Abstract
The main purpose of this work is to study the effect of a new process of accelerating which consist to couple the electrochemical process with the adsorption to remove an anionic dye, the indigo carmine. That is why, we investigated the effects of the new process of accelerating the adsorption process by using alternating current (AC) on the retention of an anionic dye, the indigo carmine. The adsorption capacity of dye (mg/g) was raised with the raise of current voltage in solution, temperature, and initial indigo carmine concentration and decreased with the increase of initial solution pH, current density, and mass of carbon. The results demonstrate that the removal efficiency of 97.0 % with the current voltage of 15 V is achieved at a current density of 0.014 A/cm2, of pH 2 using zinc as electrodes and contact time of 210 min for adsorption in the presence of AC. Concerning the adsorption without AC, the results obtained showed that for an initial concentration equal to 20 mg/L, more than 95 % amount of adsorbed dye was retained after 405 min of contact in batch system. The comparison between adsorption in the presence and absence of an alternating current shows the importance of the alternating current in the acceleration of the adsorption method and improve the performances of FILTRASORB 200. For both cases, the adsorption mechanism follows the fractal kinetics BSf(n,α) model and the Brouers–Sotolongo isotherm model provides a good fit of the experimental data for both adsorption with and without alternating current.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colorants are largely employed in textile, paper, plastic, food, and cosmetic industries (Sumanjit et al. 2013; Ong et al. 2007; Vargas et al. 2011). Several dyes and their break-down products contribute to water toxicity and constitute a menace for the environment, human, and animals (García et al. 2014). In fact, dyes are recalcitrant and hardly biodegradable and are not taken off from wastewater by classic wastewater treatment method (Bello et al. 2013) due to their complex structure and synthetic origins (Khodaie et al. 2013; Wang et al. 2008).
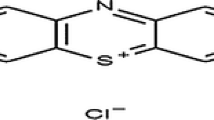
Indigo carmine (5,5′-indigodisulfonic acid sodium salt or indigotine) is a dark blue dye used primarily in textile industries for the dyeing of polyester fibers and denim (blue jeans) (Stergiopoulos et al. 2014). In addition, indigo carmine is employed as dye in food and cosmetics industry (Stergiopoulos et al. 2014). The indigo carmine is classified as a very toxic dye (Mittal et al. 2006). The contact of this dye with the human body can provoke irritations of the skin and eye, permanent damage with the cornea and the conjunctive one, gastro-intestinal irritation with nausea, vomiting, and diarrhea (Maghri et al. 2012).
The treatment of colored water was the subject of several studies (Rodrigues et al. 2013; Căilean et al. 2009; Vijayageetha et al. 2013; Ncibi et al. 2008a, b; Abidin and Rahmat 2010) whose purpose is to reduce the intensity of the color and the organic matter contained in these waters. Different processes have been proposed for the elimination of dyes and in particular the indigo carmine from wastewater such as adsorption (Ramesh and Sreenivasa 2015; Aliabadi et al. 2006; Babel and Opiso 2007; Forgacs et al. 2004), photochemical (Kirupavasam and AllenGnana Raj 2012), electrochemical (Stergiopoulos et al. 2014; El-Ashtoukhy 2013; Hammami et al. 2012), electrocoagulation (Deo Mall et al. 2013), ultrafiltration (Căilean et al. 2009), and biological methods (Karthik et al. 2014; Abidin and Rahmat 2010).
However, several of these technologies are very expensive (Mckay et al. 1987; Low et al. 1995). The conventional biological process is not very efficient because most dyes are nonbiodegradable (Stergiopoulos et al. 2014). Physical process of dyes treatment are nondestructive. It transfer dye from water into the adsorbent which generates large amount of residue (Ramesh and Sreenivasa 2015). For these reasons, researchers are encouraged to search for new technologies (Kesraoui et al. 2015; Ncibi et al. 2006, 2007; Zoughuir et al. 1998; Elkassimi et al. 1998). In this context, the alternating current appears as a new alternative, original, and very interesting in accelerating the phenomenon of adsorption and the removal of heavy metals (Shul’gin et al. 1975).
The main purpose of this work is to study the effect of a new process of accelerating by coupling of electrochemical process with adsorption to remove dyes. In order to reach this objective, the adsorption of indigo carmine (Fig.1) by activated carbon (FILTRASORB 200 which is more used in textile industries in Tunisia) in the absence and presence of alternating current (AC) was investigated. This anionic dye is considered in this paper as a model representative of the pollution of textile wastewater which is the most important source of pollution in Tunisia. The influence of several parameters such as pH, contact time, initial IC concentration, temperature, and voltage on the adsorption capacity was studied. Furthermore, mathematical modeling of the kinetics and adsorption isotherms was investigated.
Materials and methods
Materials
The FILTRASOB 200 is a commercial and granulated activated carbon, it was obtained from a textile industry (Chimitex, Tunisia). The origin of this adsorbent is bituminous whose characteristics are shown in Table 1.
Indigo carmine (or indigotin) is a blue dye (number E132) natural extract of the indigo plant. It is part of the family of indigoids. It was purchased from Sigma (purity >85 %). Stock solutions were prepared by dissolving the same quantity of IC in distilled water to give a concentration of 1 g/L. All studied solutions were prepared by diluting the stock solution with distilled water to reach the required concentrations.
Methods
The adsorption experiments have been performed in batch reactor by adding 0.5 g of activated carbon in 150 mL of IC solution with the desired concentration (20–100 mg/L), pH (1–4), and temperature (25–80 °C). All the experiments were carried out at 25 °C, except the tests made to study the effect of temperature on adsorption. The electrical part of the mounting comprises a current source which delivers an AC voltage of 2 to 15 V. It is connected to a voltmeter that allows us to read the voltage. In a 150-mL capacity cell, we plunged two zinc electrodes and the distance between two Zn electrodes has been 4 cm. The current supplied between the electrodes is controlled by an ammeter. Identical mounting to the previous but without the electric part has been used for the blank test. The initial pH was adjusted by adding dilute solutions of HCl (1 M) or NaOH (1 M). After adsorption, the residual concentrations of indigo carmine were determined by a double beam spectrophotometer (FT-IR) (Camspec M550) at a wavelength λ max of 608 nm. The estimation of indigo carmine elimination, the adsorption capacity at equilibrium time (Q) or adsorption elimination efficiency will be calculated, respectively, in accordance with the Eqs. 1 and 2 :
where
- C 0 :
-
is the initial indigo carmine concentration (mg/L)
- C i :
-
is the residual indigo carmine concentration at any time (mg/L)
- V :
-
is the volume of solution (L) and M is the mass of the sorbent (g)
At equilibrium, C i is equal to C e and Q is equal to Q e.
Results
Influence of the alternating current voltage
To study the effect of the AC voltage on the adsorption of indigo carmine by activated carbon, one series of experiments was performed on indigo carmine solutions with the concentration of 20 mg/L at pH 2. Figure 2 shows the percentage removal of the dye by activated carbon. When the AC voltage is augmented from 5 to 15 V, the elimination of indigo carmine by adsorption increases.
A remarkable increase in removal of indigo carmine by activated carbon was found by increasing AC voltage. This can be explained by the increases of the ions mobility of this solution especially the anionic dye which move to the surface of the activated carbon protonated by H+ ions in acidic medium. Hence, there is an increase in contact between the adsorbate and the adsorbent. As a consequence, there is an increase in the amount of adsorbed ions.
Influence of contact time and initial indigo carmine concentration
The influence of contact time at different initial concentrations on the adsorption of indigo carmine onto activated carbon is presented in Fig. 3. As shown, the quantity of adsorbed indigo carmine increases with time and it is left constant after an equilibrium contact time of about 2 and 4 h, respectively, with AC and without AC for the lower concentrations (i.e., 20 mg/L) and almost 10 and 30 h, respectively, with AC and without AC for the higher concentrations (100 mg/L). Moreover, raising the indigo carmine concentration from 10 to 100 mg/L allows the activated carbon to increase their adsorption capacities from 5.86 to 31.05 mg/g with AC and from 5.85 to 30.94 mg/g without AC, respectively, at pH 2 and a constant temperature of 25 °C. Furthermore, the adsorption capacity is similar for adsorption in the presence and absence of AC. On the other hand, in the absence of AC, the adsorption is very slow and reaches equilibrium after 30 h for concentration of 100 mg/L with adsorption capacities of 30.95 mg/g. Whereas in the presence of AC, the adsorption is very speed and equilibrium is reached after only 10 h for the same concentration with adsorption capacity of 31.5 mg/L.
In each case, it was noted that adsorption of indigo carmine was rapid during the first hours and then it became slower to finally attained saturation. The higher adsorption rate during the initial period may be due to the high number of sites availables for the sorption, and no less than 96 % of total dye was removed. After this phase, the number of sites accessible for adsorption decreased such that the colorant molecules needed longer periods for to access the most inaccessible sites (Kesraoui et al. 2015; Ben Hamissa et al. 2010).
When comparing between adsorption in the presence and absence of the AC, only the speed of absorption has changed, while the adsorption capacity is almost the same for both. This is probably due to the existence of the electric field which has increased the speed of movement of the molecules of the indigo carmine.
Influence of initial pH
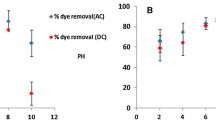
The initial solution pH constitutes a significant parameter in any adsorption study. The influence of pH on indigo carmine adsorption by activated carbon was investigated by tests performed on solutions of different pH (from 2 to 10) as shown in Fig. 4. The dye adsorption in the presence and absence of AC raised as the pH of the solution reduced over the pH range 2–10. The results show that the equilibrium adsorption capacity is maximum at pH 2 for both methods (5.60 mg/g with AC and 4.09 mg/g without AC).
Acidity is a very important parameter in the adsorption reaction. The pH of solution will control the amplitude of the electrostatic charges conferred by the ionized dye molecules (Ould Brahim et al. 2014; Rajurkar and Desa 2015). The indigo carmine and the activated carbon can have functional groups could be protonated or deprotonated to form various surface charges at different pH, which leads to electrostatic attraction or repulsion between the charged adsorption and adsorbents (Sivaramakrishna et al. 2014; Ben Douissa et al. 2014). In case of this study, the attraction of the positively charged surface of the AC at acidic pH on the negative sites of this anionic dye (Ben Hamissa et al. 2013) is independent of alternating current.
Influence of salt addition
The study of salt addition is intended, on the one hand, to simulate the industrial dye baths, on the other hand, to investigate the effect of ionic strength on adsorption phenomena. In fact, textile industry often added salts to improve the fixation of the dye on the tissue. As shown in Fig. 5, the adsorbed amount of the indigo carmine decreased when adding 0.5 g NaCl for adsorption in the presence and absence of AC.
In theory, when the electrostatic forces between the surface of the adsorbent and the adsorbate ions are favorable, a raise in ionic strength decreases the adsorption capacity. Inversely, when the electrostatic forces are repulsive, a raise in the ionic strength increases the adsorption capacity (Newcombe and Drikas 1997; Alberghina et al. 2000). The similar results were obtained with our both processes. The addition of 0.5 g NaCl at pH 2 allowed a significant decrease in the amount adsorbed of 28.5 and 11 %, respectively, in the presence and absence of AC. Sodium ions Na+ highly mobile appear discharging anionic dyes and decrease their attachment to the positive surface sites of activated carbon (Krika et al. 2012).
Influence of temperature
The indigo carmine elimination capacity by the activated carbon was also investigated at various temperatures (298, 313, and 353 K). The study of the effect of temperature on the adsorption of the indigo carmine by activated carbon for initial concentration of 20 mg/L and a pH 2 is shown in Fig. 6. A raise of temperature is followed by a raise of adsorption. Therefore, the viscosity of the solution decreased and the mobility of the adsorbate increased.
A small raise of adsorption capacity of the indigo carmine by activated carbon was observed with increasing temperature. This result indicates that the adsorption was endothermic (Ncibi et al. 2008b; Bilgin Simsek et al. 2015). This behavior may be due to the increase of adsorbate ions mobility with temperature which lightly improves the elimination of indigo carmine from the solution by activated carbon (Al-Khatib et al. 2012). Similar performances were got in other studies for the adsorption of Congo red dye onto date palm leaf base (Ghadah 2014) and adsorption of remazol brilliant blue by seed activated carbon (Ahmad et al. 2015).
Thermodynamic analysis
Thermodynamic parameters such as free energy change (ΔG°), enthalpy change (ΔH°), and entropy change (ΔS°) for the adsorption of indigo carmine by activated carbon were calculated according to the Eqs. 3 and 4 (Ucun et al. 2008):
where
- R :
-
is the gas constant (8.314 J mol−1 K−1)
- T :
-
is the absolute temperature (K)
- K c :
-
is the apparent equilibrium constant defined according to the Eq. 5 (Yurtsever and Sengil 2009):
where
- C ad,eq :
-
is the concentration of adsorbed dye at equilibrium (mg/L)
- C r,eq :
-
is the remaining concentration dye at equilibrium (mg/L).
The thermodynamic was studied at 298, 313, and 353 K. The thermodynamic parameters like Gibbs free energy ΔG° (kJ mol−1), enthalpy ΔH° (kJ mol−1), and entropy ΔS° (J mol−1 K−1) were shown in Table 2.
The negative value of ΔG° shows the spontaneous nature of adsorption of indigo carmine onto activated carbon for all investigated temperatures (Acosta et al. 2016; Aljeboree et al. 2014; Bouhamed et al. 2012). ΔG° is varying from −8.63 to −11.21 kJ mol−1, and from −7.85 to −11.43 kJ mol−1 in the presence and absence of AC, respectively. Furthermore, these values being within the range −20 to 0 kJ mol−1 (Ben Hamissa et al. 2010), this process can be considered as physisorption. ΔH° has a positive value which confirms the endothermic character of the adsorption process (Ben Hamissa et al. 2013; Bouhamed et al. 2012). The positive value of ΔS° suggests the increasing randomness at solid/liquid interface in the course of the adsorption process taking place in the internal structure of indigo carmine removal onto activated carbon (Bouhamed et al. 2015; Ben Hamissa et al. 2013; Bouhamed et al. 2012). Similar findings have been found by other investigators for the adsorption of dyes on various adsorbents (Travlou et al. 2013; Sun et al. 2013; Salleh et al. 2011; Matheswaran and Karunanithi 2007). These results indicate also that the alternating current has no significant influence on the thermodynamic of the adsorption, the values of the free energy are maintained essentially the same.
Kinetic data analysis
To forecast the mechanism of the indigo carmine adsorption, two kinetic models were analyzed: the pseudo-first-order and the pseudo-second-order models.
The model of pseudo-first-order and the pseudo-second-order equation (Saleh 2015; Ho 2004; Lagergren 1898; Ho and McKay 1998) were employed to fit the experimental data of the colorant adsorption before to attain equilibrium. The linearized form of this model is determined by Eq. 6:
where
- t :
-
is the time (min)
- k 1 :
-
is the equilibrium rate constant of pseudo-first-order sorption (min−1).
This equation can be also written according to the Eq. 7:
and define h 1 = k 1 Q e as the initial adsorption rate expressed in (mg g−1 min−1) is the initial adsorption rate (Lagergren 1898).
Contrary to the pseudo-first-order model, the pseudo-second-order model is used to a larger interval of time (Ncibi et al. 2006; Ho and McKay 1999). The pseudo-second-order is founded on the adsorption capacity of the solid phase is described by the Eq. 8:
where
- k 2 :
-
is the equilibrium rate constant of pseudo-second-order adsorption (g/mg min)
- \( {h}_2={k}_2.{Q}_{\mathrm{e}}^2 \) :
-
as the initial adsorption rate expressed in (mg g−1 min−1).
The kinetics of indigo carmine adsorption was studied before reaching equilibrium by both models previously described. The modeling results expressed by the rate constants, the calculated equilibrium adsorption capacities, and the linear regression coefficients obtained at all concentrations were presented in Table 3.
The correlation coefficients for the first-order kinetic model obtained at all studied initial concentration and without AC were low. While the second-order model gives satisfactory fit with the experimental data related to the adsorption of indigo carmine onto activated carbon with high R 2 (0.992). These results demonstrate that the pseudo-second-order model is able to describe satisfactorily the kinetic behavior of indigo carmine adsorption by activated carbon and without AC. Similar phenomenon processes have been found in the adsorption of direct dyes on activated carbon prepared from date pits-A (Mahmoudi et al. 2015), sawdust (Malik 2004), and adsorption of Congo red dye on activated carbon from coir pith (Namasivayam and Kavitha 2002).
As regards the indigo carmine adsorption onto activated carbon in the presence of AC, the order of kinetic model is poorly defined. In fact, the first-order kinetic model was obtained at 20 and 100 mg/L, while at 40, 60, and 80 mg/L, the second-order model was better. In order to improve the kinetic study and determine accurately the order of kinetic adsorption of indigo carmine onto activated carbon, the Brouers–Sotolongo fractal (BSf) kinetics modeling has been used.
On the other hand, the initial adsorption rate (h 1 and h 2) and the rate constant (k 1 and k 2) were found to increase using AC. This result indicates that the rates of indigo carmine adsorption onto activated carbon are much faster when using AC.
BSf(n,α) kinetics modeling
Brouers–Sotolongo model generalized fractional kinetic equation was designed to obtain a universal function for the kinetics of complex systems characterized by stretched exponential and/or power law behaviors. The Brouers–Sotolongo model unifies and generalizes previous theoretical attempts to describe the “fractal kinetic.” Many details on the mathematical development of this model and its application are presented in Brouers and Sotolongo (Brouers 2014a, b; Brouers and Al-Musawi 2015). The pseudo BSf (n,α) sorption kinetics equation is given by Eq. 9:
where
- α :
-
as the fractal time exponent
- n :
-
is an effective non-integer reaction order
- τ c :
-
a characteristic time
- Q e :
-
is the sorbed quantity at saturation
- Q t :
-
is the sorbed quantity at any time
Equation 9 is a solution of a fractal differential equation (Brouers and Al-Musawi 2015; Brouers and Sotolongo-Costa 2006) and the half sorption time (the time at which half of the sorbed material has been adsorbed is a function of the three quantities a, n, and τ c (Eq. 10):
Kinetic parameters were calculated from fits of BSf(n, α) model and they are showed in Tables 4 and 5. The best-fit model was selected according to the nonlinear regression correlation coefficient (R 2) and listed in Table 5.
Based on the R 2 correlation coefficients provided by pseudo-first-order, pseudo-second-order (Table 4), and BSf model (Table 5), it is clear that the BSf model is the best-fitting kinetic model for all studied initial indigo carmine concentrations and for both processes.
We have verified that for this type of well-behaved experimental data the other regression parameters (covariance, adjusted Rsquare …) of the nonlinear fitting program of “mathematica 10” software follow the same order.
Moreover, it appears that with the data reported in Table 4, the largest values of the recursion parameter R 2 are obtained with the BSf(1,α) function for both processes. A similar phenomenon has been found by other researchers (Ben Hamissa et al. 2013; Ncibi et al. 2008a).
However, it would be easy to compare τ c values calculated from BSf(1,α) in the presence and absence of AC. As a matter of fact, for 20 mg/L in the presence of AC, the τ c (64.30) is clearly shorter than the τ c in absence of AC (114.99). A similar result has been found for all concentration. Hence, the results from BSf(n,α) showed clearly that the AC allows an increase of the speed of the adsorption reaction.
Adsorption isotherm modeling
In order to optimize dyes adsorption process, it is useful to find the best correlation for the equilibrium curves. The isotherms modeling were investigated using four equilibrium models: Langmuir, Freundlich, Temkin, and Brouers-Sotolongo. The mathematical expressions are listed in Tables 6 and 7.The graphic correlation between the experimental data and the theoretical models for the adsorption process is shown in Fig. 7a and b for indigo carmine in the presence and absence of AC, respectively. The isotherms fit and their parameters were, respectively, followed and calculated using nonlinear regressions analysis.
First, Table 7 shows that the experimental data were better described by the Brouers–Sotolongo for both processes. In fact, the Brouers–Sotolongo isotherm model presents the best fit by offering the highest nonlinear R 2 (0.988 without AC and 0.994 with AC). In addition, results show clearly that Freundlich model is not appropriate to fit the isotherm curves for adsorption of indigo carmine onto activated carbon. The Freundlich results show that the utilization of this model gives the lowest R 2 (0.7814 without AC and 0.7949 with AC). Similar results were obtained in other studies (Brouers and Al-Musawi 2015).
Comparison between coupling of electrochemical process with adsorption and other adsorption process using activated carbon for IC.
To determine the efficiency of our process (coupling of electrochemical process with adsorption process) compared to others adsorption process to eliminate IC from aqueous solutions, a comparison was realized according to the adsorption capacity (Q m) (Table 8).
Table 8 shows that the coupling of electrochemical process with adsorption process could be an effective method to eliminate IC when compared to the other study using activated carbon to remove IC.
Several studies have found the values of Q m for the adsorption of IC lower than the coupling of electrochemical process with adsorption process such as Secula et al. (2011); Odogu et al. (2016); Rehman et al. (2014), and Lakshmi et al. 2009.
Conclusion
The absorption tests of the indigo carmine on the activated carbon (FILTRASORB 200) were carried out in the absence and in the presence of AC in different conditions of AC voltage, pH, temperature, and concentration. The effectiveness of the AC in the increased speed of adsorption was demonstrated. The best dye adsorption was found at pH 2; it is 99 % for the highest initial concentration 100 mg/L and at a voltage of 15 V. The addition of NaCl decreased greatly the amount adsorbed because of the reduction of the electrostatic forces between the adsorbate and the adsorbent. The thermodynamic study showed that the adsorption of indigo carmine is a spontaneous and endothermic process and while the kinetic study showed that the process follows a kinetic of BSf(1,α) model. The adsorption isotherm studies showed that the Brouers–Sotolongo model presents a good fit to the experimental data. AC has a little effect on the parameters of the adsorption equilibrium; however, it has a great effect on the speed of adsorption that it accelerates. Therefore, we succeed to improve the performance of FILTRASORB 200 by increasing the speed of the adsorption process and reduce the processing time.
References
Abidin FCZA, Rahmat NR (2010) Multi-stage ozonation and biological treatment for removal of azo dye industrial effluent. Int J Environ Sci Dev 1(2):193–198
Acosta R, Fierro V, Martinez de Yuso A, Nabarlatz D, Celzard A (2016) Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 149:168–176. doi:10.1016/j.chemosphere.2016.01.093
Ahmad MA, Ahmad N, Bello OS (2015) Adsorption kinetic studies for the removal of synthetic dye using durian seed activated carbon. J Dispers Sci Technol 36:670–684. doi:10.1080/01932691.2014.913983
Alberghina G, Bianchini R, Fichera M, Fisichella S (2000) Dimerization of Cibacron Blue F3GA and other dyes: influence of salts and temperature. Dyes Pigments 46:129–137 jest.2012.42.53
Aliabadi M, Morshedzadeh K, Soheyli H (2006) Removal of hexavalent chromium from aqueous solution by lignocellulosic solid wastes. Int J Environ Sci Technol 3:321–325. doi:10.1007/BF03325940
Aljeboree AM, Alshirifi AN, Alkaim AF (2014) Kinetics and equilibrium study for the adsorptionof textile dyes on coconut shell activated carbon. Arab J Chem. doi:10.1016/j.arabjc.2014.01.020
Al-Khatib L, Fraige F, Al-Hwaiti M, Al-Khashman O (2012) Adsorption from aqueous solution onto natural and acid activated bentonite. Am J Environ Sci 8:510–522. doi:10.3844/ajessp.2012.510.522
Babel S, Opiso ME (2007) Removal of Cr from synthetic wastewater by sorption into volcanic ash soil. Int J Environ Sci Technol 4:99–107. doi:10.1007/BF03325967
Bello OS, Bello IA, Adegoke KA (2013) Adsorption of dyes using different types of sand. A Rev S Afr J Chem 66:117–129
Ben Amor H, Mabrouk A, Talmoudi N (2015) Preparation of activated carbon from date stones:optimization on removal of indigo carmine from aqueous solution using a two-level full factorial design. Int J Eng Res Gen Sci 3:6–17
Ben Douissa N, Dridi-Dhaouadi S, Mhenni MF (2014) Study of antagonistic effect in the simultaneous removal of two textile dyes onto cellulose extracted from Posidonia oceanica using derivative spectrophotometric method. J Water Process Eng 2:1–9. doi:10.1016/j.jwpe.2014.03.004
Ben Hamissa AM, Brouers F, Ncibi MC, Seffen M (2013) Kinetic modeling study on methylene blue sorption onto Agave americana fibers: fractal kinetics and regeneration studies. Sep Sci Technol 48:1–9. doi:10.1080/01496395.2013.809104
Ben Hamissa AM, Lodi A, Seffen M, Finocchio E, Botter R, Converti A (2010) Sorption of Cd(II) and Pb(II) from aqueous solutions onto Agave americana fibers. Chem Eng J 159:67–74. doi:10.1016/j.cej.2010.02.036
Bilgin Simsek E, AvcıTuna AO, Beker U (2015) A statistical approach for arsenic adsorption onto Turkey clinoptilolite. Environ Sci Pollut Res 22:3249–3256. doi:10.1007/s11356-014-2975-8
Bouhamed F, Elouear Z, Bouzid J (2012) Adsorptive removal of copper(II) from aqueous solutions on activated carbon prepared from Tunisian date stones: equilibrium, kinetics and thermodynamics. J Taiwan Inst Chem Eng 43:741–749. doi:10.1016/j.jtice.2012.02.011
Bouhamed F, Elouear Z, Bouzid J, Ouddane B (2015) Multi-component adsorption of copper, nickel and zinc from aqueous solutions onto activated carbon prepared from date stones. Environ Sci Pollut Res (ICIME 2014). doi:10.1007/s11356-015-4400-3
Brouers F (2014a) Statistical foundation of empirical isotherms. Open J Stat 4:687–701. doi:10.4236/ojs.2014.49064
Brouers F (2014b) The fractal (BSf) kinetics equation and its approximations. J Mod Phys 5:1594–1601. doi:10.4236/jmp.2014.516160
Brouers F, Al-Musawi TJ (2015) On the optimal use of of isotherm models for the characterization of biosorption of lead onto algae. J Mol Liq 212:46–51. doi:10.1016/j.molliq.2015.08.054
Brouers F, Sotolongo O, Marquez F, Pirard JP (2005) Microporous and heterogeneous surface adsorption isotherms arising from Levy distributions. Physica A: Stat Mechan Appl 349(1):271–282. doi:10.1016/j.physa.2004.10.032
Brouers F, Sotolongo-Costa O (2006) Generalized fractal kinetics in complex systems (application to biophysics and biotechnology). Physica A 368:165–175. doi:10.1016/j.physa.2005.12.062
Căilean D, Barjoveanu G, Musteret CP, Sulitanu N, Manea LR, Teodosiu C (2009) Reactive dyes removal from wastewater by combined advanced treatment. Environ Eng Manag J, 8(3):503–511. http://omicron.ch.tuiasi.ro/EEMJ/pdfs/vol8/no3/25.
Deo Mall I, Taneja N, Thakur CK (2013) Treatment of indigo carmine dye bearing wastewater by electrocoagulation. 2nd International Conference on Environment, Agriculture and Food Sciences (ICEAFS’2013) August 25–26, 2013 Kuala Lumpur (Malaysia)
El-Ashtoukhy ESZ (2013) Removal of indigo carmine dye from synthetic wastewater by electrochemical oxidation in a new cell with horizontally oriented electrodes. Int J Electrochem Sci 8:846–858
Elkassimi M, Meziane D, Abouarnadasse S, Azizi H (1998) Elimination des colorants de l’industrie de textile par le charbon de bois. Proceeding de la 2ème coférence Maghrébine de Génie des Procédés:555–558
Forgacs E, Cserhatia T, Oros G (2004) Removal of synthetic dyes from wastewaters, a review. Environ Int 30:953–971. doi:10.1016/j.envint.2004.02.001
Freundlich H (1906) Über die adsorption in losungen. Z Phys Chem 57:385–470
García ER, Medina RL, Lozano MM, Pérez IH, Valero MJ, Maubert FAN (2014) Adsorption of azo-dye orange II from aqueous solutions using a metal-organic framework material: iron- benzenetricarboxylate. Materials 7:8037–8057. doi:10.3390/ma7128037
Ghadah A (2014) Removal of Congo red dye from aqueous solution by date palm leaf base. Am J Appl Sci 11(9):1553–1557. doi:10.3844/ajassp.2014.1553.1557
Hammami S, Oturan MA, Oturan N, Bellakhal N, Dachraoui M (2012) Comparative mineralization of textile dye indigo carmine by photo-Fenton process and anodic oxidation using boron-doped diamond anode. Desalin Water Treat 45:297–304
Ho YS (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59(1):171–177. doi:10.1023/B:SCIE.0000013305.99473.cf
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70(2):115–124. doi:10.1016/S0923-0467(98)00076-1
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. doi:10.1016/S0032-9592(98)00112-5
Karthik V, Saravanan K, Bharathi P, Dharanya V, Meiaraj C (2014) An overview of treatments for the removal of textile dyes. J Chem Pharm Sci 7:301–307
Kesraoui A, Moussa A, Ben Ali G, Seffen M (2015) Biosorption of alpacide blue from aqueous solution, by lignocellulosic biomass: Luffa cylindrica fibers. Environ Sci Pollut Res, (ICIME 2014). doi:10.1007/s11356-015-5262-4
Khodaie M, Ghasemi N, Moradi B, Rahimi M (2013) Removal of methylene blue from wastewater by adsorption onto ZnCl2 activated corn husk carbon equilibrium studies. Journal of Chemistry, ID 383985 (2013) 6. Doi:10.1155/2013/383985
Kirupavasam EK, AllenGnana Raj G (2012) Photocatalytic degradation of amido black-10B using nano photocatalyst. J Chem Pharm Res 4(6):2979–2987
Krika F, Azzouz N, Ncibi MC (2012) Removal of hexavalent chromium from aqueous media using Mediterranean Posidonia oceanica biomass: adsorption studies and salt competition investigation. Int J Environ Res 6(3):719–732
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 24(4):1–39
Lakshmi UR, Srivastava VC, Mall ID, Lataye DH (2009) Rice husk ash as an effective adsorbent: evaluation of adsorptive characteristics for indigo carmine dye. J Environ Manag 90(2):710–720. doi:10.1016/j.jenvman.2008.01.002
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc. 2221. Doi: 10.1021/ja02268a002
Low KS, Lee CK, Tan KK (1995) Biosorption of basic dye by water hyacinth roots. Bioresour Technol 52:79–83. doi:10.1016/0960-8524(95)00007-2
Maghri I, Kenz A, Elkouali M, Tanane O, Talbi M (2012) Textile dyes removal from industrial waste water by Mytilus edulis shells. J Mater Environ Sci 3(1):121–136
Mahmoudi K, Hosni K, Hamdi N, Srasra E (2015) Kinetics and equilibrium studies on removal of methylene blue and methyl orange by adsorption onto activated carbon prepared from date pits—a comparative study. Korean J Chem Eng 32(2):274–283. doi:10.1007/s11814-014-0216-y
Malik PK (2004) Dye removal from wastewater using activated carbon developed from sawdust: adsorption equilibrium and kinetics. J Hazard Mater 113:81. doi:10.1016/j.jhazmat.2004.05.022.
Matheswaran M, Karunanithi T (2007) Adsorption of Chrysoidine R by using fly ash in batch process. J Hazard Mater 145(1–2):154–161. doi:10.1016/j.jhazmat.2006.11.006
McKay G, Ramprasad G, Mowli P (1987) Desorption and regeneration of dye colours from low-cost materials. Water Res 21:375–377. http://hdl.handle.net/1783.1/35920.
Mittal J, Mittal L, Kurup L (2006) Batch and bulk removal of hazardous dye, indigo carmine from wastewater through adsorption. J Hazard Mater 137(1):591–602
Namasivayam C, Kavitha D (2002) Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dyes Pigments 54:47. doi:10.1016/S0143-7208(02)00025-6
Ncibi MC, Altenorc S, Seffen M, Brouerse F, Gaspard S (2008a) Modelling single compound adsorption onto porous and nonporous sorbents using a deformed Weibull exponential isotherm. Chem Eng J 145:196–202. doi:10.1016/j.cej.2008.04.001
Ncibi MC, Mahjoub B, Seffen M (2006) Studies on the biosorption of textile dyes from aqueous solutions using Posidonia oceanica (L.) leaf sheaths fibres. Adsorpt Sci Technol 24:461–473. doi:10.1016/j.jhazmat.2006.06.029
Ncibi MC, Mahjoub B, Seffen M (2007) Kinetic and equilibrium studies of methylene blue biosorption by Posidonia oceanica (L.) fibres. J Hazard Mater B139:280–285. doi:10.7202/019166ar
Ncibi MC, Mahjoub B, Seffen M (2008b) Étude de la biosorption du chrome (VI) par une biomasse méditerranéenne Posidoniaoceanica (L.) delile. Rev Sci Eau J Water Sci 21(4):441–449. doi:10.7202/019166ar
Newcombe G, Drikas M (1997) Adsorption of NOM activated carbon: electro-static and non-electrostatic effects. Carbon 35:1239–1250. doi:10.1016/S0008-6223(97)00078-X
Odogu AN, Daouda K, Desiré BBP, Nsami NJ, Mbadcam KJ (2016) Removal of indigo carmine dye (ic) by batch adsorption method onto dried cola nut shells and its active carbon from aqueous medium. Int J Eng Sci Res Technol 5(3):874–887. doi:10.5281/zenodo.48382
Ong ST, Lee CK, Zainal Z (2007) Removal of basic and reactive dyes using ethylenediamine modified rice hull. Bioresour Technol 98:2792–2799. doi:10.1016/j.biortech.2006.05.011
Ould Brahim I, Belmedani M, Belgacem A, Hadoun H, Sadaoui Z (2014) Discoloration of azo dye solutions by adsorption on activated carbon prepared from the cryogenic grinding of used tires. Chem Eng Trans 38:121–126. doi:10.3303/CET1438021
Rajurkar NS, Desa A (2015) Removal of crystal violet from aqueous solutions using Chitosan and Saraca indica leaves. J Appl Chem. 4(5):1446–1455. http://www.joac.info/
Ramesh TN, Sreenivasa VP (2015) Removal of indigo carmine dye from aqueous solution using magnesium hydroxide as an adsorbent. J Mater 10. doi:10.1155/2015/753057
Rehman R, Zafar J, Nisar H (2014) Adsorption studies of removal of indigo caramine dye from water by formaldehyde and urea treated cellulosic waste of Citrus reticulata peels. Asian J Chem 26(1):43–47. doi:10.14233/ajchem.2014.15305
Rodrigues CS, Madeira LM, Boaventura RA (2013) Treatment of textile dye wastewaters using ferrous sulphate in a chemical coagulation/flocculation process. Environ Technol 34(5–8):719–729. doi:10.1016/j.jhazmat.2009.08.027
Salleh MAM, Mahmoud DK, Karim WA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solidwastes: a comprehensive review. Desalination 280(1–3):1–13. doi:10.1016/j.desal.2011.07.019
Secula S, Cagnon B, Creţescu I, Diaconu M, Petrescu S (2011) Removal of an acid dye from aqueous solutions by adsorption on a commercial granular activated carbon: equilibrium, kinetic and thermodynamic study. M Chem Chem Eng, Biotechnol, Food Ind 12(4):307–322
Shul’gin LP, Kosyakov AL, Kochetkova RD, Petra VI (1975) Inventor’s Certificate N°529124, Byull.Izobret
Sivaramakrishna L, Sivasankar Reddy M, Jagadeesh M, Wan Zuhairi WY, Taha MR, Varada Reddy A (2014) Evaluation of biomass, Indian Jujuba Seed (IJS) for removal of Congo red. Am J Environ Sci 10:374–382. doi:10.3844/ajessp.2014.374.382
Stergiopoulos D, Dermentzis K, Giannakoudakis P, Sotiropoulos S (2014) Electrochemical decolorization and removal of indigo carmine textile dye from wastewater. Glob NEST J 16(3):499–506
Sumanjit K, Rani S, Mahajan RK (2013) Adsorption kinetics for the removal of hazardous dye Congo red by biowaste materials as adsorbents. Journal of Chemistry. ID 628582 (2013) 12
Sun D, Zhang Z, Wang M, Wu Y (2013) Adsorption of reactive dyes on activated carbon developed from Enteromorpha prolifera. Am J Anal Chem 4:17–26. doi:10.4236/ajac.2013.47A003
Saleh TA (2015) Isotherm, kinetic, and thermodynamic studies on Hg(II) adsorption from aqueous solution by silica-multiwall carbon nanotubes. Environ Sci Pollut Res 22:16721–16731. doi:10.1007/s11356-015-4866-z
Temkin MI (1941) Adsorption equilibrium and kinetics of process on non homogeneous surfaces and in the interaction between adsorbed molecules. J Phys Chem 15:296–233
Travlou NA, Kyzas GZ, Lazaridis NK, Deliyanni EA (2013) Graphite oxide/chitosan composite for reactive dye removal. Chem Eng J 217:256–265. doi:10.1016/j.cej.2012.12.008
Ucun H, Bayhan YK, Kaya Y (2008) Kinetic and thermodynamic studies of the biosorption of Cr(VI) by Pinus sylvestris Linn. J Hazard Mater 153:52. doi:10.1016/S0960-8524(02)00086-X
Vargas AMM, Cazetta AL, Kunita MH, Silva TL, Almeida VC (2011) Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): study of adsorption isotherms and kinetic models. Chem Eng J 168:722–730. doi:10.1016/j.cej.2011.01.067
Vijayageetha VA, Pandia Rajan A, Arockiaraj SP, Annamalai V, Janakarajan VN, Saravana Balaji MD, Dheenadhayalan MS (2013) Treatment study of dyeing industry effluents using reverse osmosis technology. Research Journal of Recent Sciences ISSN 2277–2502 (3) (ISC-2013) 58–61. www.isca.in
Wang L, Zhang J, Wang A (2008) Removal of methylene blue from aqueous solution using chitosan-g-poly(acrylic acid L)/montmorillonite superadsorbent nanocomposite. Colloids Surf A Physicochem Eng Asp 322(1–3):47–53. doi:10.1016/j.colsurfa.2008.02.019
Yang B, Liu Y, Li Z, Lei L, Zhou J, Zhang X (2016) Preferential adsorption of pentachlorophenol from chlorophenols-containing wastewater using N-doped ordered mesoporous carbon. Environ Sci Pollut Res (2016) 23:1482–1491. doi:10.1007/s11356-015-5384-8
Yurtsever M, Sengil IA (2009) Biosorption of Pb(II) ions by modified quebracho tannin resin. J Hazard Mater 163:58–64. doi:10.1016/j.jhazmat.2008.06.077
Zoughuir H, Khalef H, Bouras O, Chenouf N, Belkaiss D. (1998) Traitement des eaux résiduaires colorées de l’unité de SOITEX de Boufarik par adsorption sur argiles modifiées. Proceeding de la 3ème conférence Maghrébine de Génie des Procédés, Tome 3 pp 296–299
Acknowledgments
The authors of this study express their sincere thanks to the FP4BATIW project and the Laboratory of Energy and Materials for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kesraoui, A., Selmi, T., Seffen, M. et al. Influence of alternating current on the adsorption of indigo carmine. Environ Sci Pollut Res 24, 9940–9950 (2017). https://doi.org/10.1007/s11356-016-7201-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7201-4