Abstract
Ostrinia furnacalis (Guenée) and Helicoverpa armigera (Hübner) are the most important pests of maize in China. A laboratory study and a 2-year field study on the efficacy of transgenic maize expressing the Cry1Ac protein BT38 against O. furnacalis and H. armigera were performed. We found that the husks, kernels, and silks of BT38 showed significant efficacy against larvae of O. furnacalis and H. armigera. In the field, when neonate larvae of O. furnacalis and H. armigera were on plants at different growth stages and when levels of leaf-damage or number of damaged silks were used to score efficacy, we found that BT38 showed significant insecticidal efficacy against O. furnacalis and H. armigera, but the non-Bt maize did not show significant efficacy against either pest. These results suggest that the insecticidal efficacy of Bt maize expressing the Cry1Ac protein could be useful in the integrated pest management of these key maize pests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Genetically modified (GM) crops are plants that have had a target gene, isolated from plants, microorganisms, or animals, transferred into the crop genome through various methods, making it inherently stable and lending the crop new genetic characteristics such as insect resistance, disease resistance, stress resistance, high yield, and high quality (Ferré et al. 2008). Isolated from bacterium Bacillus thuringiensis (Bt) and imported into the maize genome, the target gene BT is able to address the need of the maize for new genetic resistance against insect pests.
Transgenic technology has benefits that traditional and conventional cultivation technology cannot offer (Gould 1998). Maize (Zea mays L.) is one of the most important cereal crops in the world, both for human food production and animal husbandry (Yang and Song 2001). The most serious pests of maize in China have been, until recently, the Asian corn borer Ostrinia furnacalis (Guenée) and the cotton bollworm, Helicoverpa armigera (Hübner). Chemical insecticides are, or were until recently widely applied to control these pests, which led to further problems such as pest resistance, pest outbreaks, and higher pesticide residues in the crop. GM maize (with the addition of various Bt proteins) can also be used to control these maize pests (Koziel et al. 1993, Yang and Song 2001, He et al. 2003a, b). Since the first transgenic maize plants expressing the Cry1Ab protein were reported by Koziel et al. (1993), a total of 10 transgenic maize lines have been made commercially available (Comas et al. 2013). All of these transgenic maize lines have been designed to control the European corn borer, Ostrinia nubilalis (Hübner), and other lepidopteran pests by expressing Cry1Ab (events 176, Bt11, MON810, MON809, and MON802), Cry1Ac (event DBT418), Cry9C (event CBH351), or Cry1Fa2 (event TC1507) proteins. Substantial research has shown Bt cotton expressing Cry1Ac to have good control effects on H. armigera, an important cotton pest in China (Wu et al. 2008). Considering the remarkable adaptability of insects to toxins and other control tactics, the evolution of resistance in target pests to Bt toxic proteins endangers the future efficiency of Bt maize (Carrière et al. 2010). Although the majority of target pest species remain susceptible to Bt maize, several cases of evolved resistance have been reported in five species of lepidopteran pests: Helicoverpa punctigera to Bt cotton producing Cry1Ac and Cry2Ab, Helicoverpa zea to Bt cotton producing Cry1Ac and Cry2Ab, Spodoptera frugiperda to Bt maize producing Cry1F, Pectinophora gossypiella to Bt cotton producing Cry1Ac, and Busseola fusca to Bt maize producing Cry1Ab, (Carrière et al. 2010). Natural selection will create the evolution of resistance to Bt maize in three situations, which are as follows: offspring of surviving insects on Bt maize, different fitness continually affecting survival rate on Bt maize, and varied individual survival rate on Bt maize (Benardi et al., 2014). Because of temporal and spatial overlap of Bt maize in crop-production systems in China, such overlap may enhance the threat of H. armigera and O. nubilalis to the evolution of resistance to Bt maize. However, there are no data available in the literature demonstrating the efficacy of Bt maize expressing Cry1Ac against H. armigera and O. nubilalis.
The purpose of this study was to systemically evaluate the effects of Bt maize expressing Cry1Ac against O. furnacalis and H. armigera under laboratory and field conditions so as to provide information to incorporate the use of Bt maize varieties into integrated pest management plans against these invertebrates.
Materials and methods
Maize varieties
Bt maize BT38 and its corresponding non-transformed near isoline Z58 (non-Bt maize) as a control were used for the experiments. Both were provided by the China National Maize Center (Haidian, Beijing).
Sources of experimental insects
Neonate larvae of the Asian corn borer (O. furnacalis) were provided by the Institute of Plant Protection, Chinese Academy of Agricultural Sciences (Haidian, Beijing). Neonate cotton bollworm (H. armigera) larvae were obtained from a laboratory colony from the China Agricultural University (Haidian, Beijing).
Expression analysis of Cry1Ac proteins in Bt maize
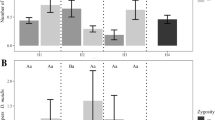
Leaves were collected from BT38 and Z58 at the 6~8-leaf stage; silks and kernels were collected from BT38 and Z58 at blossom stage (Table 1), then weighed and kept at −20 °C until Cry protein levels were analyzed. The concentration of Cry1Ac in maize leaves, silks, and kernels was measured by ELISA with Cry1Ac detection kits from EnviroLogix (Portland, ME). Kits were identified as zxfQualiPlateTM Kit for Cry1Ab/Cry1Ac—AP 003 CRBS. The samples were diluted at a rate of 1:20 (milligram sample: microliter PBST buffer) and fully ground by mortar and pestle. ELISA was performed according to the manufacturer’s instructions.
Bioassay with Asian corn borer and bollworm
Neonate larvae of Asian corn borer and cotton bollworm were tested on the husks, kernels, and silks of maize. Samples of plant tissues were punched out into 2-cm long rectangle pieces and then put into the wells of Cultrex 24 Well BME Cell plates obtained from the Corning Company (NY, USA); two neonate larvae were put into each of 24 cells. Each treatment had three replicates. The Cultrex 24 Well BME Cell plates with larvae were placed in an incubator and held at 27 ± 1 °C and 14 L: 10Dh photoperiod. Larval survival was recorded each day for 8 days following the treatment.
Field experiment design
Bt maize BT38 and its corresponding non-transformed near isoline Z58 (non-Bt maize) were grown in the field under unprotected conditions during the 2012–2013 growing seasons at the Shangzhuang Experimental Station of China Agricultural University, located to the east of Xinlitun village, Haidian District, Beijing (altitude, 47 m; 116°17′ 52.84″ E; 39°57′ 52.84″ N). Average annual temperature at the site is 12.6° C, and the climate is semi-arid with a total yearly rainfall of 168 ± 8 mm.
Bt and non-Bt corn plots were arranged in a randomized block design with three replications, respectively. Each plot measured 10 by 15 m and contained 10 rows with 60 cm between rows and 25 cm between plants within a row. A 3-m-wide strip of buffer separated each plot from its neighbors, planted with non-Bt corn (Fig. S1). No herbicide or insecticide has ever been used in the experiment fields. In 2012 and 2013, corn was planted on June 5 and May 10, respectively.
Insect inoculation and sampling methods
Asian corn borer
Maize was artificially infested with Asian corn borer neonate larvae following the method outlined by He et al. (2003a, b). The infestation was carried out at the mid-whorl leaf and silk stage. At the mid-whorl leaf stage, each of 40 selected plants in each plot was artificially infested with about 60 neonate larvae using a Bazooka applicator (Beijing, China, GDHY). Leaf damage was measured 14 days later, and an assessment was made of the relative level of leaf damage by classifying the level of damage to each leaf on a nine-point scale (Chang 2007). Also, at the silk stage, the first silks of each plant were infested with about 60 neonate larvae per silk mass with a Bazooka applicator, and again after 3 days to ensure successful infestation.
Cotton bollworm
Maize foliage was artificially infested with 20~30 cotton bollworm neonate larvae on each of 40 plants selected in each plot. Also, at the silk stage, the first silks of each plant were infested with neonate larvae with a Bazooka applicator, and again after 3 days. Silk damage levels were assessed by classifying the level of damage using a nine-point scale (Chang 2007).
Data analysis
Data were analyzed using an analysis of variance by Nick Longford (Gerber and Voelkl 1997). Data on Cry proteins in plant leaves were analyzed using one-way analysis of variance (ANOVA) and Tukey’s HSD test. Data on survival percentages of O. furnacalis and H. armigera were arcsine square root transformed to fit a normal distribution and then analyzed using ANOVA. Data on damage levels of BT38 and Z8 by O. furnacalis and H. armigera in field were analyzed using Tukey’s test with SPSS 19.0 software.
Results
Cry proteins in Bt maize
Leaves, husks, kernels, and silks of the BT38 strain all produced Cry1Ac protein, and its concentration in these plant parts was 480.30 to 587.38, 282.25, 257.45, and 319.38 ng/g fresh weight (FW), respectively (F = 22.49; df = 6,15; P < 0.05). The higher concentration of Cry1Ac protein was found at the jointing stage of Bt maize (Table 1). No Cry1Ac protein was detected in any samples from Z58.
BT38 varietal effects in the laboratory
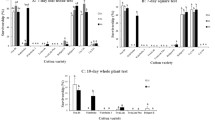
The survival of Asian corn borer was <10 % within 5–8 days after being placed on BT38 (expressing the Cry1Ac protein) husks, kernels, and silks, but >65 % within 5–8 days after feeding on the same tissues of the corresponding non-transformed near isoline Z58 (non-Bt maize). This difference indicates that Bt maize BT38 confers significant mortality to Asian corn borer larvae (F = 6.785; df = 2, 6; P = 0.032) (Fig. 1).
Similarly, the survival of cotton bollworm was <10 % after 6–7 days of feeding on BT38 husks, kernels and silks, but >70 % after 6–7 days feeding on the same tissues of the corresponding non-transformed near isoline Z58 (non-Bt maize). Bt maize BT38 thus showed significant insect mortality to cotton bollworm (F = 7.631; df = 2, 6; P = 0.026) (Fig. 2).
BT38 varietal effect in the field
Asian corn borer
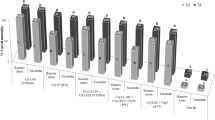
In 2012, the average leaf damage level on a scale from 0 to 8 (highest) (Table S1) and silk damage to BT38 maize expressing the Cry1Ac protein from Asian corn borer were 2.60 ± 0.10 and 2.13 ± 0.03 out of eight, respectively, while on the non-transformed parental variety (Z58) leaf and silk damage was 6.70 ± 0.06 and 6.23 ± 0.12 (F = 0.65, df = 119, P < 0.05), respectively, significantly different from BT38. Thus, BT38 maize expressing the Cry1Ac protein showed efficacy against Asian corn borer, while Z58 was susceptible to Asian corn borer in the field tests (Fig. 3). In 2013, Asian corn borer damage to leaves and silks of BT38 maize was 2.49 ± 0.1 and 4.28 ± 0.31, respectively, while the average damage levels on leaves and silks of Z58 was 5.78 ± 0.23 and 6.07 ± 0.01, respectively (F = 0.38, df = 119, P < 0.05), significantly different from damage to BT38 maize (Fig. 3).
Leaf- and silk-damage level of BT38 and Z58 by Ostrinia furnacalis in the field in different years. Values with same letter in the same year are not significantly different according to analysis of variance and Tukey’s test at the P = 0.05 level of significance. HE highly efficacious, E efficacious, ME moderately efficacious, LE low efficacious
Cotton bollworm
In 2012, the average damage level on silks of BT38 from cotton bollworm was 2.43 ± 0.15, while the average level on Z58 was 4.02 ± 0.29 (F = 0.61, df = 119, P < 0.05) significantly different from that of Bt maize. In 2013, cotton bollworm damage to silks of BT38 maize was 0.89 ± 0.07, while the average damage levels to silks of Z58 were 3.52 ± 0.39 (F = 0.47, df = 119, P < 0.05) significantly higher than damage to BT38 maize. Using the average damage level on silks as an evaluation criterion, BT38 showed significant efficacy against cotton bollworm (Fig. 4).
Silk-damage levels of BT38 and Z58 by Helicoverpa armigera in the field in different years. Values with same letter in the same year are not significantly different according to analysis of variance and Tukey’s test at the P = 0.05 level of significance. HE highly efficacious, E efficacious, ME moderately efficacious, LE low efficacious
Discussion
Cotton and maize varieties expressing Cry1 or Cry2 proteins targeting lepidopteran pests are the principal GM crops grown worldwide. Many lepidopteran species have demonstrated high sensitivity to the Cry1 protein (James, 2013). For example, Cry1Ab, Cry1Aa, and Cry1Ac toxic proteins caused high mortality to O. nubilalis, O. furnacalis, and Diatraea saccharalis. Moreover, no significant differences in LC50 and LC90 values were found between species (Tan et al. 2011). However, Heliothis virescens (F.), Chrysodeixis includes (Walker), and Helicoverpa zea (Boddie) displayed higher tolerance to the Cry1Ac toxin than some other populations of lepidopteran pests (Benardi et al., 2014).
Our laboratory tests found that Bt maize variety BT38 expressing the Cry1Ac protein caused significant mortality in O. furnacalis and H. armigera (Figs. 1 and 2). Similar results were also found in the Asian corn borer, O. furnacalis by Wang et al. (2014). Our field tests found that the level of damage from Asian corn borer and cotton bollworm to leaves or silks on Bt maize expressing the Cry1Ac protein was significantly lower than that of its isoline hybrid parental non-transformed line Z58 (Figs. 3 and 4).
As a spray, products containing Bt toxins have existed for many years. But short residuals in the field and the consequent need for repeated use over a growing season have limited the use of Bt insecticides as spray applications (Comas et al. 2014). Bt maize, in contrast, expresses the Bt-protein continuously and eliminates these problems (Ferré et al. 2008). Bt crops have not only reduced insecticide usage but also increased the use of such biological control agents as generalist predators (Cao et al. 2014). The efficacy of Bt maize depends on the high expression of Bt protein in the specific feeding sites of neonate larvae, which change as the maize plant grows. From the mid-whorl stage on, most larvae feed in unfurled leaves until silk is formed. Control of Asian corn borer at this stage is therefore of great importance to ensure a high yield (Gould 1998). Cry1Ac genes express insecticidal proteins in both young foliage and silk (Bravo et al. 2007), and our study demonstrates that the insecticidal protein is highly toxic to Asian corn borer larvae feeding on this tissue (Fig. 3). These results are similar to the level of efficacy against European corn borer of some Bt maize lines expressing the Cry protein (Huang et al. 1999).
Bt-cotton expressing the Cry1Ac protein has shown high efficacy against cotton bollworm in the field in other studies (Huang et al. 1999). In our study, Bt maize expressing the Cry1Ac protein was generally or highly effective against cotton bollworm, while the non-transformed near isoline maize Z58 showed significantly different efficacy from BT38 in the field experiments (Fig. 4). Bt maize expressing the Cry1Ab protein also showed high efficacy against cotton bollworm in past field trials (Yang and Song 2001). Nevertheless, continuous usage of Bt maize may lead to field-evolved resistance by pests, which has been documented in several populations of major targeted pests (Cao et al. 2014). The refuge strategy suggested by various theoretical mathematical models, would, if widely applied, be useful in delaying the development of pest resistance to Bt maize (Cao et al. 2014).
Our results suggest that Bt maize expressing the Cry1Ac protein can provide a means of potential control in the integrated pest management of the important maize pests, Asian corn borer and cotton bollworm, in maize fields and thereby reduce the use of chemical insecticides. However, the mechanisms of insect control of the Bt maize and resistance management require further study.
References
Benardi O, Sorgatto RJ, Barbosa AD, Domingues FA, Dourado PM, Carvalho RA, Martinelli S, Head GP, Omoto C (2014) Low susceptibility of Spodoptera cosmioides, Spodoptera eridania and Spodoptera frugiperda (Lepidoptera: Noctuidae) to genetically-modified soybean expressing Cry1Ac protein. Crop Prot 58:33–40
Bravo A, Gill SS, Soberon M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423–435
Cao GC, Feng HQ, Guo F, Wu KM, Li XC, Liang GM, Desneux N (2014) Quantitative analysis of fitness costs associated with the development of resistance to the Bt toxin Cry1Ac in Helicoverpa armigera. Sci Rep 4:5629
Carrière Y, Crowder DW, Tabashnik BE (2010) Evolutionary ecology of insect adaptation to Bt crops. Evol Appl 3:561–573
Chang X (2007) Resistance of transgenic Bt maize on armyworm. Acta Phytophy Sin 34:225–228
Comas J, Lumbierres B, Pons X, Albajes R (2013) Ex-ante determination of the capacity of field tests to detect effects of genetically modified corn on nontarget arthropods. J Econ Entomol 106:1659–1668
Comas C, Lumbierres B, Pons X, Albajes R (2014) No effects of Bacillus thuringiensis maize on nontarget organisms in the field in southern Europe: a meta-analysis of 26 arthropod taxa. Transgenic Res 23:135–143
Ferré J, Rie V, MacIntosh SC (2008) Insecticidal genetically modified crops and insect resistance management (IRM), (pp. 41–85). In: Romeis J, Shelton AM, Kennedy GG (eds) Integration of insect-resistant genetically modified crops within IPM programs. Springer, Netherlands
Gerber SB, Voelkl KE (1997) The SPSS guide to the new statistical analysis of data. Springer-Verlag, New York
Gould F (1998) Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu Rev Entomol 43:701–726
He KL, Wang ZY, Wen LP, Bai XS, Zhou RD, Zhu QH (2003a) Field evaluation of Asian maize borer control in hybrid of transgenic maize event MON 810. Agr Sci China 2:1363–1368
He KL, Wang ZY, Zhou DR, Wen LP, Song YY, Yao ZY (2003b) Evaluation of transgenic Bt corn for resistance to the Asian com borer (Lepidoptera: Pyralidae). J Econ Entomol 96:935–940
Huang F, Buschman LL, Higgins RA, et al. (1999) Inheritance of resistance to Bacillus thuringiensis toxin (Dipel ES) in the European corn borer. Science 284:965–967
James C (2013) Global status of commercialized biotech/GM crops: 2013, ISAAA brief no. 46. ISAAA, Ithaca, NY
Koziel MG, Beland GL, Bowman C, Carozzi NB, Crenshaw R, Crossland L, Dawson J, Desai N, Hill M, Kadwell S, Launis K, Lewis K, Maddox D, Mcpherson K, Meghji MR, Merlin E, Rhodes R, Warren GW, Wright M, Evola SV (1993) Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Nat Biotechnol 11:194–200
Tan SY, Cayabyab BF, Alcantara EP, Ibrahim YB, Huang F, Blankenship EE, Siegfried BD (2011) Comparative susceptibility of Ostrinia furnacalis, Ostrinia nubilalis and Diatraea saccharahis (Lepidoptera: Crambidae) to Bcaillus thuringiensis Cry1 toxins. Crop Prot 30(2011):1184–1189
Wang YQ, He KL, Fan J, Wang YD, Zhang TT, Wang ZY, Bai SX (2014) Resistance of transgenic Bt corn variety BT799 to the Asian corn borer. J Appl Entomol 51(3):636–642
Wu K, Lu Y, Feng H, Jiang Y, Zhao J (2008) Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 321:1676–1678
Yang CY, Song JC (2001) Progress of Bt maize and its resistance to pest insects. Maize Sci 9:88–93
Acknowledgments
The authors greatly appreciated the English editing by Van Driesche Scientific Editing. This research was funded by the Special Fund for Transgenic Crop Research of China (2016ZX08011-003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Yi-ping Chen
Rights and permissions
About this article
Cite this article
Chen, HX., Yang, R., Yang, W. et al. Efficacy of Bt maize producing the Cry1Ac protein against two important pests of corn in China. Environ Sci Pollut Res 23, 21511–21516 (2016). https://doi.org/10.1007/s11356-016-7340-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7340-7