane (APTES, 99 %), toluene (99.8 %), ethanol and acetone (>99 %), were purchased from Sigma-Aldrich (Chemie, Steinheim, Germany). HCl (35 % w/w, d = 1187 g/ml) and NaOH (>98 %) were from Carlo Erba (Milano, Italy). Glyphosate, NaCl (for ionic strength control) and H2SO4 (95–97 %, d = 1.84 g/ml) were from Sigma-Aldrich. High-purity water (18.2 MΩ cm resistivity at 25 °C), produced by an Elix-Milli Q Academic system (Millipore, Vimodrone, MI, Italy) was used for standard and eluent preparation.
Instrumentation
The following chromatographic equipment (from Dionex, Thermo Scientific, Sunnyvale, CA, USA) was used: a 4000i chromatograph (25-μL injection loop) with a conductivity detector and IonPac AG16 (50 × 4 mm) and IonPac AS16 (250 × 4 mm) columns. The mobile phase was a NaOH solution. Detection was performed by chemical suppressed conductivity (regenerant solution: 50 mM H2SO4) and a 4 mm-AMMS III membrane suppressor. Eluent flow rate was 1.0 mL/min. Chromatographic data were collected by PeakNet 2.8 software.
For calibration curve, a stock 100 mg/L glyphosate solution was used to prepare standard solutions (six levels ranging from 0.1 to 15 mg/L). Each level was injected in triplicate.
Leaching of Fe from the sorbent was evaluated by a iCAP-Q ICP-MS instrument (Thermo Scientific) at m/z 56.
Ion-exchange method for glyphosate determination
The adsorption properties of the prepared materials towards glyphosate were evaluated by a chromatographic method based on anion-exchange mechanism optimized for the purpose. The required features for this method were robustness, accuracy, precision, and sensitivity.
Additionally, in view of testing the sorbent performance with environmental waters, the method should be selective for glyphosate even in the presence of anions commonly found in waters. To this aim, the IonPac AS16 column was chosen due to its high capacity and for its specificity for polyvalent ions. Due to column hydroxide selective functional groups, a NaOH eluent was chosen. Isocratic elutions of glyphosate in the presence of Cl−, NO3 −, SO4 2− and PO4 3− ions were performed in the range 20–35 mM NaOH (see Fig. S2 of the Supplementary Data). According to the results obtained, 20 mM NaOH ensured a baseline resolution among the peaks of phosphate and glyphosate ions, with a total analysis run time of approximatively 25 min. Figures of merit of the method are described in the Supplementary data section.
Fe-NH2-SBA-15 preparation and characterization
Fe-NH2-SBA-15 was prepared starting from SBA-15 by two consecutive stages: (i) formation of iron oxide nanoparticles and (ii) surface functionalization with amino groups. The details of the synthesis are reported as Supplementary data.
As regards stage (i), the iron oxide phase was formed inside the SBA-15 porous structure starting from the impregnation with Fe(NO3)3 solution (Parma et al. 2012). The obtained sample is labeled: Fe-SBA-15.
As regards stage (ii), Fe-SBA-15 was functionalized with APTES. The obtained sample is labeled: Fe-NH2-SBA-15. SBA-15 as such was also functionalized and the sample obtained (named NH2-SBA-15) used as reference.
Scanning transmission electron microscopy (STEM) images were recorded with a Merlin instrument (Zeiss, Germany) equipped with an EDAX detector (Oxford Istruments, Abingdon, Oxfordshire, UK). For EDAX analysis, the sample has been metallized with Cr.
Nitrogen adsorption isotherms were measured using a Quantachrome (FL, USA) AUTOSORB-1 instrument. Prior to nitrogen adsorption, samples were outgassed (393 K, 5 h). BET specific surface areas (SSA) were calculated in the relative pressure range 0.04–0.1, and the pore size distribution was determined through the density functional theory (DFT) method, using the NLDFT equilibrium model.
Zeta potential measurements were performed using a Nano-ZS90 Zetasizers Nano Series Malvern Instruments (Alfatest, Rome, Italy) using disposable polystyrene zeta cells.
Experimental design (DOE) for adsorption process
A 23 factorial design was used to study the effects of the factors and their interactions which can play a role during the adsorption process and can affect glyphosate removal efficiency. The selected independent factors were (i) pH of solution containing the herbicide, (ii) ionic strength of solution, and (iii) the ratio between the mass of glyphosate (mg) and the mass of Fe-NH2-SBA-15 (g) used during adsorption experiments (hereafter called “C” ratio). The (−1) and (+1) levels were the following: pH: 2.1 and 6.0; I = 8 ⋅10−3 M and 1.2 ⋅10−2 M; C: 0.1 and 0.3.
Experimental design was applied to Fe-NH2-SBA-15, to the intermediate material (Fe-SBA-15), and to the unmodified SBA-15 to ascertain single contribution of iron, amine, and mesoporous silica, respectively, to the adsorption of glyphosate (see Adsorption mechanisms and recovery of glyphosate section).
Adsorption experiments
Adsorption experiments within the DOE were repeated three times for each sorbent (Fe-NH2-SBA-15, Fe-SBA-15 and SBA-15). I and pH of herbicide solution were chosen according to planned experiments in the DOE. The pH values were adjusted by HCl and NaOH. In order to obtain the C ratio required by the DOE, the volume solution was changed accordingly.
More details are reported as Supplementary data.
Dynamic adsorption under the optimized adsorption conditions
Based on the experimental conditions optimized through the DOE, adsorption dynamic tests were also set. Poly-Prep® Chromatography Columns in polypropylene, 9 cm high, conical 0.8 × 4 cm, 10 mL (Bio-Rad, Hercules, CA) were filled with 0.1 g of Fe-NH2-SBA-15. The glyphosate standard solution was flown through the material by a peristaltic pump at a flow rate of 0.6 ml/min. The treated solution was then filtered and analyzed by IC to evaluate glyphosate adsorption percentage according to the procedure described in the Ion-exchange method for glyphosate determination section.
Recovery of glyphosate
For the Fe-NH2-SBA-15 regeneration test, based on the pH-dependent retention mechanisms (see Results and discussion section), 12.5 mM NaOH was used. Desorption experiments were performed also on Fe-SBA-15 and on unmodified SBA-15.
More details are reported as Supplementary data.
Real samples
The performance of Fe-NH2-SBA-15 was tested on drinking water (Turin, Italy) and on wastewater inlet and effluent of a membrane biological reactor (MBR) that includes nitrification, denitrification and filtration steps (Prato, Italy). The MBR, placed after a series of classical primary treatments, receives leachate from various landfills, and its effluent is delivered at the beginning of a second complex treatment line consisting basically of activated sludge system that receive also mixed urban-industrial wastewater. The effluent of this latter treatment line is discharged in surface water and must fulfill the requirements of regulation. The samples where characterized for chemical oxygen demand (COD), biochemical oxygen demand (BOD5), total suspended solids (TSS), and N and total P content. Samples were collected in two different dates (25/06–05/07/12) and stored at −15 °C. Wastewater samples were initially analyzed by IC in order to verify the absence of glyphosate at the quantitation limit of the method (100 μg/L). Subsequently, samples were spiked with 2 mg/L glyphosate and the experimental conditions optimized through the DOE (pH 2.1, C = 0.35) were applied. The samples were then filtered (0.45 μm) and analyzed by IC to evaluate the percentage of glyphosate absorbed. For each sample, removal procedure was tested in triplicate. In parallel, a blank (wastewater spiked with glyphosate and stirred overnight without the presence of Fe-NH2-SBA-15) was also run in order to exclude the presence of biodegradation phenomena.
Results and discussion
Material characterization
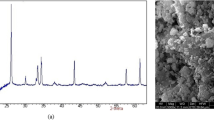
Specific surface area (SSA), obtained by BET model, pore volume, and DFT pore size for SBA-15, Fe-SBA-15, and Fe-NH2-SBA-15 are reported in Table 1. The inclusion of iron oxide and subsequent functionalization with APTES reduced, as expected, the SSA, the pore volume and the mean pore size. Results obtained from STEM analysis on Fe-NH2-SBA-15 (Fig. 1) reveal the presence of clusters (black spots) inside the mesoporous silica, in agreement with the reduction of pore volume. EDAX analysis on the same sample (data not reported) showed the presence of Fe, with an average concentration of 12.7 % w/w, (based on 3 measurements), which well compares with Fe nominal fraction (14.7 % w/w).
The dissociation constant (pKa = 7.56) and the ion exchange capacity (0.342 meq/g) for the Fe-NH2-SBA-15 sorbent were evaluated by potentiometric titration with HCl according to Soldatov (Soldatov 2000). The pK value obtained was about 3 pK units lower than the bulk solution value (pK = 10.6 (Weast 1989)). This value is in agreement with the pK of APTES monolayer produced on silicon substrate, and it is characteristic of surface amino groups (Zhang et al. 1998).
Optimization of adsorption conditions through DOE
DOE is usually chosen in order to estimate the influence of predefined variables on the performance of a system by a predefined number of experiments. In screening studies, the variables with the largest influence on the procedure can be identified, thus allowing for obtaining experimental conditions for the highest response. In some instances, statistical experimental design has been applied to optimization of adsorption processes (Affouri et al. 2015; Bouchemal et al. 2012; Darwish et al. 2015; Geyikçi and Büyükgüngör 2013; Royer et al. 2010). The factorial design is preferable respect to a one variable at a time approach because this latter does not guarantee to reach the real optimum and it is valid only if the variables are totally independent from each other (Leardi 2009).
According to the characteristics of the adsorption process to be optimized, the factors detailed in Experimental design (DOE) for adsorption process section were chosen and a 23 DOE was planned within the following (−1) and (+1) levels: pH: 2.1 and 6.0; I = 8 ⋅10−3 M and 1.2 ⋅10−2 M; C: 0.1 and 0.3.
The parameter values were chosen according to the following considerations. The pH range studied accounts for any chemical modification of both silica and glyphosate forms. Higher pH values, that can be found in some kind of waters like treated wastewater plants effluents (e.g., from 6 to 9) were not considered since no significant variations in the nature of the adsorbate-adsorbent interactions are expected at pH values higher than 6. The I values were representative of average compositions of local drinking water, whereas C values were chosen in order to avoid saturation of the ion-exchange sites of Fe-NH2-SBA-15 calculated by titration. Differently from other applications (Geyikçi and Büyükgüngör 2013; Royer et al. 2010), in this study, the amount of the target compound to be removed and the amount of sorbent were correlated in a single parameter, the “C” ratio, without increasing the number of experiments.
The design matrix, the coded variables, and the response obtained (percentage of adsorbed glyphosate, %GLYads as calculated by eq. 1S) for Fe-NH2-SBA-15 are shown in Table 1S of Supplementary data.
Main and interactive (two-factor and/or three-factor interactions) effects of the parameters investigated were calculated by the Yates’ algorithm (Morgan 1991), whereas the effects were interpreted by means of a normal probability plot (Table 2S of Supplementary data). Data in Table 2S reveals that the main effects of pH, C, and the interaction effect of pH-C are significant for the adsorption of glyphosate onto Fe-NH2-SBA-15. In detail, DOE highlights a negative correlation between pH and glyphosate adsorption (higher pH means lower adsorption percentages). Full discussion on DOE is given in the Supplementary data.
The following conditions pH = 2.1, I = 1 ⋅10−2 M and C = 0.35 were considered optimal and tested under batch conditions in high purity water spiked with 2 mg/L glyphosate. The results obtained indicated a quantitative removal of glyphosate (residual concentration below the LOD), thus confirming the hypothesis derived by the DOE.
Adsorption mechanisms and recovery of glyphosate
The development of materials to be used for purification of water matrices should consider not only the adsorption of target molecules, but also the possibility to desorb the pollutant and reuse the material for consecutive adsorption cycles.
In order to set up proper experimental conditions to recover glyphosate from Fe-NH2-SBA-15, we considered necessary to ascertain the mechanisms involved in the adsorption of glyphosate on Fe-NH2-SBA-15. Therefore, the DOE was repeated on Fe-SBA-15 and SBA-15 as well (Table 2).
SBA-15 showed poor adsorption (14.8 ± 0.9 %) only at acidic pH, whereas the adsorption becomes negligible at pH 6. The decrease of retention at increasing pH suggests that hydrogen-bonding and ionic interactions strongly affect the pollutant-sorbent interaction.
More in detail, the partial retention observed at pH 2.1 is explained by hydrogen-bonding interactions since at this pH value glyphosate is present mainly in its neutral zwitterionic form (see form B in Fig. S1) and silica is supposed to have a surface charge close to zero. Increasing the pH, repulsive ionic interactions do not allow adsorption, since glyphosate is ionized (monovalent or divalent anions, see forms C, E, and D in Fig. S1) and silica surface is expected to become negatively charged (Braga et al. 2011).
The presence of iron oxide inside the mesoporous silica (Fe-SBA-15) provides interaction sites for binding phosphate group of glyphosate through the formation of monodentate and bidentate species (Sheals et al. 2002) and, hence, an enhancement of glyphosate retention is expected. The DOE for Fe-SBA-15 showed quantitative retention at pH 2.1, where the zwitterionic form is present and silica is around its zero charge, thus supporting the initial hypothesis. At pH 6, retention decreases to 76 %. In order to explain this behavior, zeta potential (ξ) measurements were performed on Fe-SBA-15 dispersed in aqueous solutions at the two different pH values. Results obtained pointed out that at pH 2.1 the ξ value measured (4.42 mV) is in agreement with a positively charged surface, which is apparently ascribed to iron oxide clusters. Under these conditions, retention of glyphosate on Fe-SBA-15 is reasonably due to the ionic and complexation interactions between the cationic surface and negatively charged phosphonic group of glyphosate. Differently, at pH 6, the ξ value measured (−13.8 mV) reveals a negatively charged surface, partially due to the dissociated silanols and to the iron oxide phase (ξiron oxide phase, pH 6 = −10 mV) that decreases the affinity between the Fe-SBA-15 surface and glyphosate.
The dependence observed for glyphosate retention on pH is in agreement with what highlighted by Zeng et al. (2008) for the adsorption of phosphate on high-surface area iron-oxide based sorbents and by Cui et al. (Cui et al. 2012a) for the adsorption of glyphosate on MnO2-SBA-15 composite. As proposed by Zeng, at pH 4–7 adsorption of phosphate moieties on iron oxide based sorbents is due to the presence of both monodentate (FeOPO3 2−, logK = 16.9) and binuclear bidentate complexes ((FeO)2PO2 −, logK = 23.4), this last species being dominant for more acidic pH values. Mono and bidentate complexes of glyphosate on goethite surface were characterized also by Sheals et al. (Sheals et al. 2002). The adsorption yields obtained for Fe-SBA-15 at the two pH values studied are consistent with the presence of the two above mentioned different complexes, according to their stability constants.
The presence of amino groups in Fe-NH2-SBA-15 causes a different behavior. Retention is significant (about 97 %) only at acidic pH, and it is dependent upon analyte/adsorbent concentration ratio, whereas at pH 6 retention is around 16 %.
Although zeta potential measurements at pH 6 on Fe-NH2-SBA-15 (ξ = −12.8 mV) would suggest a retention behavior comparable with the one observed for Fe-SBA-15, the minor adsorption observed could be ascribed to a minor (almost halved) SSA and pore volume (177 m2/g and 0.28 cm3/g for Fe-NH2-SBA-15 against 382 m2/g and 0.51 cm3/g for Fe-SBA-15, see Table 1).
In order to evaluate the contribution of −NH2 moieties to the retention, adsorption of glyphosate was measured at pH 2.1 also for SBA-15 functionalized with APTES (NH2-SBA-15), observing an adsorption percentage of 28 % (Table 2), which accounts for pure anion-exchange interactions as well as hydrogen bonding, as previously highlighted for SBA-15.
The same anion-exchange interaction may be hampered at pH 6 due to interaction of cationic sites with dissociated silanols.
Based on the hypothesized adsorption mechanisms, tests for the evaluation of desorption of glyphosate were performed for Fe-NH2-SBA-15. A 12.5 mM NaOH solution was chosen according to the pH dependent adsorption observed. Recovery experiments were performed for Fe-SBA-15 and SBA-15 as well. The results obtained (Fig. 2) pointed out a complete recovery of glyphosate. Desorption was ascribed both to the reduced ion exchange capacity exhibited by Fe-NH2-SBA-15 at very basic pH (due to the deprotonation of amino groups, pKa = 7.6) and due to the competitive effect of the OH−counter ion in the ion exchange equilibrium. The competitive adsorption of OH− for phosphate groups of glyphosate was also observed for surface NH3 + groups in polystyrene resin (Xiao and Wen 2016), under anion-exchange mechanism.
Desorption recovery of 74.5 % was achieved for Fe-SBA-15, indicating a competitive effect of OH− vs glyphosate in the complexation equilibria with iron sites and/or a dissociation of glyphosate in the liquid phase. Complexation of iron by bidentate bridging (Weng et al. 2012), which is less dependent on pH variations (Cui et al. 2012a), may account for the fraction of glyphosate not eluted.
In conclusion, we propose a retention mechanism which is mainly based on complexation for Fe-SBA-15, and on complexation together with anion-exchange interactions for Fe-NH2-SBA-15.
On the basis of the results obtained from desorption experiments, the rationale of developing Fe-NH2-SBA-15 as sorbent of tunable affinity is justified by the complete reactivation of the substrate.
Reuse of Fe-NH2-SBA-15 sorbent
The Fe-NH2-SBA-15 sorbent was tested under repeated cycles of glyphosate removal from water and subsequent regeneration.
Results obtained after five adsorption and release cycles, Fig. 3, show maintained performances with quantitative adsorption and desorption percentages. To the best of our knowledge, this is the first case in which a substrate proposed for glyphosate removal is evaluated for reuse (Cui et al. 2012a; Cui et al. 2012b; Mohsen Nourouzi et al. 2010; Shoval and Yariv 1979; Zhu et al. 2015) or in which a complete regeneration is achieved (Salman et al. 2012).
Adsorption performance of Fe-NH2-SBA-15 after five repeated cycles of adsorption/regeneration. Tests were performed under adsorption conditions, as described in Fig. 2
Applications to the removal of glyphosate from real samples
The performance of the Fe-NH2-SBA-15 was tested for local drinking water (Torino, Italy) spiked with 2 mg/L glyphosate, as well as for a more complex water matrix, i.e., a wastewater inlet and effluent of a MBR treatment, the composition of which is reported in Table 3.
The results obtained showed that, for both drinking and wastewater samples, the removal of glyphosate was quantitative (glyphosate was not observed below the detection limit).
It is worth noting that for the inlet sample, ionic strength is outside the experimental range investigated by the DOE, showing the robustness of the DOE approach, provided that pH conditions are controlled. In fact, despite the high carbon load and inorganic ions content (N-NO3 − presence and total P content that was averaging from 140 and 40 mg/L for MBR inlet and effluent, respectively), Fe-NH2-SBA-15 does not suffer from matrix interferences during adsorption of glyphosate.
In Fig. S4, typical chromatograms obtained for wastewater samples analyzed before and after the adsorption process are shown.
Dynamic adsorption under the optimized experimental conditions
The adsorption of glyphosate by columns packed with Fe-NH2-SBA-15 was tested (see Dynamic adsorption under the optimized adsorption conditions section) in dynamic conditions. The treated solution was injected in the ion chromatographic system for glyphosate quantification. The results obtained showed that also at dynamic conditions, which can be favorable for separating solid and liquid phases in a plant, the removal percentage was quantitative, since residual glyphosate concentration was below the quantitation limit. The same treated solution was analyzed by ICP-MS to evaluate possible leaching of Fe, which resulted to be 0.9 mg/L (value below effluent limitation guidelines set by Italian regulation Decree 152/2006).
To simulate regeneration of the Fe-NH2-SBA-15 sorbent under dynamic conditions, a solution of 12.5 mM NaOH was continuously flown for 15 min (0.6 mL/min) and then analyzed to evaluate the amount of glyphosate recovered, which was quantitative.
Conclusions
A functionalized iron oxide/SBA-15 sample (Fe-NH2-SBA-15) was synthesized and investigated for the adsorption of glyphosate from water matrices under the assumption of selective contributions of the single components of the material. Adsorption and desorption measurements, performed also for the materials obtained at the intermediate stages of synthesis of Fe-NH2-SBA-15, allowed us to highlight the following remarks:
-
at pH 2, all the materials exhibit better removal performance with respect to pH 6. At high pH, the negatively charged surface of the material hardly interact with the negatively charged glyphosate;
-
all the materials containing iron exhibit removal performance higher than materials that do not contain it;
-
the surface behavior of Fe-NH2-SBA-15 involves both complexation and anion-exchange interactions.
The performance observed for the sorbent Fe-NH2-SBA-15 (quantitative adsorption and complete recovery of glyphosate through diluted NaOH solution) is worth of note, mostly considering that the sorbent can be regenerated and reused with unaltered performances for at least five cycles.
The best performance of Fe-NH2-SBA-15 was obtained at pH 2.1, that is far from pH conditions usually adopted in drinking and wastewater treatment processes. Nevertheless, very acidic pH (i.e., 1.9–2.5) has been measured in environmental water intended for urban use, including irrigation and human consumption, as a consequence of peculiar geochemical composition and anthropic activities (Appleyard et al. 2004; Fisher 2002). Furthermore, the optimal acidic pH of Fe-NH2-SBA-15 is compatible with some industrial activities (e.g., tannery) that may re-use acidic water for their processes.
References
Affouri A, Eloussaief M, Kallel N, Benzina M (2015) Application of Tunisian limestone material for chlorobenzene adsorption: characterization and experimental design. Arab J Geosci 8:11183–11192. doi:10.1007/s12517-015-2011-4
Appleyard S, Wong S, Willis-Jones B, Angeloni J, Watkins R (2004) Groundwater acidification caused by urban development in Perth, Western Australia: source, distribution, and implications for management. Soil Research 42:579–585. doi:10.1071/SR03074
Assalin MR, De Moraes SG, Queiroz SC, Ferracini VL, Duran N (2009) Studies on degradation of glyphosate by several oxidative chemical processes: ozonation, photolysis and heterogeneous photocatalysis. Journal of Environmental Science and Health Part B 45:89–94
Benbrook CM (2016) Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28:1
Borggaard OK, Gimsing AL (2008) Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 64:441–456. doi:10.1002/ps.1512
Bouchemal N, Azoudj Y, Merzougui Z, Addoun F (2012) Adsorption modeling of orange G dye on mesoporous activated carbon prepared from Algerian date pits using experimental designs. Desalin Water Treat 45:284–290. doi:10.1080/19443994.2012.692042
Braga PRS, Costa AA, De MacEdo JL, Ghesti GF, De Souza MP, Dias JA, Dias SCL (2011) Liquid phase calorimetric-adsorption analysis of Si-MCM-41: evidence of strong hydrogen-bonding sites. Microporous Mesoporous Mater 139:74–80. doi:10.1016/j.micromeso.2010.10.020
Carneiro RT et al. (2015) Removal of glyphosate herbicide from water using biopolymer membranes. J Environ Manag 151:353–360. doi:10.1016/j.jenvman.2015.01.005
Chemical Abstracts Service Scifinder, version (2015)
Chemicalize (2016). www.chemicalize.org. Accessed Aug 07 2016
Chen F, Wu Q, Lü Q, Xu Y, Yu Y (2015) Synthesis and characterization of bifunctional mesoporous silica adsorbent for simultaneous removal of lead and nitrate ions. Sep Purif Technol 151:225–231. doi:10.1016/j.seppur.2015.07.024
Cooper C, Burch R (1999) Mesoporous materials for water treatment processes. Water Res 33:3689–3694. doi:10.1016/S0043-1354(99)00095-0
Coupe RH, Kalkhoff SJ, Capel PD, Gregoire C (2012) Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag Sci 68:16–30. doi:10.1002/ps.2212
Cui H, Li Q, Qian Y, Zhang Q, Zhai J (2012a) Adsorption of aqueous glyphosate n-phosphonomethylglycine by manganese oxides/mesoporous silica SBA-15 composite at high salinity condition. Asian J Chem 24:2685–2690
Cui H, Li Q, Qian Y, Zhang Q, Zhai J (2012b) Preparation and adsorption performance of MnO2/PAC composite towards aqueous glyphosate. Environ Technol 33:2049–2056. doi:10.1080/09593330.2012.660641
Darwish NN, Zubaidi IA, Sayed YE, Shareefdeen Z (2015) Factorial design analysis for adsorption of sulfur compounds from diesel oil on activated charcoal. In: 6th International Conference on Modeling, Simulation, and Applied Optimization, ICMSAO 2015 - Dedicated to the Memory of Late Ibrahim El-Sadek. doi: 10.1109/ICMSAO.2015.7152231
Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64:319–325
EPA (Environmental Protection Agency) (2015) Drinking Water Treatability Database, available at http://iaspub.epa.gov/tdb/pages/contaminant/treatmentSummary.do;jsessionid=F0HoNehqcxFtju2Wjvz_MLUvhIMkMDg3RnrpMbXRiVPKkakmSJIv!1249989703
European Food Safety Authority (EFSA) (2015) Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J 13:4302
European Parliament and the Council of the European Union (2008) Directive 2008/105/EC: Environmental quality standards in the field of water policy, amending and subsequently repealing council directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC
Fisher RS (2002) Groundwater quality in Kentucky: pH Kentucky Geological Survey, Information Circular 6 Series XII
Gasnier C, Dumont C, Benachour N, Clair E, Chagnon M-C, Séralini G-E (2009) Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 262:184–191. doi:10.1016/j.tox.2009.06.006
Geyikçi F, Büyükgüngör H (2013) Factorial experimental design for adsorption silver ions from water onto montmorillonite. Acta Geodyn Geomater 10:363–370. doi:10.13168/AGG.2013.0035
Guyton KZ et al. (2015) Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. The Lancet Oncology 16:490–491. doi:10.1016/S1470-2045(15)70134-8
Horth H (2010) Monitoring results for surface and groundwater. European Glyphosate Environmental Information Service. Available at http://www.egeis-toolbox.org/documents/11%20Detection%20in%20SW%20and%20GW%20draft%20v3.pdf
ISPRA (2014) Rapporto nazionale pesticidi nelle acque (dati 2011–2012), available at http://www.isprambiente.gov.it/files/pubblicazioni/rapporti/Rapporto_208_2014.pdf
Jia D, Zhou C, Li C (2011) Adsorption of glyphosate on resin supported by hydrated iron oxide: equilibrium and kinetic studies. Water Environment Research 83:784–790
Jönsson J, Camm R, Hall T (2013) Removal and degradation of glyphosate in water treatment: a review. Journal of Water Supply: Research and Technology - AQUA 62:395–408. doi:10.2166/aqua.2013.080
Kolpin DW, Thurman EM, Lee EA, Meyer MT, Furlong ET, Glassmeyer ST (2006) Urban contributions of glyphosate and its degradate AMPA to streams in the United States. Sci Total Environ 354:191–197. doi:10.1016/j.scitotenv.2005.01.028
Krenkova J, Foret F (2011) Iron oxide nanoparticle coating of organic polymer-based monolithic columns for phosphopeptide enrichment. J Sep Sci 34:2106–2112
Leardi R (2009) Experimental design in chemistry: a tutorial. Anal Chim Acta 652:161–172. doi:10.1016/j.aca.2009.06.015
Manassero A, Passalia C, Negro A, Cassano A, Zalazar C (2010) Glyphosate degradation in water employing the H2O2/UVC process. Water Res 44:3875–3882
Mohsen Nourouzi M, Chuah TG, Choong TSY (2010) Adsorption of glyphosate onto activated carbon derived from waste newspaper. Desalin Water Treat 24:321–326. doi:10.5004/dwt.2010.1461
Morgan E (1991) Chemometrics: experimental design (analytical chemistry by open learning). vol Copyright (C) 2016 American Chemical Society (ACS). All Rights Reserved. John Wiley and Sons,
Parma A et al. (2012) Structural and magnetic properties of mesoporous SiO2 nanoparticles impregnated with iron oxide or cobalt-iron oxide nanocrystals. J Mater Chem 22:19276–19288. doi:10.1039/C2JM32314A
Powles SB (2008) Evolved glyphosate-resistant weeds around the world: lessons to be learnt. Pest Manag Sci 64:360–365
Royer B, Lima EC, Cardoso NF, Calvete T, Bruns RE (2010) Statistical design of experiments for optimization of batch adsorption conditions for removal of reactive red 194 textile dye from aqueous effluents. Chem Eng Commun 197:775–790. doi:10.1080/00986440903359004
Salman JM, Abid FM, Muhammed AA (2012) Batch study for pesticide glyphosate adsorption onto palm oil fronds activated carbon. Asian J Chem 24:5646–5648
Sarich C (2015) Global Research, Natural Society, Sri Lanka’s newly elected President Bans Glyphosate effective immediately, available at http://naturalsociety.com/sri-lankas-newly-elected-president-bans-glyphosate-effective-immediately/#ixzz3kO5z51rV
Sheals J, Sjöberg S, Persson P (2002) Adsorption of glyphosate on goethite: molecular characterization of surface complexes. Environmental science & technology 36:3090–3095
Shenderovich IG et al. (2003) Pyridine-15 N A mobile NMR sensor for surface acidity and surface defects of mesoporous silica. J Phys Chem B 107:11924–11939. doi:10.1021/jp0349740
Shoval S, Yariv S (1979) The interaction between Rounup (glyphosate) and montmorillonite. Part II. Ion exchange and sorption of iso-propylammonium by montmorillonite. Clay Clay Miner 27:29–38
Soldatov VS (2000) A simple method for the determination of the acidity parameters of ion exchangers. React Funct Polym 46:55–58. doi:10.1016/S1381-5148(00)00041-9
Speth TF (1993) Glyphosate removal from drinking water. Journal of Environmental Engineering (United States) 119:1139–1157. doi:10.1061/(ASCE)0733-9372(1993)119:6(1139)
Sprankle P, Meggitt WF, Penner D (1975) Rapid inactivation of glyphosate in the. Soil Weed Science 23:224–228. doi:10.2307/4042278
Struger J, Thompson D, Staznik B, Martin P, McDaniel T, Marvin C (2008) Occurrence of glyphosate in surface waters of southern Ontario. Bull Environ Contam Toxicol 80:378–384. doi:10.1007/s00128-008-9373-1
The Healthy Home Economist (2014) Dutch Parliament bans Roundup, France and Brazil to follow. http://www.thehealthyhomeeconomist.com/roundup-banned-netherlands-france-brazil-likely-soon-follow/
Tomlin CDS (2006) The pesticide manual: a world compendium. 14th edn., Hampshire
Vencill W (2002) Weed Science Society of America, Lawrence, KS Herbicide Handbook:231–234
Villeneuve A, Larroudé S, Humbert JF (2011) Herbicide contamination of freshwater ecosystems: impact on microbial communities. In: Stoytcheva M (ed.) Pesticides - Formulations, Effects, Fate. Available from: http://www.intechopen.com/books/pesticides-formulations-effects-fate/herbicide-contamination-of-freshwater-ecosystems-impact-on-microbial-communities. InTech. doi:10.5772/13515.
Vunain E, Meijboom R (2014) Mesoporous materials as potential absorbents for water purification. In: Application of nanotechnology in water research. John Wiley & Sons, Inc., New York, pp. 269–284. doi:10.1002/9781118939314.ch10
Weast RC (1989) CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data. CRC Press, Boca Raton
Weng L, Van Riemsdijk WH, Hiemstra T (2012) Factors controlling phosphate interaction with iron oxides. J Environ Qual 41:628–635. doi:10.2134/jeq2011.0250
World Health Organization (2005) Glyphosate and AMPA in drinking-water
Wu Z, Zhao D (2011) Ordered mesoporous materials as adsorbents. Chem Commun 47:3332–3338. doi:10.1039/c0cc04909c
Xiao G, Wen R (2016) Comparative adsorption of glyphosate from aqueous solution by 2-aminopyridine modified polystyrene resin, D301 resin and 330 resin: influencing factors, salinity resistance and mechanism. Fluid Phase Equilib 411:1–6. doi:10.1016/j.fluid.2015.11.026
Yuan G (2014) An insight into glyphosate trend agronews, available at http://newsagropages.com/News/NewsDetail-13358htm
Zeng H, Fisher B, Giammar DE (2008) Individual and competitive adsorption of arsenate and phosphate to a high-surface-area iron oxide-based sorbent Environmental Science and Technology 42:147–152. doi:10.1021/es071553d
Zhang H, He H-X, Wang J, Mu T, Liu Z-F (1998) Force titration of amino group-terminated self-assembled monolayers using chemical force microscopy. Applied Physics A 66:S269–S271
Zhang Y et al. (2014) Hydrophobic bifunctionalized hexagonal mesoporous silicas as efficient adsorbents for the removal of Orange IV. RSC Adv 4:49783–49788. doi:10.1039/c4ra08632e
Zhu X, Li B, Yang J, Li Y, Zhao W, Shi J, Gu J (2015) Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-based MOFs of UiO-67. ACS Appl Mater Interfaces 7:223–231. doi:10.1021/am5059074
Acknowledgments
M.C.B. and M.D.B. thank Drs. E. Coppini and D. Fibbi (Gida S.p.A., Prato, Italy) for the supply of the wastewater samples. Financial support from Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR, Italy) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 316 kb)
Rights and permissions
About this article
Cite this article
Rivoira, L., Appendini, M., Fiorilli, S. et al. Functionalized iron oxide/SBA-15 sorbent: investigation of adsorption performance towards glyphosate herbicide. Environ Sci Pollut Res 23, 21682–21691 (2016). https://doi.org/10.1007/s11356-016-7384-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7384-8