Abstract
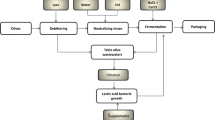
The production of table olives is a significant economic activity in Mediterranean countries. Table olive processing generates large volumes of rinsing water that are characterized by high organic matter and phenol contents. Due to these characteristics, a combination of more than one technology is imperative to ensure efficient treatment with low operational cost. Previously, biological filters were combined with electrooxidation to treat table olive washing water. Although this combination was successful in reducing pollutant loads, its cost could be further reduced. Constructed wetlands could be an eligible treatment method for integrated table olive washing water treatment as they have proved tolerant to high organic matter and phenol loads. Two pilot-scale horizontal subsurface constructed wetlands, one planted and one unplanted, were combined with a biological filter and electrooxidation over a boron-doped diamond anode to treat table olive washing water. In the biological filter inlet, chemical oxygen demand (COD) concentrations ranged from 5500 to 15,000 mg/L, while mean COD influent concentration in the constructed wetlands was 2800 mg/L. The wetlands proved to be an efficient intermediate treatment stage, since COD removal levels for the planted unit reached 99 % (mean 70 %), while the unplanted unit presented removal rates of around 65 %. Moreover, the concentration of phenols in the effluent was typically below 100 mg/L. The integrated trickling filter-constructed wetland-electrooxidation treatment system examined here could mineralize and decolorize table olive washing water and fully remove its phenolic content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The annual global production of table olives is estimated at approximately 1.5 million tons (Kyriacou et al., 2005; International Olive Council, 2002), with the majority based on Mediterranean countries (mostly Spain, Italy and Greece) (Beltran-Heredia et al., 2000). Table olives can be classified into three types based on their colour, green, black and black, through oxidation (Beltran-Heredia et al., 2000). In Greece, the annual table olive production is estimated at 45,000 tons of black olives and 20,000 tons of green olives that are processed in approximately 75 factories (Kyriacou et al., 2005; Kopsidas, 1992). Table olive processing wastewater (TOWW) is a serious environmental threat due to its seasonal production and its high organic and phenolic loads (Kotsou et al., 2004). Typical characteristics of TOWW include a wide range of pH values (from 3.6 to 13.2), high loads of suspended solids (0.03–0.4 g/L) and high organic matter content (BOD5 of 0.1–6.6 g/L and chemical oxygen demand (COD) of 0.3–16.2 g/L) (Kotsou et al., 2004; Kopsidas, 1992).
TOWWs are usually retained in evaporation ponds, causing bad odours and in some cases surface and groundwater pollution (Beltran-Heredia et al., 2000). However, in recent years, a great variety of treatment methods have been tested to treat TOWW, including the following: aerobic degradation systems (Benitez et al., 1997), anaerobic digestion (Borja et al., 1993; Wheatley, 1990), ozone oxidation (Rivas et al., 2000), Fenton oxidation (Rivas et al., 2003), a combination of UV radiation and hydrogen peroxide, as well as photo-Fenton (Benitez et al., 2001), and combined biological and chemical treatments (Kotsou et al., 2004; Benitez et al., 2001; Rivas et al., 2001; Beltran-Heredia et al., 2000; Benitez et al., 1997).
Biological methods have been recognized as inexpensive and effective processes (Kotsou et al., 2004). Although aerobic biological treatment methods are more efficient, these processes are limited by the unbearable cost of the continuously provided mechanical aeration. However, recent research has shown that trickling filters can successfully aerobically degrade COD and phenols from olive mill and table olive mill wastewater at relatively low cost (Tatoulis et al., 2016; Michailides et al., 2011). Constructed wetlands are also considered a promising technology to treat wastewater due to their low cost, simple operation and maintenance, and favourable appearance (Shutes, 2001). Although constructed wetlands have been used to treat a variety of wastewater, until now, only a few attempts to treat olive mill wastewater have been published (Gikas et al., 2013; Herouvim et al., 2011; Kapellakis et al., 2012; Bubba et al., 2004). Both trickling filters and constructed wetlands are biological systems that do not require mechanical aeration.
The concept of coupling chemical and biological methods for industrial wastewater treatment has been recognized as a promising approach for effluents that cannot be treated effectively by single processes (Comninellis et al., 2008). Industrial effluents usually contain high concentrations of several organic pollutants, including biodegradable, biorecalcitrant and toxic fractions; in this respect, the manner in which processes can be coupled is case-specific. In recent years, advanced electrochemical oxidation processes have been tested for wastewater treatment as a single process or a part of process integration (Martínez-Huitle et al., 2015; Radjenovic and Sedlak, 2015; Ganzenko et al., 2014). In the case of agro-industrial effluents that usually contain biodegradable fractions, electrochemical oxidation is used as a post-treatment step to polish the final stream prior to discharge. The efficiency of hybrid systems comprising aerobic/anaerobic biological treatment followed by electrochemical oxidation over boron-doped diamond anodes has been demonstrated for the treatment of cheese whey (Katsoni et al., 2014a), olive pomace leachate (Katsoni et al., 2014b; Grafias et al., 2010) and table olive wastewater (Tatoulis et al., 2016).
The aims of this work were to study the effectiveness of pilot-scale horizontal subsurface flow (HSF) constructed wetlands on TOWW treatment in a Mediterranean climate and optimize a hybrid system for TOWW treatment. TOWW was pre-treated by a biological trickling filter, while effluent from the constructed wetlands was post-treated using electrooxidation. In this way, pollutant concentration was reduced further before effluent reuse or disposal, and operation cost was minimized. The combination of two low-cost biological methods (a trickling filter and a constructed wetland) will further increase biological pollutant reduction at minimal cost. In addition, the lowered pollutant load will reduce the operational cost of the electrooxidation polishing stage.
Materials and methods
Description of the biological filter
TOWW was first treated in a pilot-scale biological filter (Tatoulis et al. 2016), which had a working volume of 6 L and was operated as a sequencing batch reactor with recirculation (draw-fill mode) of 0.5 L/min. Final dissolved COD concentrations in the filter effluent ranged from 500 to 4000 mg/L, depending on initial COD concentrations (i.e. 5500, 7500 and 15,000 mg/L), and total phenol effluent concentrations ranged from 100 to 500 mg/L (Tatoulis et al., 2016).
Description of pilot-scale constructed wetland units
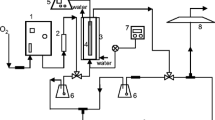
Two identical pilot-scale HSF wetlands (Fig. 1) were constructed at the Department of Environmental and Natural Resources Management, located in Agrinio, Western Greece. The two units comprised high-density poly-ethylene (HDPE) tanks with the following inner dimensions: 72 cm long, 33 cm wide (surface area = 0.24 m2) and 35 cm deep. Both units were filled with fine gravel (D 50 = 6 mm). One unit was planted (OG-P) with common reeds (Phragmites australis) obtained from local streams, and the other was kept unplanted (OG-U) as the control. To achieve rapid vegetation growth, the OG-P unit was planted with three reed stems (12 reeds/m2). Both pilot-scale units were equipped with inlet and outlet hydraulic structures similar to those used in full-scale systems. Wastewater inflowed through a perforated plastic pipe (diffuser) placed across the entire width of the upstream side of the tank. These diffusers were fastened onto the tank so that they were perfectly horizontal, thus ensuring the uniform distribution of wastewater across the tank. The outlet was an orifice (1/4 in. diameter) located at the base of the downstream end of the unit, connected to a U pipe. The elevation of the downstream end of this pipe, from which water overspilled, controlled the downstream water surface level in the tank at the upper surface of the porous medium (i.e. at about 35 cm). Overspilled wastewater was collected in a 35-L plastic tank for proper disposal. Void volume was estimated by draining the tanks and measuring the water volume of each. Water volume and mean porosity for OG-P were 13.5 L and 29 %, respectively, and 14 L and 30 %, respectively, in the OG-U unit. The units were operated from July 2014 to April 2015 with a hydraulic residence time (HRT) of 4 days, from May to June 2015 with an HRT of 2 days and from July to November 2015 with an HRT of 8 days.
Electrooxidation experiments
Batch electrooxidation experiments were performed in a double-walled, cylindrical glass vessel with a liquid capacity of 0.2 L. A rectangular electrode (16 cm2) made of boron-doped diamond was used as the anode and the cathode was made of stainless steel. A control unit was used to keep the TOWW temperature constant at 30 ± 2 °C. It should be noted that the electrooxidation experiments were conducted at lower current densities (between 16.1 and 125 mA/cm2) than in a previous study (Tatoulis et al., 2016) because the effluent obtained from the OG-P wetland unit had a mean COD value of just 450 mg/L.
Evapotranspiration assessment
Evapotranspiration (ET) was assessed on a daily basis. Each morning the outlet storage tanks were emptied and the volume of the treated wastewater was measured. The volume of the wastewater introduced into the units was also measured on a daily basis. Influent and effluent volumes, as well as precipitation values, were then used to assess ET. During days of high temperatures and solar radiation, ET values reached 15.5 L/day (i.e. 13.6 mm/day), exceeded wastewater influent volumes and lead to a reduction in unit water level. These high values of ET lead to wastewater volume condensation and the overestimation of effluent concentrations. Thus, it was imperative to correct effluent concentrations in terms of ET and precipitation to prevent their under- or overestimation. Nevertheless, in real conditions, high ET values will increase pollutant concentration but also decrease effluent volumes. For this reason, a post-treatment stage (in this case electrochemical oxidation) is most likely needed to bring the final effluent pollutant concentrations within the legislation limits for disposal.
Water quality monitoring
Water samples were collected from the influent and the effluent sampling points of each unit with a frequency equal to the HRT. pH, electrical conductivity (EC) and dissolved oxygen (DO) were measured in situ. DO, pH and EC were measured using a CONSORT C 835, multi-parameter analyzer. Meteorological data were obtained from the meteorological station of the National Observatory of Athens located in Agrinio and for the entire operation period, the mean air temperature was 19.2 °C and total precipitation was 1314 mm.
Dissolved COD was monitored measuring the absorbance of the sample after dichromate digestion (HANNA instruments C9800 reactor) at 150 °C for 2 h in the presence of silver and mercury sulphates (closed reflux method) (APHA, 1989). The absorbance was measured by a HANNA C99 Multi-parameter bench photometer. Total organic carbon (TOC) was measured by an Aurora 1030W analyzer (OI Analytical). Total phenolic compounds (with respect to syringic acid) were determined spectrophotometrically using a Boeco (Germany, S-20) spectrophotometer, according to the Folin-Ciocalteu method (Waterman and Mole, 1994).
Results and discussion
Constructed wetlands
Statistics (i.e. mean and standard deviation) of the physicochemical parameters measured in the influents and effluents of both pilot-scale wetland units are presented in Table 1. Mean pH values increased from 5.8 in the influent to approximately 6.4 in the effluent. This can be attributed either to biological oxidation or the dilution of carbonate salts in the porous media. EC showed a slight increase in the effluent values of both units due to the extensive ET, while DO concentrations decreased, as expected, due to microbial activity and pollutant oxidation. Nevertheless, in most cases, DO concentrations remained above 3 mg/L, indicating that aerobic conditions prevailed in the pilot-scale wetlands.
Figure 2 presents time series charts for influent and effluent concentrations of COD for the OG-P (planted) and OG-U (unplanted) wetland units. Effluent concentrations were corrected by removing precipitation and ET volumes. A trickling filter was used to pre-treat the TOWW (Tatoulis et al., 2016) before it entered the wetlands. Mean influent and effluent concentrations recorded in the OG-P were 2750 ± 900 and 900 ± 800 mg/L, respectively, and 2800 ± 940 and 1000 ± 900 mg/L, respectively, in the OG-U unit. In both units, COD effluent concentrations presented fluctuations, which can be attributed to the following: (a) the initial wetland commissioning phase (approximately the first 2 to 3 months of operation), (b) HRT alteration, as wetland units need to adjust to the new loads, (c) temperature decrease and plant die off, which takes place mostly during winter months, and/or (d) influent concentration fluctuations which can shock constructed wetlands. As reported by Herouvim et al. (2011), organic matter is mainly removed by aerobic or anaerobic processes, and this is verified in the present study as organic matter removal in the planted (mean of 70 %) and unplanted units (mean of 62 %) did not show any significant differences (ANOVA p value = 0.678 > 0.05). Nevertheless, vegetation has a positive effect on organic matter removal as it increases the available surface area for microbial growth and dissolved oxygen concentrations. Furthermore, as reported in previous studies (Herouvim et al., 2011; Akratos and Tsihrintzis, 2007), the presence of vegetation in constructed wetlands increases organic matter removal during winter months when compared to non-vegetated wetland units.
Another crucial operational parameter for constructed wetlands is HRT, as minimum HRTs lead to smaller wetland surface area requirements. Three different HRTs that have been previously used for organic matter removal in HSF constructed wetlands (i.e. 2, 4 and 8 days) were applied in the present study (Sultana et al., 2016; Akratos and Tsihrintzis, 2007). The effect of HRT on COD removal was assessed by performing one-way ANOVA analysis in specific data sets, where temperature values were not significantly different (p = 0.007 > 0.05) and COD influent concentrations were almost identical. For both units (OG-P and OG-U), the one-way ANOVA results showed that an HRT of 2 days leads to significantly lower (p = 0 for OG-P and OG-U) COD removal efficiencies (48 % for OG-P and 40 % OG-U) compared to the removal efficiencies achieved with an HRT of 4 days (95 % for OG-P and OG-U). On the contrary, no significant differences (p = 0.376 for OG-P and 0.073 for OG-U) were recorded for HRTs of 4 and 8 days (90 % for OG-P and 78 % OG-U). As reported by Sultana et al. (2016), an HRT of 4 days is sufficient to safely remove organic matter when COD influent concentrations are below 2500 mg/L.
COD removal is temperature dependent thus implying that organic matter removal is mostly a result of aerobic and anaerobic bacterial activity (Akratos and Tsihrintzis, 2007), as bacteria increase their function when air temperature rises. To statistically assess the effect of temperature on COD removal, the results obtained from experiments conducted with an HRT of 4 days were divided into two data sets (above and below 15 °C) (Sultana et al., 2016; Akratos and Tsihrintzis, 2007). While temperature did not significantly affect the unplanted unit (OG-U) (p = 0.678), the planted unit (OG-P) was significantly affected by temperature (p = 0.026). The dependence of COD removal efficiency on influent concentration observed in these experiments was expected, since microbes cannot degrade very high organic matter concentrations and, at the same time, reeds cannot provide the oxygen concentrations needed to degrade all the organic matter. The data, therefore, imply that in these pilot-scale constructed wetlands, organic matter was removed by microbial activity.
One of the main pollution issues associated with olive processing is the presence of phenols, which have rather toxic effects on the environment. Figure 3 presents time series charts for phenol influent and effluent concentrations in the OG-P and OG-U units, respectively. Both units seem to be rather effective at removing phenols as mean removal efficiencies were 78 and 75 % for OG-P and OG-U. Fluctuations in effluent concentrations seem to follow fluctuations in influent concentrations (Fig. 3). Similar to COD removal, the effect of HRT on phenol removal was also assessed using one way-ANOVA and respective data sets (described above). Although phenol influent concentrations fluctuated greatly, especially when pilot-scale units operated under 8-day HRTs, effluent concentrations remained stable and were not affected by HRT alterations (OG-P-p = 0.06; OG-U-p = 0.387), thus indicating that CW units can achieve stable operation.
Electrooxidation post-treatment
Effluents from the constructed wetlands with relatively low COD concentrations (i.e. when operated with an HRT of 8 days) were post-treated by electrooxidation in order to remove residual pollutants before final disposal. For that period, the mean concentrations of COD and total phenols recorded in the effluents were 450 and 85 mg/L, respectively. Figure 4a shows the effect of the applied current density on COD removal. Almost complete COD removal (i.e. 94 %) was achieved after 90 min of electrooxidation at 62.5–125 mA/cm2 and this decreased to 76 and 67 %, respectively, when the two lower densities of 31.2 and 16.1 mA/cm2 were applied. Since the permissible COD discharge limit for industrial effluents is 125 mg/L (Gazette of the Government (GR) 2011/354Β), this value can be achieved after 45 min of electrooxidation at 62.5 mA/cm2 (indicated by the dashed line in Fig. 4a). In addition to COD removal, fast decolourization occurred between 45 and 60 min for all the currents tested (Fig. 4b). When effluent from the trickling filter was fed directly into the electrooxidation unit, the time needed to achieve the permissible discharge limit ranged between 60 and 240 min at 187–250 mA/cm2 (Tatoulis et al., 2016), depending on the efficacy of the trickling filter’s operation.
Figure 5 shows COD evolution profiles at different initial COD concentrations during electrooxidation at 62.5 mA/cm2. This was performed by diluting a sample (COD concentration of 450 mg/L) obtained from the planted wetland unit (OG-P) with water. Shorter treatment times were needed to achieve complete removal at lower initial concentrations, i.e. for the run at 220 mg/L COD, 15–30 min sufficed to bring the effluent to the permissible discharge limit. The evolution of TOC is also given in Fig. 5 for the undiluted experiment. The fact that the extent of mineralization (i.e. 76 %) after 90 min is lower than COD removal (i.e. 94 %) implies that the remaining organic matter is highly oxidized.
Anodic oxidation over non-active boron-doped diamond electrodes is characterized by improved mineralization rates compared to active anodes. This is ascribed to the fact that the electrogenerated hydroxyl radicals are weakly adsorbed onto the anode surface, and, therefore, they are available to oxidize the organic matter to carbon dioxide and water (Martínez-Huitle et al., 2015; Comninellis et al., 2008). To assess the technical feasibility of electrooxidation, figures-of-merit such as the average current efficiency (ACE), the specific energy consumption (SEC in kWh/g COD removed) and the electric energy per order (Eo in kWh/m3) can be employed as follows (Radjenovic and Sedlak, 2015; Bolton et al., 2001):
where COD0 and COD t (in mg/L) correspond to concentrations at t = 0 and t = t (seconds), respectively, I is the applied current (A), U is the applied voltage (V), F is the Faraday constant (96,487 C/mol) and V is the liquid volume (m3).
At the conditions where the permissible COD discharge limit is attained (i.e. ΔCOD = 314 mg/L, I = 1 A, U = 27.9 V and t = 2700 s), an ACE value of 28 % is computed from Eq. (1), implying that other electrochemical reactions, such as oxygen evolution, occur concurrently with COD reduction. This said, the cost of removing 1 g of COD is computed to just 1.7 Euro cents according to Eq. (2) and taking into account that the average cost of energy for industrial use in Greece is 0.05 €/kWh. On the other hand, the cost of electrooxidation would rise to about 3 Euro cents per gramme of COD removed in the absence of a constructed wetland step (Tatoulis et al., 2016). The operational cost of the biological filter per unit volume of effluent treated has been estimated at 0.09 Euro/m3 (Michailides et al., 2011), while the operational cost of constructed wetlands is negligible. Conversely, the cost for electrooxidation increases by two orders of magnitude (i.e., 9.5 Euro/m3) according to Eq. (3).
The proposed trickling filter-wetland-electrooxidation hybrid system
Based on the results derived from this work, as well as the results obtained when the biological filter was coupled only with electrooxidation (Tatoulis et al., 2016), a flexible hybrid system is proposed for the treatment of table olive washing water (TOWW). This system comprises a biological filter, a horizontal subsurface flow (HSF) constructed wetland and electrooxidation. As a rule of thumb, each of the three sequential stages should achieve a COD decrease by an order of magnitude. Specifically, the system may consist of the following:
-
1)
A biological trickling filter with mixed, aerobically grown indigenous cultures which is capable of treating TOWW with initial COD concentrations of up to 15 g/L (Tatoulis et al., 2016). The efficiency of the filter depends on the type of operating mode (i.e. batch or sequencing batch operation with recirculation) and on the hydraulic retention time. However, decolourization cannot be achieved even under the most favourable conditions when COD effluent is as low as 0.5 g/L. Therefore, a suitable post-treatment step should be applied to improve the quality of the final discharge in terms of residual pollutant content (Tatoulis et al., 2016).
-
2)
HSF constructed wetlands can be used as an intermediate polishing step for the biologically pre-treated TOWW. The use of constructed wetlands prior to electrooxidation leads to a final polishing step that can operate at mild currents and short treatment times.
-
3)
Electrochemical oxidation is deemed suitable as a polishing step to decolourize the effluent and lower the final COD value to under the permissible limit of 125 mg/L for municipal and industrial effluents (Gazette of the Government (GR) 2011/354Β). Although electrooxidation over a boron-doped diamond anode can treat biologically pre-treated effluents at concentrations of 2–5 g/L COD, this would require several hours at high current densities (up to 250 mA/cm2); thus, its combination with a biological filter and a constructed wetland may reduce the overall treatment cost.
Conclusions
A novel integrated TOWW treatment system is proposed, comprising a biological filter, an HSF constructed wetland and electrooxidation. This combination can fully mineralize and decolourize TOWW, which can then be safely disposed. Although a biological filter and electrooxidation have been successfully combined for TOWW treatment in the past (Tatoulis et al., 2016), their combination with constructed wetlands is likely to reduce the treatment cost of electrooxidation from up to 3 Euro cent per gramme of COD removed to 1.7 Euro cent per gramme of COD removed. Nevertheless, constructed wetlands have high surface area requirements; thus, when land availability is limited, the combination of a biological filter only with electrooxidation will be favoured. Constructed wetlands were used to treat TOWW for the first time and from these initial pilot-scale experiments, it can be concluded that:
HSF wetlands treating TOWW achieved significant COD (70 and 62 % for the OG-P and OG-U units, respectively) and total phenol (78 and 75 % for the OG-P and OG-U units, respectively) removal. COD removals are further enhanced (90 and 78 % for the OG-P and OG-U units, respectively) when HRTs are longer than 4 days.
The similar removal efficiencies recorded between the planted and unplanted wetland units indicate that organic matter was mainly removed by biological processes. Nevertheless, the presence of plant vegetation did slightly increase COD removal, thus proving the positive effect of vegetation on constructed wetland operation.
References
Akratos CS, Tsihrintzis VA (2007) Effect of temperature, HRT, vegetation and porous media on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecol Eng 29:173–191
APHA (1989) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington D.C
Beltran-Heredia J, Torregrosa J, Dominguez JR, Garcia J (2000) Aerobic biological treatment of black table olive washing wastewater: effect of an ozonation stage. Process Biochem 35:1183–1190
Benitez FJ, Acero JL, Gonzalez T, Garcia J (2001) Organic matter removal from wastewater of the black olive industry by chemical and biological procedures. Process Biochem 37:257–265
Benitez FJ, Beltran-Heredia J, Torregrosa J, Acero JL, Cercas V (1997) Aerobic degradation of olive mill wastewaters. Appl Microbiol Biotechnol 47:185–188
Bolton JR, Bircher KG, Tumas W, Tolman CA (2001) Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems. Pure Appl Chem 73:627–637
Borja R, Martin A, Garrido A (1993) Anaerobic digestion of black olive wastewater. Bioresour Technol 45:27–32
Bubba MD, Checchini L, Pifferi C, Zanieri L, Lepri L (2004) Olive mill wastewater treatment by a pilot-scale subsurface horizontal flow (SSF-h) constructed wetland. Ann Chim 94:875–887
Comninellis C, Kapalka A, Malato S, Parsons SA, Poulios I, Mantzavinos D (2008) Advanced oxidation processes for water treatment: advances and trends for R&D. J Chem Technol Biotechnol 83:769–776
Ganzenko O, Huguenot D, van Hullebusch ED, Esposito G, Oturan MA (2014) Electrochemical advanced oxidation and biological processes for wastewater treatment: a review of the combined approaches. Environ Sci Pollut Res 21:8493–8524
Gazette of the Government (GR) 2011/354Β. (In Greek)
Gikas GD, Tsakmakis ID, Tsihrintzis VA (2013) Treatment of olive mill wastewater in pilot-scale natural systems. Proc. of 8th Int. Con. of EWRA “Water resources management in an interdisciplinary and changing context”, Porto, Portugal, 26–29 June 2013, paper #232, pp 1207–1216
Grafias P, Xekoukoulotakis NP, Mantzavinos D, Diamadopoulos E (2010) Pilot treatment of olive pomace leachate by vertical-flow constructed wetland and electrochemical oxidation: an efficient hybrid process. Water Res 44:2773–2780
Herouvim E, Akratos CS, Tekerlekopoulou AG, Vayenas DV (2011) Treatment of olive mill wastewater in pilot-scale vertical flow constructed wetlands. Ecol Eng 37:931–939
International Olive Council (2002) The world market for table olives. Olivae 92:24–28
Kapellakis IE, Paranychianakis NV, Tsagarakis KP, Angelakis AN (2012) Treatment of olive mill wastewater with constructed wetlands. Water 4:260–271
Katsoni A, Mantzavinos D, Diamadopoulos E (2014a) Coupling digestion in a pilot-scale UASB reactor and electrochemical oxidation over BDD anode to treat diluted cheese whey. Environ Sci Pollut Res 21:12170–12181
Katsoni A, Mantzavinos D, Diamadopoulos E (2014b) Sequential treatment of diluted olive pomace leachate by digestion in a pilot scale UASB reactor and BDD electrochemical oxidation. Water Res 57:76–86
Kopsidas GC (1992) Wastewater from the preparation of table olives. Water Res 26:629–631
Kotsou M, Kyriakou A, Lasaridi K, Pilidis G (2004) Integrated aerobic biological treatment and chemical oxidation with Fenton’s reagent for the processing of green table olive wastewater. Process Biochem 39:1653–1660
Kyriacou A, Lasaridi KE, Kotsou M, Balisa C, Pilidis G (2005) Combined bioremediation and advanced oxidation of green table olive processing wastewater. Process Biochem 40:1401–1408
Martínez-Huitle CA, Rodrigo MA, Sirés I, Scialdone O (2015) Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chem Rev 115:13362–13407
Michailides M, Panagopoulos P, Akratos CS, Tekerlekopoulou AG, Vayenas DV (2011) A full-scale system for aerobic biological treatment of olive mill wastewater. J Chem Technol Biotechnol 86:888–892
Radjenovic J, Sedlak DL (2015) Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ Sci Technol 49:11292–11302
Rivas FJ, Beltran FJ, Gimeno O (2000) Joint treatment of wastewater from table olive processing and urban wastewater. Integrated ozonation-aerobic oxidation. Chem EngTechnol 23:177–181
Rivas FJ, Beltran FJ, Gimeno O, Alvarez P (2001) Chemical-biological treatment of table olive manufacturing wastewater. J Environ Eng 127:611–619
Rivas FJ, Beltran FJ, Gimero O, Alvarez P (2003) Treatment of brines by combined Fenton’s reagent-aerobic biodegradation. II. Process modeling. J Hazard Mater 96:277–290
Shutes RBE (2001) Artificial wetlands and water quality improvement. Environ Int 26:441–447
Sultana M-Y, Mourti C, Tatoulis T, Akratos CS, Tekerlekopoulou AG, Vayenas DV (2016) Effect of hydraulic retention time, temperature, and organic load on a horizontal subsurface flow constructed wetland treating cheese whey wastewater. J Chem Technol Biotechnol 91:726–732
Tatoulis TI, Zapantiotis S, Frontistis Z, Akratos CS, Tekerlekopoulou AG, Pavlou S, Mantzavinos D, Vayenas DV (2016) A hybrid system comprising an aerobic biological process and electrochemical oxidation for the treatment of black table olive processing wastewaters. Int Biodeterior Biodegrad 109:104–112
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. In: Lawton JH, Likens GE (eds) Methods in ecology. Blackwell Scientific Publications, Oxford
Wheatley A (1990) Anaerobic digestion: a waste treatment technology. Elsevier Applied Science, Barking
Acknowledgments
The second author gratefully acknowledges a post-doctoral scholarship from the Hellenic State Scholarships Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Tatoulis, T., Stefanakis, A., Frontistis, Z. et al. Treatment of table olive washing water using trickling filters, constructed wetlands and electrooxidation. Environ Sci Pollut Res 24, 1085–1092 (2017). https://doi.org/10.1007/s11356-016-7058-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7058-6