Abstract
Continuous monitoring of chemicals in the environment is important to control their fate and to protect human health, flora, and fauna. Perfluoroalkyl acids (PFAAs) have been detected frequently in different environmental compartments during the last 15 years and have drawn much attention because of their environmental persistence, omnipresence, and bioaccumulation potential. Water is an important source of their transport. In the present study, distributions of PFAAs in river water, wastewater treatment plant (WWTP) effluent, and tap water from eastern part of Germany and western part of Kenya were investigated. Eleven perfluorocarboxylic acids (PFCAs) and five perfluorosulfonic acids (PFSAs) were analyzed using liquid chromatography/tandem mass spectrometry. Sum of mean concentrations of eight PFAAs detected in drinking tap water from Leipzig was 11.5 ng L–1, dominated by perfluorooctanoic acid (PFOA, 6.2 ng L–1). Sums of mean riverine concentrations of PFAAs detected in Pleiße/White Elster, Saale, and Elbe (Germany) were 24.8, 54.3, and 26.8 ng L–1, respectively. Annual flux of PFAAs from River Saale was estimated to be 164 ± 23 kg a–1. The effluent of WWTP in Halle was found to contain four times higher levels of PFAAs than river water and was dominated by perfluorobutane sulfonate (PFBS) with 32 times higher concentration than the riverine level. It advocates that WWTPs are the point source of contaminating water bodies with PFAAs, and short-chain PFAAs are substituting long-chain homologues. Sums of mean riverine concentrations of PFAAs in Sosiani (Kenya) in samples from sparsely populated and densely populated areas were 58.8 and 109.4 ng L–1, respectively, indicating that population directly affected the emissions of PFAAs to surface waters. The discussion includes thorough review and comparison of recently published literature reporting occurrence of PFAAs in aqueous matrices.

Perfluoroalkyl acids in aqueous matrices
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perfluoroalkyl acids (PFAAs) are aliphatic acids in which all the hydrogen atoms bound to carbon chain are substituted with fluorine atoms in such a manner that they consist of a perfluoroalkyl moiety (C n F2n + 1–) in addition to the acid functional group such as –COOH (perfluoroalkyl carboxylic acid, PFCA) or –SO3H (perfluoroalkane sulfonic acid, PFSA) (Buck et al. 2011; Cousins 2013). Because of unrivalled characteristics such as hydrophobicity, oleophobicity, great chemical and thermal stability, and surface activity, PFAAs and their precursors have been used in several products such as nonstick cookware, cosmetics, varnishes, lubricants, fire extinguishers, and coatings of paper, textiles, carpets, and leather (Kissa 2001; Buck et al. 2011; Cousins 2013). Thousands of tons of PFAAs and their precursors have been produced in North America, Europe, China, and Japan, and alarming amounts have been released to the environment via direct and indirect sources (Prevedouros et al. 2006; Paul et al. 2009).
In 2001, reports of widespread presence of PFAAs in human blood (Hansen et al. 2001) and wildlife even in fewer urbanized locations such as the Arctic and the North Pacific Oceans (Giesy and Kannan 2001) have raised the level of awareness about PFAAs. Since then, scientists have assiduously studied the environmental fate and toxicity of these substances. PFAAs with 8-carbon atoms, i.e., perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), are the most widely studied PFAAs because of their omnipresence, persistence, bioaccumulation potential, and toxicological effects (Perkola and Sainio 2013), and both are important components of “exposome” (Wild 2005). PFOS and its salts have been included in the annex B of Stockholm Convention on persistent organic pollutants (UNEP 2009), and major producers such as 3M have stopped its production (USEPA 2000). However, manufacturing of PFOS and its precursors is still going on in some parts of the world (Xie et al. 2013; Chen et al. 2015; Löfstedt Gilljam et al. 2015). PFOA was planned to be voluntarily eliminated from service until the end of last year according to the Stewardship program (USEPA 2006). Even when the production of PFOS and PFOA is completely stopped, emissions from products may continue for several years (Lindim et al. 2015).
Extensive evidence compels that it is almost impossible to avoid exposure to PFAAs and their precursors because of their ubiquitous presence (Miralles-Marco and Harrad 2015). Dietary intake (Herzke et al. 2013; Vestergren et al. 2013), drinking water (Eriksson et al. 2013), dust ingestion, and inhalation (Shoeib et al. 2016) are the major direct exposure pathways (Zushi et al. 2012). Figure 1 shows in a simplistic manner how human beings can be exposed to PFAAs. Once these substances find their way to enter the body, they can bind with blood protein with estimated half-lives of human serum elimination to be 3.8 and 5.4 years for PFOA and PFOS, in that order (Olsen et al. 2007). Levels in humans are influenced by several factors such as gender, age, smoking, BMI, race, and ethnicity (Ode et al. 2013). It has been found that PFAAs can cause hepatotoxicity, carcinogenicity, immunotoxicity, developmental toxicity, and endocrine disruption (Kjeldsen and Bonefeld-Jørgensen 2013; Long et al. 2013). The underlying biochemical mechanism of toxic action of PFAAs is not fully understood, but it may be associated with the activation of nuclear receptor peroxisome proliferator-activated receptor alpha (Lau 2012).
PFAAs draw further attention because several poly- and perfluoroalkyl substances (PFAS) such as fluorotelomer alcohols (FTOH) and perfluorooctane sulfonamide (FOSA) biodegrade aerobically to PFAAs (Keränen et al. 2013; Liu and Mejia Avendaño 2013). PFAAs can be frequently detected in water because of their hydrophilic functional group, low vapor pressure, and moderate sorption (Paul et al. 2009). It can be expected that several dozens of omnipresent PFASs may eventually end up as PFAAs in rivers, streams, lakes, and seas. Riverine concentrations of PFAAs vary with the flow and temperature of water, and main sources of their presence in surface waters are wastewater treatment plants (WWTPs) (Filipovic and Berger 2015), surface runoff (Perkola and Sainio 2013), and dry and wet deposition (Rayne et al. 2009). Several authors have reported the surface water concentrations of PFAAs, which vary depending on several factors such as sampling location, season, industrial growth status of the country, ongoing manufacturing practices, human population, and their wealth status (local gross domestic product (GDP) (Lindim et al. 2015): for instance, recently reported riverine concentrations of PFASs (ΣPFAS) in Germany are 7.9–18 ng L–1 during summer and 4.1–249 ng L–1 during winter (Zhao et al. 2014), while in China 12.8–9540 ng L–1 during summer and 1.77–2180 ng L–1 during winter (Zhu et al. 2015). Other examples are: India 1.79–18.65 ng L–1 (Sharma et al. 2016), Italy 1–2886 ng L–1 (Valsecchi et al. 2015), Japan 49.20–260.90 ng L–1 (Niisoe et al. 2015), Kenya 0.4–109.63 ng L–1 (Orata et al. 2009), Korea 1.17–40.63 ng L–1 (Lam et al. 2014), Spain 49.20–260.90 ng L–1 (Lorenzo et al. 2016), and Vietnam 1–17 ng L–1 (Duong et al. 2015).
Domestic and industrial WWTPs play a major role in contaminating surface waters with PFAAs (Oliaei et al. 2013). Higher levels of perfluorobutane sulfonic acid (PFBS), PFOS, and PFOA can be frequently detected in WWTP effluents compared to influents because of widespread presence of PFAA precursors in incoming waste streams and subsequently their microbial degradation during treatment processes (Liu and Mejia Avendaño 2013). PFAAs in WWTPs are either from ongoing primary emissions or because of their recirculation. Filipovic and Berger (2015) found that tap water is an important source of PFAA recirculation and contributed more than 40 % of PFSAs and 30 % of PFCAs to WWTP influents. It is, therefore, necessary to distinguish between actual emissions and recirculation, especially when elevated levels of PFAAs are observed in tap water. Importance of tap water increases further as it is a main drinking water source in many countries. Regular use of drinking water even with low levels of PFAAs can considerably increase total human exposure with a serum/drinking water ratio of approximately 100:1 (Post et al. 2012). Situation worsens dramatically when infants are fed on milk from mothers drinking contaminated water or with the formula prepared with contaminated water because so-called low levels may not be pernicious for adults but can retard growth in toddlers. Because of the reasons given, continuous monitoring of PFAAs, especially effluent of WWTPs, rivers, and drinking water, is obligatory for controlling and managing their transport behavior and associated risks to human health and environment (DWI 2009; UNEP 2009; EU Directive 2013; USEPA SNUR 2015; CEPA 2016).
In the present study, different aqueous samples have been analyzed to measure the concentrations of 11 PFCAs and 5 PFSAs using UHPLC-MS/MS. Tap water has been collected only from one location in Leipzig (Germany) and therefore may not be considered to represent the quality of tap water in the whole city. Two locations have been selected in Leipzig (Germany) to collect surface water samples, one at River Pleiße (a right tributary of the River Weiße Elster or White Elster) and the other at River White Elster, which is a right tributary of the River Saale and eventually flows into it in Halle. Samples were taken from River Saale in Halle (Germany), which is a left-bank tributary of the River Elbe. More samples were collected from an effluent of municipal wastewater treatment plant (Halle). Additionally, water samples were supplied from River Sosiani running through the town of Eldoret in Kenya. To our best knowledge, limited information is available regarding the occurrence of PFAAs in surface waters of areas mentioned above. Further samples were grabbed from River Elbe close to the border of Czech Republic. A 500-mL water sample from IJ, Amsterdam (Netherlands) was provided by IVM Institute of Environmental Studies during sixth inter-laboratory study (van der Veen et al. 2014).
Materials and methods
Chemicals, reagents, and standards
Standard solutions of PFAAs (2 μg mL–1) and mass-labeled standards (50 μg mL–1) in methanol were provided by Wellington Laboratories Inc., Canada. Methanol (LiChrosolv®), acetonitrile (LiChrosolv®), formic acid (Emsure®, 98–100 %), ammonium acetate (Emsure®, ACS Reag.), and HPLC grade water (LiChrosolv®) were purchased from Merck, Germany. Thomas Geyer GmbH & Co. KG and Riedel-de Haën, Germany provided acetone (99.8 %) and ammonia (25 %), respectively. PFAAs were quantified using the internal standard method. Standard solutions were prepared with successive dilution of stock solution of PFAAs by adding a methanol/water (60:40) mixture to it following addition of mass-labeled internal standards making total volume of the solution 200 μL in suitable LC plastic vials with plastic caps. Calibration was performed prior to analysis of samples and continuously checked during the analysis. In addition to calibration standards and samples, laboratory reagent blank (LRB) and field reagent blank (FRB) were analyzed. LRBs were prepared using double-distilled water and were analyzed like real samples. FRBs were made of double-distilled water placed in plastic containers and were treated as samples in all respects, including transport and exposure to sampling site, storage, and analysis.
Surface water samples
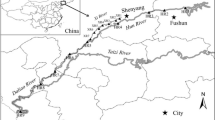
Surface water samples were collected in thoroughly washed and dried high-density polyethylene (HDPE) bottles from several locations. Methanol (5 mL) and mass-labeled surrogates were added to each bottle. Prior to collect sample, bottles were washed three times with sampling water. Temperature and pH of water were noted at the sampling site. Bottles were closed tightly, placed in an icebox, and transferred to the laboratory where they were stored at 4 °C in refrigerator (Shoemaker et al. 2009). It is recommended that water samples should be extracted within 14 days and extracts should be analyzed within 28 days after extraction (Shoemaker et al. 2009). Furthermore, recovery measurements and method validation were carried out using laboratory fortified blank (LFB), prepared by spiking double-distilled water with PFAAs (25 ng L–1). Details of all the sampling sites and their coordinates are shown in Fig. 2. In short, ten samples each from two different locations at River Saale, Germany, ten samples from an effluent of a treatment plant in Halle, Germany, two to six samples from three separate locations at River Sosiani, Kenya, two to four samples from River Elster and Pleiße in Leipzig, Germany, two samples from a location at River Elbe, and two tap water samples from our department were collected and analyzed at the start of 2015. Additionally, IVM Institute for Environmental Studies provided water sample from IJ, Amsterdam during sixth inter-laboratory study at the end of 2013.
Different steps involved in analysis of PFAAs. Table contains details of the sampling sites, number of samples n, and geographic coordinates of sampling locations. Further details are in Electronic Supplementary Material
Solid phase extraction (SPE)
Water samples (500 mL) were passed through Chromabond® HR-XAW columns (150 mg, 6 mL) without filtration. Conditioning, washing, and elution were carried out following DIN 38407-42 (2011) standard method with some modifications. SPE cartridge was conditioned with 1 % ammonia in methanol (5 mL) followed by methanol (5 mL) and finally twice with double-distilled water (5 mL). As soon as the water passed through, sample was loaded in the cartridge using a vacuum pump at a flow rate of approximately 5–10 mL min–1. Afterwards, sample bottle was washed with 10 mL double-distilled water, which was transferred into the cartridge. In the end, cartridge was washed with double-distilled water (5 mL), acetone/acetonitrile/formic acid (50:50:1, 5 mL), and methanol (2 mL) in that order. The pump was kept on for 15 min to further remove liquid out of the cartridge completely. Afterwards, PFAAs were eluted with methanol (2 mL), 1 % ammonia in methanol (4 mL), and methanol (4 mL) in that order at a slow flow rate (2–3 mL min–1) in thoroughly washed plastic tubes. The extracts were evaporated to reduce volume under a gentle stream of nitrogen in heated water bath (40 °C). Methanol/water (60:40) mixture was added to each tube following addition of mass-labeled internal standards and was vortexed to dissolve PFAAs. Following that, contents of the tubes were transferred into designated plastic vials with plastic caps, which were placed in the LC autosampler in proper order together with blanks and calibration standards.
Analysis with UHPLC-MS/MS
UHPLC-MS/MS analysis was performed with Agilent 1290 system equipped with binary pump SL 1200 and triple quadrupole MS 6460 in negative ESI mode using multiple reaction monitoring (MRM). Ten-microliter solution was injected to a column (Zorbax Eclipse XDB-C18 Rapid Resolution 100 mm, 1.8 μm) at 30 °C. Mobile phase was 5 mmol L–1 ammonium acetate in HPLC grade water (A) and 0.05 % acetic acid in methanol (B) with a flow rate 0.4 mL min–1. The mobile phase was kept at 30 % of B in start for 2 min followed by gradual change to 90 % of B in next 2 min, held at this point for a minute, and eventually sharply back to initial state (30 % B) in the end. Before analysis of samples, method was optimized, and parameters such as recovery rates and detection limits were calculated. Different steps involved in the analysis of PFAAs in surface water samples are shown in Fig. 2.
Quality assurance
Plastic apparatus and vials were used throughout the work and contact with Teflon was avoided as much as practically possible. Apparatus has been washed rigorously with methanol and double-distilled water and dried at 70 °C overnight prior to use. Polypropylene vials and caps were used to prevent contamination of the sample from PTFE-coated septa. However, such vials do not reseal completely; therefore, vials were not used multiple times, and fresh calibration standards were used each time. Laboratory and field blanks have been analyzed to confirm the potential background interference. To reduce background interference, some researchers have used PFAS isolator columns prior to injector to trap contaminants in mobile phase and delay their elution (Vestergren et al. 2013).
Results and discussion
PFAAs are distributed in surface waters all over the world, and their distribution pattern depends on hydrophobicity or chain length and the nature and acidity of the acid functional group (Nordén et al. 2013) as well as industrial and development status of the area. Perfluoroalkyl chain length is crucial to define the physicochemical behavior of PFAAs, but its “phobic” nature makes the polar functional group main culprit for the widespread presence of PFAAs in surface waters. Here, distribution pattern of 16 PFAAs in different types of water samples (riverine, effluent, and tap) is evaluated using UHPLC-MS/MS. Gradient program was optimized to separate and quantify 11 PFCAs and 5 PFSAs using standards composed of linear isomers of PFAAs and mass-labeled internal standards. Details of the analytical parameters of the current investigation of PFAAs in surface waters, precursor and product ions, retention times, detection limits, and percentage recoveries for six replicates of 25 ng L–1 solutions of PFAAs are provided in Table 1. Method detection limits (MDL), as calculated by adding standard deviation of seven replicates of LRB to concentration of respective PFAAs if any, were in the range 0.04–1.33 ng L–1 for PFCAs and 0.04–0.26 ng L–1 for PFSAs. Two transitions for PFSAs were monitored, implying the most abundant product ion for quantification and the other for qualification.
Tap water is used for drinking, washing, cooking, and flushing in many parts of the world and is a potential direct exposure source of PFAAs to humans. During analysis of tap water from Leipzig, PFOA (6.2 ng L–1) was the major PFAA found among eight other PFAAs and covered 53 % of total load (ΣPFAA = 11.5 ng L–1). Other PFAAs found in tap water were perfluorododecanoic acid (PFDoDA; 1.8 ng L–1), PFBS (1.3 ng L–1), perfluorohexanoic acid (PFHxA; 0.9 ng L–1), perfluorobutanoic acid (PFBA; 0.6 ng L–1), perfluorononanoic acid (PFNA; 0.4 ng L–1), perfluorodecanoic acid (PFDA; 0.3 ng L–1), and perfluoroheptanoic acid (PFHpA; 0.2 ng L–1). PFDoDA was detected in all samples, LRB, FRB, and HPLC grade water (1.5 ng L–1), and therefore may be taken as background contamination from an unknown source. Perfluoropentanoic acid (PFPeA) and perfluorohexane sulfonic acid (PFHxS) were observed below detection limit, and perfluoroheptane sulfonic acid (PFHpS), PFOS, perfluorodecane sulfonic acid (PFDS), perfluoroundecanoic acid (PFUnDA), perfluorotridecanoic acid (PFTrDA), and perfluorotetradecanoic acid (PFTeDA) were not detected. A rough comparison between recent distribution profiles of PFAAs in tap water/drinking water samples from different countries is shown in Fig. 3. Values range from about 2 ng L–1 in Faroe Islands to 36 ng L–1 in Brazil. Frequently detected PFAAs in drinking water/tap water around the world are PFBA, PFBS, PFHxA, PFHxS, PFHpA, PFOA, PFOS, PFNA, and PFDA (Thompson et al. 2011; Eriksson et al. 2013; Filipovic and Berger 2015; Schwanz et al. 2016; Sharma et al. 2016). General conception is that bottled water is safer than tap water; however, in a recent investigation by Schwanz et al. (2016), PFAAs were frequently detected in bottled waters from France (ΣPFAA = 14.9 ng L–1), Spain (ΣPFAA = 11.3 ng L–1), and Brazil (ΣPFAA = 15.0 ng L–1) and one sample from France went beyond 100 ng L–1. Dopants used in manufacturing of PET water bottles as hypothesized by the authors and contaminated source water for manufacturing bottled water can be responsible for the presence of PFAAs as PTFE plumber tapes are frequently used in sealing pipe threads.
Profile of PFAAs in tap water/drinking water from various locations: Leipzig, Germany (this study); France; Brazil; Spain (Schwanz et al. 2016); Australia (Thompson et al. 2011); Faroe Islands (Eriksson et al. 2013); India (Sharma et al. 2016); and Sweden (Filipovic and Berger 2015). Reference for China and USA (Mak et al. 2009). For multiple locations, only one has been randomly selected or average values have been used. The concentration values does not represent drinking water/tap water quality of the whole country. Numerical data is provided in Electronic Supplementary Material
Although the concentrations of PFAAs in drinking water in most parts of the world (roughly 10–15 ng L–1) are well below the threshold levels (for instance, 200 ng L–1 for PFOS, 300 ng L–1 for PFOA), continued exposure to even relatively low concentrations can considerably increase total human exposure with a serum to drinking water ratio of about 100:1 (for PFOA) (Post et al. 2012). For example, for background serum level of about 4 ng mL–1, use of drinking water containing 10 ng L–1 PFOA is expected to increase mean serum levels by about 25 % (Post et al. 2012). Recently, relationship between blood levels of residents of Bochum and Duisburg, Germany and use of drinking water contaminated with PFAAs is studied (Wilhelm et al. 2015). Authors concluded that the PFOA plasma levels in children were in coherence with the consumption of tap water, and it contributed significantly to blood plasma concentrations of PFAAs even at relatively low exposure levels. Unfortunately, conventional water treatment processes are unable to remove PFAAs completely. Coagulation, flocculation, sedimentation, and chlorination are not useful against PFAAs. Biodegradation and oxidation may turn out to be counterproductive and may degrade precursors to corresponding PFAAs, thus increasing total load of PFAAs in the finished waters (Ye et al. 2014; Rahman et al. 2014). Granular activated carbon, nano-filtration, and reverse osmosis are efficacious against most PFAAs, but removal capabilities decrease in case of short-chain homologues. Anion exchangers are exceptionally useful for removal of PFAAs, but the process may not be cost-effective (Rahman et al. 2014). Combination of high-priced multiple strategies such as activated carbon sorption and high-pressure membranes may remove PFAAs at treatment facilities, but water coming out of consumer’s tap may not be the same because of PTFE in the plumbing system.
Investigation of riverine levels of PFAAs is useful to study their transport behavior. In Fig. 4, distribution profiles of PFAAs in samples from River Pleiße, Elster, Saale, and Elbe in Germany and River Sosiani in Kenya are shown. Distribution profiles of PFAAs of the rivers in eastern Germany are similar in terms of analytes detected but with a variable order of concentration levels of individual homologues. PFOA was the dominating PFAA in Pleiße/White Elster and Saale and covers 56 % (13.9 ng L–1) and 39 % (20.9 ng L–1) of total load 24.8 and 54.3 ng L–1 (ΣPFAA), in that order. Other PFAAs in Pleiße/White Elster were PFDA (5.0 ng L–1), PFNA (1.3 ng L–1), PFBS (1.2 ng L–1), PFOS (1.0 ng L–1), PFHxA (0.9 ng L–1), PFUnDA (0.4 ng L–1), PFBA (0.4 ng L–1), PFHxS (0.4 ng L–1), and PFHpA (0.3 ng L–1). However, the order of PFAAs in terms of concentration levels in Saale was PFDoDA (8.3 ng L–1), PFBS (7.5 ng L–1), PFHxA (4.0 ng L–1), PFNA (3.7 ng L–1), PFUnDA (3.1 ng L–1), PFDA (2.2 ng L–1), PFOS (2.2 ng L–1), PFPeA (1.0 ng L–1), PFHpA (0.9 ng L–1), and PFBA (0.5 ng L–1). PFHpS, PFDS, PFTrDA, and PFTeDA were not detected in any sample. Unexpected levels of PFDoDA and PFUnDA were observed in samples from Elbe, Bad Schandau, which can be attributed to localized contamination on sampling day. Descending order of PFAAs in terms of concentration levels in Elbe was PFDoDA (6.8 ng L–1), PFBS (4.3 ng L–1), PFUnDA (3.7 ng L–1), PFDA (2.8 ng L–1), PFBA (2.6 ng L–1), PFOA (2.4 ng L–1), PFHxA (1.5 ng L–1), PFPeA (1.0 ng L–1), PFNA (0.9 ng L–1), PFHxS (0.6 ng L–1), and PFHpA (0.2 ng L–1). Dufková et al. (2012) have observed considerable levels of PFDoDA (4–14 ng L–1) and PFUnDA (3–7 ng L–1) during analysis of PFAAs in samples from Vltava and Elbe in Czech Republic using solid phase extraction followed by analysis with gas chromatograph coupled with mass spectrometer in negative chemical ionization mode (SPE-GC-NCI-MS). As the sampling site in the present study was very close to the border of Czech Republic where Elbe enters Germany, it can be assumed that these long-chain analytes may travel with Elbe from Czech Republic into Germany. On the other hand, Zhao et al. (2014) have observed fairly low levels of PFUnDA in Elbe eventually entering North Sea, whereas PFDoDA was not reported. These observations are in accordance with the physicochemical behavior of PFAAs as partitioning of PFAAs from water phase to sediments increases and solubility decreases with increasing chain length. This suggests that PFUnDA and PFDoDA [log K OC = 4.73 and 5.01, respectively (Chen et al. 2015)] may be present in Elbe earlier but may end up in sediments and soils on their way to North Sea. On the other hand, relatively shorter chain homologues flow with the river into sea.
Profile of PFAAs in surface water samples from Germany (Pleiße/White Elster, Leipzig; Saale, Halle; Elbe, Bad Schandau) and Kenya [Sosiani, Eldoret (less populated area and populated area)]. Numerical data is provided in Electronic Supplementary Material
Annual mass flows of individual PFAAs from Saale into Elbe were calculated using data from the present study and information about water discharge provided by Flood and Water Management, Saxony Anhalt (LHW 2015). Annual discharge of PFAAs from Saale into Elbe was estimated to be 164 ± 23 kg a–1, whereas annual discharge of PFAAs from Elbe into North Sea calculated by Zhao et al. (2014) was 335 ± 100 kg a–1. Annual discharges of individual PFAAs from Saale (compared with discharges from Elbe into North Sea calculated by Zhao et al. (2014), in descending order) were PFOA 63 ± 24 (83 ± 36), PFDoDA 25 ± 1 (not reported), PFBS 23 ± 8 (69 ± 46), PFHxA 12 ± 1 (71 ± 37), PFNA 11 ± 12 (7 ± 4), PFUnDA 10 ± 1 (0.7 ± 1.3), PFDA 7 ± 1 (8 ± 11), PFOS 7 ± 4 (35 ± 14), PFPeA 3 ± 1 (31 ± 18), PFHpA 3 ± 1 (not reported), and PFBA 1 + 1 (29 ± 35) in kilograms per annum (kg a–1). During three different sampling campaigns from 2006 to 2011, a decrease in concentration levels of PFOS and an increase in PFBS were observed in River Elbe (Ahrens et al. 2009b; Ahrens et al. 2009a; Zhao et al. 2014), because PFOS has been replaced with PFBS in industrial applications and products. However, low concentration levels of PFOS could still be observed despite restrictions on PFOS in Germany might be arising from environmental recirculation (Filipovic and Berger 2015) or from sources which were used several years ago but are still present because of resistance of PFOS to degradation.
River Sosiani is a stony river in Eldoret, Kenya and is a main source of water supply in town but is drying up because of excessive pollution. Environmentalists fear that the cutting down of trees and industrial discharge will dry out the river which is already 60 % dried up and is considered “dead,” severely deprived of aquatic life (AllAfrica.com 2013). The distribution profile of PFAAs in Sosiani is different from both Saale and Elbe and is dominated by long-chain PFCAs. Samples from the sparsely populated area were less contaminated (ΣPFAA = 58.8 ng L–1) in comparison to samples from densely populated area (ΣPFAA = 109.4 ng L–1), but profile of PFAAs in terms of analytes detected was similar. Eldoret is an important city in western Kenya with approximately 300,000 inhabitants. Population is an important parameter to estimate emissions of PFAAs in surface waters along with others such as local GDP and emissions from WWTPs, and it has a considerable impact on PFAA’s loads in surface waters (Lindim et al. 2015). Descending order of concentration levels of PFAAs in Sosiani in sparsely populated area was PFDoDA (23.3 ng L–1), PFDA (10.8 ng L–1), PFUnDA (10.5 ng L–1), PFNA (8.6 ng L–1), PFOA (1.6 ng L–1), PFPeA (1.3 ng L–1), PFHxA (1.3 ng L–1), PFBA (0.6 ng L–1), PFHpA (0.4 ng L–1), and traces of PFHxS and PFTrDA. On the other hand, similar analytes were detected in samples from populated area but with higher concentrations, such as PFDoDA (31.6 ng L–1), PFNA (25.9 ng L–1), PFDA (21.6 ng L–1), PFUnDA (15.9 ng L–1), PFOA (8.8 ng L–1), PFHxA (2.5 ng L–1), PFPeA (1.3 ng L–1), PFHpA (1.0 ng L–1), PFBA (0.7 ng L–1), and traces of PFTrDA. Some years ago, Orata et al. (2009) reported presence of PFOS (0.4–13.2 ng L–1) and PFOA (0.4–11.7 ng L–1) in Lake Victoria, Kenya suggesting domestic and industrial discharge as the main point source of contamination. Recently, Chirikona et al. (2015) found that discharge from hospitals contributed significant amounts of PFAAs to water systems in Lake Victoria Basin, and long-chain PFAAs were dominant.
The sample from sixth inter-laboratory study (ILS) was analyzed after a year that led to erroneous results possibly because of sorption of PFAAs to container walls. Shoemaker et al. (2009) have recommended that the water samples for the analysis of PFAAs should be stored at 6 °C or below but not to be frozen and not for more than 14 days. In the present case, the sample was not opened or unsealed and was placed in refrigerator at 4 °C but for a year. Concentration levels of PFCAs fell down by 55 % and PFSAs by 80 % as compared to statistically assigned values by the sample providers (van der Veen et al. 2014). It advocates that the concentrations of PFAAs in samples stored over a long period would be erroneous. However, distribution profile of PFAAs in surface water sample from IJ, Amsterdam is typically dominated by PFOA, PFOS, PFBS, and PFBA.
Potential environmental and toxicological impacts of long-chain PFAAs have led to phase out the production of PFOS and its precursors in 2002, followed by inclusion in the list of persistent organic pollutants and tighter regulation of its production and use. PFBS has taken over PFOS in many countries. Similarly, use of PFOA was expected to diminish until 2015, and in some parts of the world, PFOA is being replaced with PFBA. A graph is plotted to look into comparative emission levels of PFBS against PFOS and PFBA against PFOA in surface water samples from different parts of the world, sampled, and analyzed in last 5 years (Fig. 5). Bars are arranged in chorological order, extreme left being from 2015. It appears that PFBS is dominating PFOS in most parts of the world except Ebro, Spain where PFBS was not detected against minute quantities of PFOS (2.2 ng L–1). However, in fish samples from Ebro River, PFBS (69 %) was the most abundant PFAA after PFOS (81 %) (Lorenzo et al. 2016). Other exceptions are Taihu Lake, China (Chen et al. 2015) and Tama River, Japan (Ye et al. 2014) where PFOS concentrations were significantly higher than PFBS. Taihu Lake is in Eastern China and covers densely populated and industrialized regions. Tama River is one of the major rivers in Japan and 50 % of the river comes from effluents of treatment plants, which convert precursors to corresponding PFAAs during chemical oxidation. On the other hand, PFBA is catching up on PFOA but a comparison of relative distribution of both in surface water samples suggests that primary emissions of PFOA are still taking place in addition to environmental recirculation (Fig. 5). Other than sampling locations of current study, PFOA dominated PFBA at many other locations including Weser and Elbe (Germany), Xiaoqing (China), Arno and Po (Italy), and Tama River (Japan). PFBA levels were not reported for Liao River (China) with total PFAA load 44.4–781 ng L–1 dominated by PFBS (75.7 %) and PFOA (9.9 %) and Taihu Lake (China) with total PFAA load 17.2–94.4 ng L–1 dominated by PFOA (39.8 %), PFHxA (30.1 %), and PFOS (16.8 %) (Chen et al. 2015). Conversely, PFBA levels were higher than PFOA in Ganges (India), Daling (China), Ebro, and Guadalquivir Rivers (Spain). Concentration of PFBA in ground water was nine times higher than corresponding river water in India (Sharma et al. 2016), suggesting that short-chain homologues have more potential to penetrate deeper and contaminate ground water resources than long-chain homologues because of smaller soil-water partition coefficients.
PFBA vs PFOA and PFBS vs PFOS in surface waters during last 5 years. Pleiße/White Elster, Saale, and Elbe, Germany and Sosiani, Kenya (this study); Ganges, India (Sharma et al. 2016); Xiaoqing, China (Shi et al. 2015); IJ, Netherlands (van der Veen et al. 2014); Arno and Po, Italy (Valsecchi et al. 2015); Tama, Japan (Ye et al. 2014); Liao and Taihu, China (Chen et al. 2015); Elbe and Weser, Germany (Zhao et al. 2014); Daling, China (Meng et al. 2014); Guadalquivir and Ebro, Spain (Lorenzo et al. 2016). Numerical data is provided in Electronic Supplementary Material
Sources of PFAAs in surface waters are generally categorized into point and nonpoint sources. Industrial or municipal WWTPs are a major point source of PFAAs because of lacking the necessary capabilities to remove peculiar contaminants like PFAAs and biodegrading the precursors to corresponding PFAAs, thus leaving behind significant levels of PFAAs in finished waters and increasing the total load of PFAAs in effluents compared to influents (Ahrens 2011; Ye et al. 2014). During the current investigation, samples were collected from the effluent of a wastewater treatment facility on River Saale in Halle and river water samples were collected 2 km before the point where effluent confluence with the river. Results are shown in Fig. 6. Total load of PFAAs in effluent (ΣPFAA = 246.9 ng L–1) was four times higher than the river water (ΣPFAA = 59.9 ng L–1), which was dominated by PFOA (26.5 ng L–1). On the other hand, WWTP effluent was dominated by PFBS (174.2 ng L–1), 32 times higher than river water (5.5 ng L–1). Concentration of PFHxS was five times higher in effluent, PFBA and PFOS four times higher, PFUnDA three times higher, and PFHpA, PFNA, and PFDoDA two times higher than river water. Traces of PFTrDA and PFTeDA were also detected in the effluent, which were not detected in river water. Möller et al. (2010) have reported PFAAs with carbon chain length up to 18 atoms in River Rhine influenced by WWTPs. High concentration of PFBS in effluent indicates the presence of C4-based substances as alternatives to C8-based substances.
WWTP effluents as point source of PFAA contamination. Numerical data is provided in Electronic Supplementary Material
Ahrens et al. (2009b) have estimated that mass flux of PFAS from Elbe into North Sea was 802 kg a–1 in 2006 and suggested that the levels of PFBS in North Sea were pointing out towards an additional source of PFBS discharge other than Elbe into North Sea. Later, Möller et al. (2010) have estimated that mass flux of PFAS from River Rhine, Meuse, and Scheldt is around 6 t a–1 in 2008, dominated by PFBS 181 ng L–1 and PFBA 335 ng L–1. This suggested that these rivers were bringing larger quantities of PFAAs to the North Sea as compared to Elbe (7–20 times less), especially PFBS and PFBA (20–100 times higher than Elbe). During these investigations, WWTPs were pointed out as an important point source of contamination of surface waters in Germany and presence of perfluorobutanesulfonamide (FBSA) and perfluoro-butanesulfonamidoethanol (FBSE) was reported, which have potential to degrade to PFBS in WWTPs, thus increasing loads of PFBS in effluents.
In Germany, River Rhine is still the major contributor of PFASs to North Sea as can be seen from recently reported emissions (ΣPFAS = 52.4 ng L–1) while PFBS still dominates over other PFAAs, constituting 37 % of total load. Elbe’s contribution is less than half of Rhine (ΣPFAS = 21.2 ng L–1), and major PFAAs are PFHxA, PFOA, PFBS, and PFOS (Heydebreck et al. 2015). During the current investigation, samples from WWTP effluent were collected in February. In another investigation to measure the seasonal variation of PFAAs in Elbe, Zhao et al. (2014) have found elevated levels of PFBS in February (0.9–238 ng L–1), higher than its concentration levels in April (0.2–7.1 ng L–1), August (1.4–3 ng L–1), and October (0.9–1.5 ng L–1). It suggests that when water is at freezing temperatures during winter, PFBS emitted by the WWTPs stays in river and travels longer distances with water than in spring, summer, or autumn when the temperature of water is higher, which facilitates volatile PFBS to escape to the atmosphere. However, this needs further investigation. High levels of PFBS may also be due to the occasional discharges (Zhao et al. 2014). Prevalence of short-chain PFBS in effluent indicates that the effects of PFOS substitution are visible in environment. Generally, long-chain PFAAs are becoming less common in water than short-chain homologues because of extensive use of later as alternatives to former.
Economic development and stringent regulations in Europe have shifted major production of PFAAs to China, which can been seen in form of elevated levels of PFAAs in Chinese surface waters, still dominated by PFOA, and relatively lower levels of PFAAs in Europe, dominated by short-chain homologues. Recently, a new PFAA (possible alternative of PFOA), namely 2,3,3,3-tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy)-propanoic acid (HFPO-DA), has been detected in Rhine, Germany (Heydebreck et al. 2015). Lack of information about physicochemical characteristics of already well-known toxic PFAAs, such as PFOS and PFOA, presence of PFAAs in form of complicated isomeric mixtures in environmental compartments, and detection and addition of new environmentally relevant PFAAs in an already lengthy list indicates that strenuous efforts are required to understand the environmental chemistry of PFAAs. Detection of elevated levels of short-chain homologues in environmental compartments in recent years necessitates that monitoring (Weiss et al. 2015) and risk assessment of these PFAAs should be taken into consideration, and comprehensive research should be carried out to understand their health effects, transportation, and fate in different environmental compartments. Global monitoring program needs to be established to measure the emission levels in developing countries where regulations are weaker than developed countries. It should be noted that the concentrations of PFAAs in surface waters reported in the current study represent snapshots for a single day. In the future, passive samplers will be used to collect time-integrated averages of PFAAs in rivers and detailed investigation of influents, effluents, and sludge from WWTPs will be carried out to understand chemistry behind the elevated levels of PFBS in effluents observed in the present study.
Conclusions
In the present study, aqueous samples from different sites located in Germany and Kenya were analyzed to determine concentrations of 16 PFAAs. PFBA, PFHxA, PFHpA, PFOA, PFNA, and PFDA were detected in all the samples, while PFHpS and PFDS were not detected in any sample. Tap water from Leipzig was dominated by PFOA (6.2 ng L–1), covering more than half of the total load of PFAAs. River Saale, Pleiße, and White Elster were also dominated by PFOA. Annual flux of PFAAs from Saale was estimated to be 164 ± 23 kg a–1. River Elbe was dominated by long-chain PFAAs at Bad Schandau, when it entered Germany, which was quite different near Hamburg, when it met North Sea (Zhao et al. 2014), and was dominated by PFBS, PFHxA, and PFOA. In Halle, WWTP effluent contained four times higher concentration of PFAAs as compared to concentrations in river water and dominated by PFBS. Long-chain PFAAs dominated River Sosiani (Kenya) and sums of average concentrations were 58.8 and 109.4 ng L–1 in samples from sparsely populated and densely populated areas, respectively. Comparison of samples from different parts of the world suggested that distribution profile of PFAAs varied significantly from location to location. Moreover, PFBS is surpassing PFOS in the aquatic matrices in most cases, while PFOA is still the dominant PFCA. Continuous monitoring and further investigation of aqueous matrices are required to understand sources and environmental and health impacts of PFAAs.
References
Ahrens L (2011) Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. J Environ Monit 13:20–31. doi:10.1039/C0EM00373E
Ahrens L, Felizeter S, Sturm R et al (2009a) Polyfluorinated compounds in waste water treatment plant effluents and surface waters along the River Elbe, Germany. Mar Pollut Bull 58:1326–1333. doi:10.1016/j.marpolbul.2009.04.028
Ahrens L, Plassmann M, Xie Z, Ebinghaus R (2009b) Determination of polyfluoroalkyl compounds in water and suspended particulate matter in the river Elbe and North Sea, Germany. Front Environ Sci Eng China 3:152–170. doi:10.1007/s11783-009-0021-8
AllAfrica.com (2013) Kenya: heavy pollution in Eldoret choking up River Sosiani. In: AllAfrica.com. http://allafrica.com/stories/201307150072.html. Accessed 9 Jan 2016
Buck RC, Franklin J, Berger U et al (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7:513–541. doi:10.1002/ieam.258
CEPA (2016) Perfluorooctane sulfonate and its salts and certain other compounds regulations. Gatineau, Canada
Chen X, Zhu L, Pan X et al (2015) Isomeric specific partitioning behaviors of perfluoroalkyl substances in water dissolved phase, suspended particulate matters and sediments in Liao River Basin and Taihu Lake, China. Water Res 80:235–44. doi:10.1016/j.watres.2015.04.032
Chirikona F, Filipovic M, Ooko S, Orata F (2015) Perfluoroalkyl acids in selected wastewater treatment plants and their discharge load within the Lake Victoria basin in Kenya. Environ Monit Assess 187:4425. doi:10.1007/s10661-015-4425-6
Cousins IT (2013) Nordic research on per- and polyfluoroalkyl substances (PFASs). Environ Sci Pollut Res 20:7926–7929. doi:10.1007/s11356-013-2000-7
DIN 38407-42 (2011) Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung - Teil 42: Bestimmung ausgewählter polyfluorierter Verbindungen (PFC) in Wasser. F42:1–43.
EU Directive (2013) Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC And 2008/105/EC as Regards Priority Substances in the Field of Water Policy. Brussels, Belgium
Dufková V, Čabala R, Ševčík V (2012) Determination of C5–C12 perfluoroalkyl carboxylic acids in river water samples in the Czech Republic by GC–MS after SPE preconcentration. Chemosphere 87:463–469. doi:10.1016/j.chemosphere.2011.12.029
Duong HT, Kadokami K, Shirasaka H et al (2015) Occurrence of perfluoroalkyl acids in environmental waters in Vietnam. Chemosphere 122:115–124. doi:10.1016/j.chemosphere.2014.11.023
DWI (2009) Guidance on the water supply (water quality) regulations 2000 specific to PFOS (perfluorooctane sulphonate) and PFOA (perfluorooctanoic acid) concentrations in drinking water. London, England
Eriksson U, Kärrman A, Rotander A et al (2013) Perfluoroalkyl substances (PFASs) in food and water from Faroe Islands. Environ Sci Pollut Res 20:7940–7948. doi:10.1007/s11356-013-1700-3
Filipovic M, Berger U (2015) Are perfluoroalkyl acids in waste water treatment plant effluents the result of primary emissions from the technosphere or of environmental recirculation? Chemosphere 129:74–80. doi:10.1016/j.chemosphere.2014.07.082
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35:1339–1342. doi:10.1021/es001834k
Hansen KJ, Clemen LA, Ellefson ME, Johnson HO (2001) Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices. Environ Sci Technol 35:766–770. doi:10.1021/es001489z
Herzke D, Huber S, Bervoets L et al (2013) Perfluorinated alkylated substances in vegetables collected in four European countries; occurrence and human exposure estimations. Environ Sci Pollut Res 20:7930–7939. doi:10.1007/s11356-013-1777-8
Heydebreck F, Tang J, Xie Z, Ebinghaus R (2015) Alternative and legacy perfluoroalkyl substances: differences between European and Chinese river/estuary systems. Environ Sci Technol 49:8386–8395. doi:10.1021/acs.est.5b01648
Keränen J, Ahkola H, Knuutinen J et al (2013) Formation of PFOA from 8:2 FTOH in closed-bottle experiments with brackish water. Environ Sci Pollut Res 20:8001–12. doi:10.1007/s11356-013-1975-4
Kissa E (2001) Fluorinated surfactants and repellents. Second. Marcel Dekker, Inc., New York
Kjeldsen LS, Bonefeld-Jørgensen EC (2013) Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res 20:8031–8044. doi:10.1007/s11356-013-1753-3
Lam N-H, Cho C-R, Lee J-S et al (2014) Perfluorinated alkyl substances in water, sediment, plankton and fish from Korean rivers and lakes: a nationwide survey. Sci Total Environ 491–492:154–162. doi:10.1016/j.scitotenv.2014.01.045
Lau C (2012) Perfluorinated compounds. Springer Basel, Basel
LHW (2015) Hydrologischer Monatsbericht Februar 2015. Magdeburg, Sachsen-Anhalt
Lindim C, Cousins IT, VanGils J (2015) Estimating emissions of PFOS and PFOA to the Danube River catchment and evaluating them using a catchment-scale chemical transport and fate model. Environ Pollut 207:97–106. doi:10.1016/j.envpol.2015.08.050
Liu J, Mejia Avendaño S (2013) Microbial degradation of polyfluoroalkyl chemicals in the environment: a review. Environ Int 61:98–114. doi:10.1016/j.envint.2013.08.022
Löfstedt Gilljam J, Leonel J, Cousins IT, Benskin JP (2015) Is ongoing sulfluramid use in South America: a significant source of perfluorooctanesulfonate (PFOS)? Production inventories, environmental fate, and local occurrence. Environ Sci Technol acs.est.5b04544. doi: 10.1021/acs.est.5b04544
Long M, Ghisari M, Bonefeld-Jørgensen EC (2013) Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int 20:8045–56. doi:10.1007/s11356-013-1628-7
Lorenzo M, Campo J, Farré M et al (2016) Perfluoroalkyl substances in the Ebro and Guadalquivir river basins (Spain). Sci Total Environ 540:191–199. doi:10.1016/j.scitotenv.2015.07.045
Mak YL, Taniyasu S, Yeung LWY, et al (2009) Perfluorinated compounds in rap water from China and several other countries. Environ Sci Technol 43:4824–4829. doi:10.1021/es900637a
Meng J, Wang T, Wang P, et al (2014) Perfluoroalkyl substances in daling river adjacent to fluorine industrial parks: Implication from industrial emission. Bull Environ Contam Toxicol 94:34–40. doi:10.1007/s00128-014-1419-y
Miralles-Marco A, Harrad S (2015) Perfluorooctane sulfonate: a review of human exposure, biomonitoring and the environmental forensics utility of its chirality and isomer distribution. Environ Int 77:148–159. doi:10.1016/j.envint.2015.02.002
Möller A, Ahrens L, Surm R et al (2010) Distribution and sources of polyfluoroalkyl substances (PFAS) in the River Rhine watershed. Environ Pollut 158:3243–3250. doi:10.1016/j.envpol.2010.07.019
Niisoe T, Senevirathna STMLD, Harada KH et al (2015) Perfluorinated carboxylic acids discharged from the Yodo River Basin, Japan. Chemosphere 138:81–88. doi:10.1016/j.chemosphere.2015.05.060
Nordén M, Berger U, Engwall M (2013) High levels of perfluoroalkyl acids in eggs and embryo livers of great cormorant (Phalacrocorax carbo sinensis) and herring gull (Larus argentatus) from Lake Vänern, Sweden. Environ Sci Pollut Res 20:8021–8030. doi:10.1007/s11356-013-1567-3
Ode A, Rylander L, Lindh CH et al (2013) Determinants of maternal and fetal exposure and temporal trends of perfluorinated compounds. Environ Sci Pollut Res 20:7970–8. doi:10.1007/s11356-013-1573-5
Oliaei F, Kriens D, Weber R, Watson A (2013) PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ Sci Pollut Res 20:1977–1992. doi:10.1007/s11356-012-1275-4
Olsen GW, Burris JM, Ehresman DJ et al (2007) Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115:1298–1305. doi:10.1289/ehp.10009
Orata F, Quinete N, Werres F, Wilken R-D (2009) Determination of perfluorooctanoic acid and perfluorooctane sulfonate in Lake Victoria Gulf water. Bull Environ Contam Toxicol 82:218–222. doi:10.1007/s00128-008-9543-1
Paul AG, Jones KC, Sweetman AJ (2009) A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ Sci Technol 43:386–392. doi:10.1021/es802216n
Perkola N, Sainio P (2013) Survey of perfluorinated alkyl acids in Finnish effluents, storm water, landfill leachate and sludge. Environ Sci Pollut Res 20:7979–7987. doi:10.1007/s11356-013-1518-z
Post GB, Cohn PD, Cooper KR (2012) Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res 116:93–117. doi:10.1016/j.envres.2012.03.007
Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH (2006) Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 40:32–44. doi:10.1021/es0512475
Rahman MF, Peldszus S, Anderson WB (2014) Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review. Water Res 50:318–340. doi:10.1016/j.watres.2013.10.045
Rayne S, Forest K, Friesen KJ (2009) Estimated congener specific gas-phase atmospheric behavior and fractionation of perfluoroalkyl compounds: rates of reaction with atmospheric oxidants, air-water partitioning, and wet/dry deposition lifetimes. J Environ Sci Health A Tox Hazard Subst Environ Eng 44:936–54
Schwanz TG, Llorca M, Farré M, Barceló D (2016) Perfluoroalkyl substances assessment in drinking waters from Brazil, France and Spain. Sci Total Environ 539:143–152. doi:10.1016/j.scitotenv.2015.08.034
Sharma BM, Bharat GK, Tayal S et al (2016) Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: emissions and implications for human exposure. Environ Pollut 208:704–713. doi:10.1016/j.envpol.2015.10.050
Shi Y, Vestergren R, Xu L, et al (2015) Characterizing direct emissions of perfluoroalkyl substances from ongoing fluoropolymer production sources: A spatial trend study of Xiaoqing River, China. Environ Pollut 206:104–112. doi:10.1016/j.envpol.2015.06.035
Shoeib T, Hassan Y, Rauert C, Harner T (2016) Poly- and perfluoroalkyl substances (PFASs) in indoor dust and food packaging materials in Egypt: trends in developed and developing countries. Chemosphere 144:1573–1581. doi:10.1016/j.chemosphere.2015.08.066
Shoemaker J, Grimmett P, Boutin B (2009) Determination of selected perfluorinated alkyl acids in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS). EPA Doc. EPA/600/R-08/092. US EPA Off. Res. Dev. EPA 537:1–50.
Thompson J, Eaglesham G, Mueller J (2011) Concentrations of PFOS, PFOA and other perfluorinated alkyl acids in Australian drinking water. Chemosphere 83:1320–1325. doi:10.1016/j.chemosphere.2011.04.017
UNEP (2009) Stockholm Convention on persistent organic pollutants. PFOS in Annex B. Stockholm, Sweden
USEPA (2000) EPA and 3M announce phase out of PFOS. In: Environ. Prot. Agency. http://yosemite.epa.gov/opa/admpress.nsf/0/33AA946E6CB11F35852568E1005246B4. Accessed 16 May 2000
USEPA (2006) 2010/2015 PFOA stewardship program. In: Environ. Prot. Agency. http://www.epa.gov/assessing-and-managing-chemicals-under-tsca/20102015-pfoa-stewardship-program. Accessed 14 Oct 2015
Usepa SNUR (2015) Significant new uses regulations: long-chain perfluoroalkyl carboxylate and perfluoroalkyl sulfonate chemical substances. United States, Washington DC
Valsecchi S, Rusconi M, Mazzoni M et al (2015) Occurrence and sources of perfluoroalkyl acids in Italian river basins. Chemosphere 129:126–134. doi:10.1016/j.chemosphere.2014.07.044
van der Veen I, Weiss J, van Hattum B (2014) 6th interlaboratory study (ILS) on perfluoroalkyl substances (PFASs) in environmental samples 2013. IVM Institute for Environmental Studies, VU University Amsterdam, Amsterdam
Vestergren R, Orata F, Berger U, Cousins IT (2013) Bioaccumulation of perfluoroalkyl acids in dairy cows in a naturally contaminated environment. Environ Sci Pollut Res 20:7959–7969. doi:10.1007/s11356-013-1722-x
Weiss J, Boer J De, Berger U, et al (2015) PFAS analysis in water for the global monitoring plan of the Stockholm Convention: set-up and guidelines for monitoring. United Nations Environment Programme (UNEP) Division of Technology, Industry and Economics, Geneva
Wild CP (2005) Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev 14:1847–1850. doi:10.1158/1055-9965.EPI-05-0456
Wilhelm M, Wittsiepe J, Völkel W et al (2015) Perfluoroalkyl acids in children and their mothers: association with drinking water and time trends of inner exposures—results of the Duisburg birth cohort and Bochum cohort studies. Int J Hyg Environ Health 218:645–655. doi:10.1016/j.ijheh.2015.07.001
Xie S, Wang T, Liu S et al (2013) Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environ Int 52:1–8. doi:10.1016/j.envint.2012.11.004
Ye F, Tokumura M, Islam MS et al (2014) Spatial distribution and importance of potential perfluoroalkyl acid precursors in urban rivers and sewage treatment plant effluent—case study of Tama River, Japan. Water Res 67C:77–85. doi:10.1016/j.watres.2014.09.014
Zhao Z, Xie Z, Tang J et al (2014) Seasonal variations and spatial distributions of perfluoroalkyl substances in the rivers Elbe and lower Weser and the North Sea. Chemosphere 129:118–125. doi:10.1016/j.chemosphere.2014.03.050
Zhu Z, Wang T, Meng J et al (2015) Perfluoroalkyl substances in the Daling River with concentrated fluorine industries in China: seasonal variation, mass flow, and risk assessment. Environ Sci Pollut Res 22:10009–10018. doi:10.1007/s11356-015-4189-0
Zushi Y, Hogarh JN, Masunaga S (2012) Progress and perspective of perfluorinated compound risk assessment and management in various countries and institutes. Clean Technol Environ Policy 14:9–20. doi:10.1007/s10098-011-0375-z
Acknowledgments
The authors acknowledge the support for a doctoral research scholarship from Higher Education Commission (HEC), Pakistan and German Academic Exchange Service (DAAD), Germany. The authors are grateful to Mr. Uwe Schröter for technical help, Mr. Stefan Kunz for help in the lab, and Mrs. Bilha Saina Chepchirchir to provide samples from Kenya. Moreover, the authors are thankful to participants of 15th EuCheMS International Conference on Chemistry and the Environment (Leipzig, Germany) for useful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Kallenborn
An erratum to this article is available at http://dx.doi.org/10.1007/s11356-016-7721-y.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
The online version of this article contains supplementary material, which is available to authorized users. (PDF 978 kb)
Rights and permissions
About this article
Cite this article
Shafique, U., Schulze, S., Slawik, C. et al. Perfluoroalkyl acids in aqueous samples from Germany and Kenya. Environ Sci Pollut Res 24, 11031–11043 (2017). https://doi.org/10.1007/s11356-016-7076-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7076-4