Abstract
A novel approach to improve the efficiency of Fenton treatment for sludge reduction through the implication of a deflocculating agent citric acid, for the exclusion of extracellular polymeric substances (EPS) from waste-activated sludge (WAS), was investigated. Deflocculation was achieved with 0.06 g/g suspended solids (SS) of citric acid dosage. Fenton optimization studies using response surface methodology (RSM) revealed that 0.5 and 0.0055 g/g SS were the optimal dosages of H2O2 and Fe2+. The addition of a cation-binding agent set the pH value of sludge to 5 which did not affect the Fenton efficiency. The results presented in this study shows the advantage of deflocculating the sludge as SS and volatile suspended solids (VSS) reductions were found to be higher in the deflocculated (53 and 63 %, respectively) than in the flocculated (22 and 34 %, respectively) sludges. Kinetic investigation of the treatment showed that the rate of the reaction was four times higher in the deflocculated sludge than control. The methodology reported in this manuscript was successfully applied to a real case were the deflocculated mediated Fenton process reduced the sludge disposal cost from 297.8 to 61.9 US dollars/ton of sludge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The disposal of the excess sludge from waste water treatment plants causes major hazards to the environment. The most common processes employed in the degradation of the sludge excess, produced in water treatment plants, are aerobic and anaerobic. The general mechanism involved minimizes the excess sludge produced which helps to decompose the cell wall of microbes into protein and microbial degradation (Ning et al. 2015a, b; Tokumara et al. 2007).
Advanced oxidation process (AOP) is one of the pioneering techniques for waste water treatment. Many researchers (Chamarro et al. 2001; Badawy and Aline 2006; Bidga 1995; Ksibi 2006) elaborated the methods based on the AOP of contaminants. The Fenton reaction has attracted greater attention due to its unique advantages such as eco-friendliness and minimization of the excess sludge.
The utilization of Fenton’s reagent is the most effective method to treat the recalcitrant compound because the hydroxyl radicals formed during the reaction of ferrous sulfate to hydrogen peroxide have the tendency to degrade the aromatic and heterocyclic rings (Metcalf and Eddy 2003; Kavitha et al. 2015a). Furthermore, Fenton reaction has many advantages such as short reaction time compared with other advanced oxidation (ultraviolet, photocatalyst) processes. The most common Fenton’s reagents are iron and H2O2 which present a number of advantages such as cost-effectiveness and non-toxicity, no mass transfer limitations due to its homogenous catalytic nature, and no energy involved as catalyst, and the process is easy to run and control (Rathod et al. 2012).
Fenton’s reagent acts as the catalytic generation of hydroxyl radicals (·OH) coming out because of the chain response between ferrous ion and hydrogen peroxide. Equations 1 to 4 detail the oxidation of organic compounds (RH) by Fenton’s reagent (Kang and Hwang 2000; Neyens and Baeyens 2003).
The results from previous literature observations reveal that the effective impact of the Fenton reaction has been extended as a viable technique on the sewage sludge processing (Ning et al. 2014; Carey 1992; Prousek 1996). Although the prelude observations of this method creates strong base and opens wide opportunities for future research, further improvements of this technology are yet to be explored.
The organic polymers of extracellular polymeric substances (EPS) are positioned about the cell surface. Flocculant characteristic of activated sludge is due to the presence of EPS, which are accountable for the formation of microbial colonies and protection of microbial cells from external factors (Leroy et al. 2008; Orgaz et al. 2006). Thus, removal of EPS from sludge matrix can enhance biomass solubilization. According to Ping et al. (2004), EPS divulges that divalent ions like Ca2+ and Mg2+ bridge the majority of proteins in EPS. In addition, these divalent ions are also associated with a fraction of carbohydrate and nucleic acid. Due to the raise in Ca2+ and Mg2+ concentrations in EPS, the carbohydrate, nucleic acid, and protein contents are observed to be increasing. Many methods such as microwave conditioning (Peng et al. 2013), electrolysis (Yuan et al. 2010), and chemical method (Ye et al. 2012) have been developed for the removal of EPS. Citric acid is the most effective cation-binding agent that removes cations such as Ca2+ and Mg2+ from sludge flocs which helps the disruption of the floc structure. Due to easily biodegradable and environment-friendly nature, citric acid is chosen for deflocculation (Wawrzynczyk et al. 2007). There are only limited studies which explore deflocculation prior to treatment methods. However, deflocculation prior to the Fenton treatment has not been documented in the literature so far. The present study focuses on investigating the efficiency of deflocculation in the subsequent treatment. Thus, in this study, a novel and new method is adopted to improve the efficiency of Fenton treatment process through deflocculation of sludge.
Methods and materials
Sludge sampling
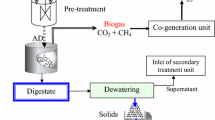
Municipal waste activated sludge was collected from the sewage treatment plant at Trivandrum (India). The collected sludge was stored at 4 °C in the laboratory for further studies. Initial characterization of the sludge is tabulated in Table 1.
Optimization of Fenton oxidation conditions by response surface methodology
Fenton oxidation conditions were optimized using response surface methodology (RSM). A central composite design (CCD), made with Design-Expert software, was employed to investigate the simultaneous effect of three independent variables: H2O2 dosage, Fe2+ dosage, and time. In this study, the optimum H2O2 dosage, Fe2+ dosage, and time for Fenton oxidation were analyzed through chemical oxygen demand (COD) and suspended solids (SS) reduction measurement of the sludge. Totally, 20 experimental runs were carried out. Based on the preliminary experiments, H2O2 dosage, Fe2+ dosage, and time varied in the range of 0.1–0.9 g/g SS, 0.001–0.01 g/g SS, and 0–60 min, respectively. The target response was COD and SS (mg/L) of sludge. To describe the response in the optimum CCD region, three factors for a full 23 factorial design with six central points, six axial points (with an axial design of ±1.63 forming an orthogonal design), and eight factorial points with 20 sets of experiments were planned.
Experimental procedure
Sludge deflocculation
The addition of citric acid with 100 mL of sludge was carried out in nine dosages (0.01 to 0.09 g/g SS concentration) using 250-mL conical flasks. The mixtures were constantly agitated in a shaker at 150 rpm for thorough mixing and incubated for 3 h (Kavitha et al. 2014a). Furthermore, the mixtures were examined for soluble EPS after proper centrifugation (10,000×g for 20 min).
Fenton oxidation
One hundred microliters of the sample was added with 0.06 g/g SS of citric acid in a 250-mL conical flask and agitated for 3 h to achieve deflocculation. Subsequently, Fe2+ (0.001 to 0.01 g/g SS) and H2O2 (0.1 to 0.9 g/g SS) were mixed in appropriate dosages to the parent solution and stirred to ensure homogeneous mixing. COD reduction and SS reduction tests were performed every 5 min in order to determine the efficiency of Fenton’s reagent which removed the organics during the chemical oxidation process. The complete experimental procedure was executed at room temperature, and the initial mixed liquor suspended solids (MLSS) of the diluted sample was set at 5000 mg/L and pH 5.
Analytical methods
Standard measurement methods were followed for COD, MLSS, and mixed liquor volatile suspended solids (MLVSS) parameters (APHA 2005). Total chemical oxygen demand (TCOD) of the sample was estimated by subjecting the homogeneous mixture of the sample to COD analysis, and soluble chemical oxygen demand (SCOD) was estimated by filtering the sample through a 0.45-μm filter and subjected to COD analysis (Kim et al. 2013). Loosely bound EPS (LB-EPS), tightly bound EPS (TB-EPS), and DNA were estimated by adopting the methods described by Yu et al. (2009). Enzyme activity (protease and amylase) measurements were calculated as per the methodology described by Kavitha et al. (2013).
Calculation of degree of deflocculation
Degree of deflocculation was calculated as per Eq. (5) to explain the extent of deflocculation process.
where
Energetic analysis and cost calculation
The cost assessment of treatment methods has to be taken into account on a pilot-scale treatment process. To evaluate the proficiency of the treatment process, energy studies and economic analysis were carried out for pilot-scale study with the experimental value obtained. For cost assessment, the calculation was made for 1 ton of sludge based on our previous study (Kavitha et al. 2015a, b). The energy employed for stirring in deflocculation and Fenton treatment was calculated based on Eq. 6 (Metcalf and Eddy 2003).
where
- P :
-
power required, W
- N p :
-
power number for impeller, unit less
- ρ :
-
density of sludge, kg/m3
- n :
-
revolutions per second, r/s
- D :
-
diameter of impeller, m
Results and discussions
Removal of EPS with citric acid
EPS accumulating on the cell surface of sludge biomass provides colloidal stability to the biomass (Burgess and Pletschke 2008). The EPS acts as a barrier and diminishes the subsequent biomass disintegration. Therefore, in the present study, EPS was removed with a cation-binding agent, citric acid, before cell disintegration. Citric acid could remove the cations that hold the EPS firmly (Wawrzynczyk et al. 2007). DNA was used as a marker to confirm the biomass disintegration.
Figure 1 depicts the impact of citric acid on LB-EPS, TB-EPS, and soluble EPS (SEPS). The amount of LB-EPS, TB-EPS, and SEPS in the untreated (without citric acid treatment) sludge was observed to be 35, 45, and 10 mg/L, respectively. After treating the sludge with citric acid, the exocellular polymers existing in TB-EPS and LB-EPS were solubilized to aqueous phase (the quantity of LB-EPS and TB-EPS got decreased and increased in soluble phase). The LB-EPS and the TB-EPS got decreased simultaneously due to the effective disruption of sludge matrix through citric acid. The citric acid removes the cations that hold the sludge EPS firmly which simultaneously releases the extracellular polymers from the TB-EPS and LB-EPS portion. Similar observation was noted by several researchers in their deflocculation studies. For example, Vimala et al. (2015) observed a simultaneous decrement in the LB-EPS and the TB-EPS while using the salt of citric acid (sodium citrate) for deflocculation of sludge matrix. Similarly, Kavitha et al. (2015b) proposed that usage of NaCl in deflocculation leads to a simultaneous decrement in the LB-EPS and the TB-EPS from the sludge matrix.
By totaling the LB-EPS and TB-EPS, the amount of removable exopolymers was calculated to be 80 mg/L. It can be seen from Fig. 1 that a notable decrease in LB-EPS (35 to 15 mg/L) and TB-EPS (45 to 18 mg/L) amount was observed up to 0.06 g/g SS of citric acid. Concurrently, an increase in soluble EPS quantity (50 mg/L) was observed up to the same dosage of citric acid (0.06 g/g SS), indicating the effective removal of extractable exopolymers. Hence, the degree of deflocculation was calculated as 80 %. Further increasing the citric acid dosage to 0.07 g/g SS, a steep increment in soluble EPS was observed (95 mg/L) which went beyond the maximal removable exopolymers (80 mg/L). This interprets the dominance of cell disintegration with the release of intracellular polymers into the medium. Based on the above discussion, it can be confirmed that the maximal removal of EPS with limited biomass disintegration was attainable only up to 0.06 g/g SS of citric acid. Additionally, the amount of soluble EPS and DNA increased with increment in dosage of citric acid up to 0.06 g/g SS. At this optimal dosage, their concentrations were noted to be 50 and 10 mg/L, respectively. An abrupt release of DNA (25 mg/L) was observed beyond 0.06 g/g SS of citric acid which specifies the biomass disintegration. The results of the present study confirm that the 0.06 g/g SS of citric acid dosage is observed to be optimal for effective removal of EPS with minimal biomass disintegration (Abac et al. 2009).
Disintegration efficiency of sludge decreases due to the immobilized exoenzymes which establish the contact probability with soluble organics. The influence of citric acid dosage on exoenzyme activity (protease and amylase) was monitored and illustrated in Fig. 1. The values of protease and amylase increased up to a dosage of 0.06 g/g SS of citric acid which was similar to the EPS release. Protease of 0.042 U/mL and amylase of 0.031 U/mL were observed at 0.06 g/g SS of citric acid dosage. The abrupt increases in protease and amylase concentrations were evident beyond the concentration of 0.06 g/g SS of citric acid which reflected the releases of intercellular enzymes by cell lysis. By this analysis, the citric acid concentration of 0.06 g/g SS is considered to be optimal for extracellular enzyme activity.
Optimization of Fenton reagent dosage and time
The central composite design was adopted for the Fenton treatment process. The efficiency of Fenton process is influenced by a variety of parameters such as H2O2 dosage, Fe2+ dosage, and time (Dewil et al. 2005; Barbusinski and Filipek 2000). In view of a strong association between the factors H2O2 dosage, Fe2+ dosage, and time on Fenton oxidation, optimization in terms of COD and SS reduction as suitable response factors was carried out.
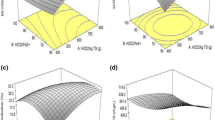
The outcome of the significant analysis of the model and its parameters is shown in Tables S1 and S2 (Supplementary information) for COD and SS reduction. The coefficients of the quadratic equation that are employed to calculate the optimum COD and SS reduction percentage were evaluated by regression analysis. The results of ANOVA analysis specifies that the calculated R 2 was found to be 0.9889 and 0.9826, respectively, which indicated a high degree of correlation between predicted and observed values. The adjusted and predicted R 2 values were found to be 0.9790 and 0.9269 for COD reduction and 0.9669 and 0.8866 for SS reduction, respectively, which indicated that the predicted R 2 value was in reasonable agreement with the adjusted R 2 value.
The quadratic equation and its coefficients are summarized as follows:
From Tables S1 and S2 (Supplementary information), a very high significant influence on the Fenton process in terms of COD and SS reduction was noted as the F values for the model were 99.82 and 62.63 with a p value of <0.0001, respectively. The outcomes showed that the H2O2 dosage and its quadratic term are most significant, whereas the Fe dosage and its quadratic term are less significant. The lack of fit value possesses F values of about 3.12 and 3.05 and p values of about 0.1190 and 0.1229 for COD and SS reduction, respectively. From the above, it can be concluded that the lack of fit is not significant and hence the model is more significant. As elaborated in the above discussion, the influence of the independent factors on the dependent parameters is illustrated in response surface plots (Figs. 2a–c and 3a–c). From Figs. 2a–c and 3a–c, the combined influence of Fe dosage, H2O2 dosage, and contact time on COD and SS reduction was assessed.
Utmost desirability of 100 % was achieved at 0.5 g/g SS of H2O2 dosage, 0.005 g/g SS of Fe2+ dosage, and time of 30 min. From the RSM results, it is specified that the three variables studied have a significant effect on the Fenton oxidation. There was an enhancement in COD and SS reduction at the central points when they were observed with the response of H2O2 dosage, Fe2+ dosage, and time as variables. The model reveals that the central point recommends the actual process optimization region. The central point corresponds to 0.5 g/g SS of H2O2 dosage, 0.005 g/g SS of Fe2+ dosage, and time of 30 min.
Effect of pH
It has been established that pH 3 is the optimum value for the Fenton reaction (Kavitha et al. 2015a). However, this highly acidic condition limits practical applicability of the Fenton process due to the demand of consumables to acidify as well as to neutralize the sample before and after the treatment. The pH of the raw sludge was measured to be 6.8. During deflocculation, the addition of citric acid changed the pH of the sludge to 5. The experiments were conducted over the pH range 3–6 to assess whether it is possible to make use of an advantage caused by citric acid. From Fig. 4a, b, the maximum amount of COD and SS reduction are observed at pH 3 and their respective values were measured to be 73 and 56 %, respectively. At pH 5, the COD and SS reduction percentage was recorded to be 71 and 53 %, respectively. The variation of pH from 3 to 5 does not cause any significant reduction in the Fenton process. To statistically validate the results, a standard t test was performed. The p value for COD and SS reduction was calculated to be 0.31 and 0.22, respectively. This shows that the COD and SS reduction between the pH range from 3 to 5 is insignificant. Considering the above factor, it has been decided to conduct further experiments by keeping the sludge at pH 5.
Effect of deflocculation on the Fenton treatment
Effect of EPS removal on the efficiency of the Fenton treatment is the focus of this investigation. In order to correlate the advantage of EPS removal, simultaneous experiments were carried out with flocculated (with EPS and Fenton treatment) and deflocculated (EPS removed and Fenton treatment) sludges. Treatment outputs such as COD and SS reduction are considered to be the main parameters for the evaluation of the Fenton process.
Total COD reduction and suspended solids reduction
COD and SS reduction results of both flocculated and deflocculated sludges are illustrated in Fig. 5a, b, respectively. Oxidative decomposition of microbial cell wall and mineralization caused by OH radicals from the Fenton reaction are observed to be the main cause for the drastic increase in the COD reduction during the first 30 min of reaction time. H2O2 concentrations have considerable impact on organic substances; at low dosages, OH radicals are highly reactive. On the other hand, at high dosages, H2O2 proceeds as a scavenger of the OH radicals. Equations 7 and 8 detail the scavenging effect of H2O2 on OH radicals (Feng et al. 2003; Qiu et al. 2013).
The COD and SS reduction was observed to be 72 and 53 % for the deflocculated sludge, whereas in the flocculated sludge, this was found to be 34 and 22 %, respectively. The results show that suspended solids and COD reduction is enhanced by deflocculation. The presently obtained SS reduction value of 53 % is comparatively higher than 24 % which was reported for a biological mode of sludge reduction (Kavitha et al. 2013). In addition, the Fenton-mediated sludge reduction happens in relatively short retention time (30 min), when compared to other treatment processes (Vimala et al. 2015). As a consequence of the factors stated above, our proposed process reduces capital investment as well as making the process more attractive for subsequent scale up. Interestingly, SCOD of the Fenton-treated sludge was found to be 100 mg/L and this gives an advantage of dewatering sludge directly. In the Fenton process, during the sludge disintegration, the SCOD release was simultaneously followed by its mineralization, which is the reason for low SCOD values (Mo et al. 2015). However, compared to other sludge disintegration methods such as physical, chemical, and biological methods, the SCOD of the sludge remains high in the order of 2000 to 5000 mg/L which is due to the focus of SCOD release only (Merrylin et al. 2014; Kavitha et al. 2014b; Bougrier et al. 2005). The presence of high SCOD increases the sludge viscosity (Rajesh Banu et al. 2011) which in turn increases the capillary suction time and it makes dewatering of the sludge difficult (Rajesh Banu et al. 2012). Hence, subsequent treatment is essential to reduce this SCOD which demands energy and cost. The MLSS value of WAS from the return line of secondary clarifier was in the range of 5 to 7 g/L (Uan et al. 2010). Since the objective of this study is to reduce the sludge from the secondary clarifier, the suitability of the optimized citric acid and Fenton dosage was further evaluated by varying the MLSS concentration to 7 g/L. The evaluation did not show any significant deviation in the citric acid nor in the Fenton dosage, as it was calculated based on the SS concentration of the sludge.
Total volatile suspended solids reduction
The organic fraction of the sludge was estimated in terms of volatile suspended solids (VSS). The effectiveness of the sludge management depends on the reduction of organic rather than inorganic portion of the sludge (Metcalf and Eddy 2003). Variations on VSS reduction for both flocculated and deflocculated samples during the Fenton treatment for different time intervals are expressed in Fig. 6. It is evident that the VSS reduction increases linearly during the initial reaction time from 0 to 30 min and attains a maximum reduction of about 34.8 and 63 % for flocculated and deflocculated sludges, respectively. It is also observed that the reduction of VSS was relatively higher in the deflocculated sludge than in the flocculated sludge. The MLVSS-to-MLSS ratio was 0.76 at the beginning of the treatment. After the treatment, the value was reduced to 0.28, indicating the sludge degradation of the organic portion by the Fenton process. This makes the sludge handling process easy as the management of inorganic compounds was comparatively easier than the organic portion of the sludge (Metcalf and Eddy 2003).
Kinetic assessment of the treatment process
The influence of the citric acid which mediated the Fenton process on sludge treatment was assessed, and the results were evaluated through kinetic analysis. The kinetic analysis was carried out based on the literature (Kavitha et al. 2014a).
Table 2 summarizes the kinetic parameters obtained through the first-order reaction. In the SS reduction, the rate constant values of the flocculated and deflocculated samples were calculated to be 0.0087 and 0.0286 per min, respectively, whereas in the COD reduction, the rate constant values were measured to be 0.0178 and 0.044 per min for the flocculated and deflocculated samples, respectively. Table 2 shows that the k value of the deflocculated sample was observed to be higher when compared to that of the flocculated sample. This implies that the reaction rate was found to be faster and rapid in the deflocculated samples due to easier accessibility of substrates for the Fenton reaction. The goodness of fit of both the sludges was between 0.92 and 0.99, indicating a better fit between the model and experiment.
Economic analysis
Field applicability of any treatment method depends upon its economic viability. Table 3 presents the data on economic analysis of the present study. In comparison to other treatment methods used for sludge reduction (ozonation, ultrasonication, and microwave irradiation), the Fenton treatment is advantageous as it demands low capital investment and easy operation. The estimation cost for the present study was based on operational cost associated with the Fenton treatment. The operational cost calculation takes into account energy and consumable cost associated with the treatment. The energy required for agitating 1 ton of the sludge during the EPS removal and the Fenton process was calculated and tabulated in Table 3. The energy cost to dewater the sludge in filter press was calculated to be 77.5 kWh (Yang et al. 2011). The total cost in US dollars were calculated to be 18.4, 20, and 17.8 to meet out the energy demand for the flocculated sludge, deflocculated sludge, and control, respectively. Similarly, the cost required for consumables was calculated and tabulated. In Table 3, from the total cost, the cost benefit achieved by reduction in SS to be disposed was subtracted (Kavitha et al. 2015b, c). Table 3 shows the net cost of −61.9 and −226.4 USD/ton that was achieved for the deflocculated sludge and flocculated sludge, respectively. It can be concluded that the Fenton process was observed to be an economically viable method for the sludge reduction.
Conclusions
The sludge was successfully deflocculated with a minimum biomass damage of 0.06 g/g SS for a citric acid dosage. The pH correction is not warranted in this study as there is no statistically significant difference between the Fenton process efficiency at its optimum pH 3 and the deflocculated sludge at medium pH 5. The high efficiency of our proposed methodology can be measured by comparing the outcome of the disintegration process in which the deflocculated sludge has the maximum COD and SS reduction percentage of 72 and 53 % compared to 34 and 22 % for the flocculated sludge. The high degree of VSS profile of the Fenton treatment indicates the degradation of the organics. The reduction in organic portion of sludge makes it easy to undergo subsequent management process such as dewatering and disposal. In terms of the cost efficiency, it can be concluded that the process is economically viable since a net cost of −61.9 and −226.4 USD/ton was achieved for the deflocculated and flocculated sludges.
References
Abac PD, Francois B, Eric VH, Piet NLL, Gilles G (2009) Extraction of extracellular polymeric substances from anaerobic granular sludges comparison of chemical and physical extraction protocols. Appl Microbiol Biotechnol 85:1589–1599
APHA (2005) Standard methods for the examination of water and wastewater, 21 edn. American Public Health Association, Washington, DC
Badawy MI, Aline M (2006) Fenton’s peroxidation and coagulation processes for the treatment of combined industrial and domestic waste water. J Hazard Mater B136:961–966
Barbusinski K, Filipek K (2000) Aerobic sludge digestion in the presence of chemical oxidizing agents part II. Fenton’s reagent. Pol J Env S 9(3):145–149
Bidga RJ (1995) Consider Fenton chemistry for wastewater treatment. Chem Eng Process 91(12):62–66
Bougrier C, Albasi C, Delgenes JP (2005) Solubilization of waste activated sludge by ultrasonic treatment. Chem Eng J 106:163–169
Burgess JE, Pletschke BI (2008) Hydrolytic enzymes in sewage sludge treatment a mini-review. Water SA (online) 34(3):343–349
Carey JH (1992) An introduction to advanced oxidation processes (AOP) for destruction of organics in waste water. Water Pollut Res J Can 27:1–21
Chamarro E, Marco A, Esplugas S (2001) Use of Fenton reagent to improve organic chemical biodegradability. Water Res 35(4):1047–1051
Dewil R, Baeyens J, Neyens E (2005) Fenton preoxidation improves the drying performance of waste activated sludge. J Hazard Mater 117(2–3):161–170
Feng JY, Hu XJ, Yue PL, Zhu HY, Lu GQ (2003) Decoloration and mineralization of reactive red HS-3B by heterogeneous photo-Fenton reaction. Water Res 27:3776–3784
Kang YW, Hwang KY (2000) Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res 34:2786–2790
Kavitha S, Adishkumar S, Yogalakshmi KN, Kaliappan S, Rajesh Banu J (2013) Effect of enzyme secreting bacterial pretreatment on enhancement of aerobic digestion potential of waste activated sludge interceded through EDTA. Bioresour Technol 150:210–219
Kavitha S, Adishkumar S, Kaliappan S, Yeom IT, Rajesh Banu J (2014a) Improving the amenability of municipal waste acitivated sludge for biological pretreatment by phase-separated sludge disintegration method. Bioresour Technol 169:700–706
Kavitha S, Jayashree C, Adish Kumar S, Kaliappan S, Rajesh Banu J (2014b) Enhancing the functional and economical efficiency of a novel combined disperser disintegration of waste activated sludge for biogas production. Bioresour Technol 173:32–41
Kavitha S, Kaliappan S, Adish Kumar S, Yeom IT, Rajesh Banu J (2015a) Effect of NaCl induced floc disruption of biological disintegration of sludge for enhanced biogas production. Bioresour Technol 192:807–811
Kavitha S, Karthika P, Rajesh Banu J, Yeom IT, Adish kumar S (2015b) Enhancement of waste activated sludge reduction potential by amalgamated solar photo-Fenton treatment. Desalin. Water Treat 1–13.
Kavitha S, Kaliappan S, Adish Kumar S, Yeom IT, Rajesh Banu J (2015c) Achieving profitable biological sludge disintegration through phase separation and predicting its anaerobic biodegradability by nonlinear regression model. Chem Eng J 279:478–487
Kim D, Cho S, Lee M (2013) Increased solubilization of excess sludge does not always result in enhanced anaerobic digestion efficiency. Bioresour Technol 143:660–664
Ksibi M (2006) Chemical oxidation with hydrogen peroxide for domestic and waste water treatment. Chem Eng J 119(2–3):161–165
Leroy C, Derbarre C, Gillebaert F, Compere C, Combes D (2008) Effect of commercial enzymes on the adhesion of a marine biofilm forming bacterium. Biofouling 24:11–22
Merrylin J, Kaliappan S, Adish Kumar S, Yeom IT, Rajesh Banu J (2014) Enhancing aerobic digestion potential of municipal waste-activated sludge through removal of extra polymeric substance. Environ Sci Pollut Res 21:1112–1123
Metcalf and Eddy (2003) Wastewater engineering treatment and reuse, fourth edn. Mc Graw Hill Publication
Mo R, Huang S, Dai W, Liang J, Sun S (2015) A rapid Fenton treatment technique for sewage sludge dewatering. Chem Eng J 269:391–398
Neyens E, Baeyen J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater B98:33–50
Ning X, Chen H, Wu J, Wang Y, Liu J, Lin M (2014) Effects of ultrasound assisted Fenton treatment on textile dyeing sludge structure and dewaterability. Chem Eng J 242:102–108
Ning X, Wen W, Zhang Y, Li R, Jiansun WY, Yang Z, Liu J (2015a) Enhanced dewaterability of textile dyeing sludge using micro-electrolysis pretreatment. J Environ Manag 161:181–187
Ning X, Feng Y, Wu J, Chenwang C, Sun J, Chang K, Zhang Y, Yang Z, Liu J (2015b) Effect of K2FeO4/US treatment on textile dyeing sludge disintegration and dewaterability 162: 81–86.
Orgaz B, Kives J, Pedregosa AM, Monistrol IF, Laborda F, Sanjose C (2006) Bacterial biofilms removal using fungal enzymes. Enzym Microb Technol 40:51–56
Peng G, Ye F, Ye Y (2013) Effects of microwave irradiation on dewaterability and extracellular polymeric substances of waste activated sludge. Water Environ Res 85:278–285
Ping GS, Yu H, Yu Z (2004) Extraction of extracellular polymeric substances from the photosynthetic bacterium Rhodopseudomonas acidophila. Appl Microbiol Biotechnol 67:125–130
Prousek J (1996) Advanced oxidation processes for water treatment. Chem Process Chem Lis 90(4):229–237
Qiu S, Xia M, Li Z (2013) Ultrasonic irradiation as pretreatment for the reduction of excess sludge by Fenton-acclimation treatment. Water Sci Technol 67(8):1701–1707
Rajesh Banu J, Uan DK, Kaliappan S, Yeom IT (2011) Effect of sludge pretreatment on the performance of anaerobic/anoxic/oxic membrane bioreactor treating domestic wastewater. Int J Environ Sci Technol 8(2):281–290
Rajesh Banu J, Uan DK, Adish Kumar S, Kaliappan S, Yeom IT (2012) A novel method of sludge pretreatment using the combination of alkalis. J Environ Biol 33:249–253
Rathod JD, Shukla RN, Singh DM (2012) Combination of advanced oxidation process and biological treatment of industrial waste water. Int J Eng Sci Technol 1(3):24–28
Tokumura M, Sekine M, Yoshinari M, Znad H, Kawase Y (2007) Photo-Fenton process for excess sludge disintegration process. Biochemistry 42(4):627–633
Uan K, Nguyen TT, Banu JR, Kim K, Yeom IT (2010) Effects of thermochemical pretreatment on the biodegradability of sludge from a biological wastewater treatment plants. Maejo Int J Sci Technol 4:250–260
Vimala AE, Kaliappan S, Adish Kumar S, Yeom IT, Rajesh Banu J (2015) Influence of deflocculation on microwave disintegration and anaerobic biodegradability of waste activated sludge. Bioresour Technol 185:194–201
Wawrzynczyk I, Recktenwald M, Norrlow O, Dey ES (2007) Solubilisation of sludge by combined chemical and enzymatic treatment. Afr J Biotechnol 6(17):1994–1999
Yang GCC, Chen M-C, Yeh C-F (2011) Dewatering of a biological industrial sludge by electrokinetics-assisted filter press. Sep Purif Technol 79(2):177–182
Ye F, Liu X, Li Y (2012) Effects of potassium ferrate on extracellular polymeric substances (EPS) and physicochemical properties of excess activated sludge. J Hazard Mater 199:158–163
Yu G, He P, LM S (2009) Characteristics of extracellular polymeric substances (EPS) fractions from excess sludges and their effects on bioflocculability. Bioresour Technol 100:3193–3198
Yuan H, Zhu N, Song L (2010) Conditioning of sewage sludge with electrolysis: effectiveness and optimizing study to improve dewaterability. Bioresour Technol 101:4285–4290
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Rights and permissions
About this article
Cite this article
Amudha, V., Kavitha, S., Fernandez, C. et al. Effect of deflocculation on the efficiency of sludge reduction by Fenton process. Environ Sci Pollut Res 23, 19281–19291 (2016). https://doi.org/10.1007/s11356-016-7118-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7118-y