Abstract
The objective of this study is to evaluate the current status of heavy metal concentrations in constructed wetland, Shaoguan (Guangdong, China). Sediments, three wetland plants (Typha latifolia, Phragmites australis, and Cyperus malaccensis), and six freshwater fish species [Carassius auratus (Goldfish), Cirrhinus molitorella (Mud carp), Ctenopharyngodon idellus (Grass carp), Cyprinus carpio (Wild common carp), Nicholsicypris normalis (Mandarin fish), Sarcocheilichthys kiangsiensis (Minnows)] in a constructed wetland in Shaoguan were collected and analyzed for their heavy metal compositions. Levels of Pb, Zn, Cu, and Cd in sediments exceeded approximately 532, 285, 11, and 66 times of the Dutch Intervention value. From the current study, the concentrations of Pb and Zn in three plants were generally high, especially in root tissues. For fish, concentrations of all studied metals in whole body of N. mormalis were the highest among all the fishes investigated (Pb 113.4 mg/kg, dw; Zn 183.1 mg/kg, dw; Cu 19.41 mg/kg, dw; 0.846 mg/kg, dw). Heavy metal accumulation in different ecological compartments was analyzed by principle component analysis (PCA), and there is one majority of grouped heavy metals concentration as similar in composition of ecological compartment, with the Cd concentration quite dissimilar. In relation to future prospect, phytoremediation technology for enhanced heavy metal accumulation by constructed wetland is still in early stage and needs more attention in gene manipulation area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
China’s constructed wetlands are under great threats from reclamation, water diversion, dam construction, pollution, resource overuse, and biological invasion, leading to severe heavy metal contamination. Metals enter the environment in various ways such as via metal mining, erosion of geological materials, fossil fuel combustion, mine effluent outfalls, and industrial runoff. Metals can contaminate water and deteriorate sediments quality and may subsequently affect human health (i.e., top consumer in tropical level) and other biological attributes due to their toxicity, bioaccumulation, and potential biomagnification in the food chains. In wetland, aquatic portion is often closely monitored areas. Most plants (Wong et al. 2003) and fishes (Yilmaz 2006) are capable of accumulating metals from either the surrounding water or dietary sources (Alam et al. 2002; Canbek et al. 2007). Also, numerous studies about these subjects are based on accumulating high levels of trace metals in different kinds of fish (Cheung et al. 2008; Leung et al. 2014). Fish muscle tissue is a good indicator for assessing metal accumulation because the concentrations of metals in muscle reflect the bioavailable concentrations of metals in fishes readily consumed by human (Burger and Gochfield 2005) and other wildlife (Arcega-Cabrera et al. 2014).
Some of the studies on a constructed wetland in Shaoguan, Guangdong, China (Lan et al. 1992; Wong et al. 2003) have highlighted the spatial and temporal trends of heavy metal pollution. In addition, Li et al. (2014) have studied foods produced on soils impacted by mining activities in Shaoguan and indicated a potential health risk due to plant uptake of metal associated with such mining, but none has studied the bioaccumulation of heavy metals with an integration of various ecological components in this wetland so far. As this is one of the most important constructed wetland where large mining facilities, including smelting complexes, and mine refineries have been located along the boundaries of the wetland, a study on different ecological components is important to monitor heavy metal exposure in wetland system near the residential area. Henceforth, the purpose of this study was to evaluate the concentrations of four selected toxic metals (Pb, Zn, Cu, and Cd) in different components collected from the Shaoguan constructed wetland in an attempt to provide an insight into the current pollution status of constructed wetland which may be helpful in the future to monitor the pattern of bioaccumulation and biomagnification of heavy metals in the ecosystem.
Materials and methods
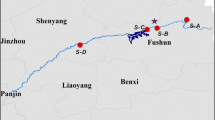
The study was carried out at Shaoguan wetland in China. Figure 1 illustrates the location and sampling points of the study constructed wetland in Shaoguan. Shaoguan is located in the subtropical region of south China. The mean annual temperature is about 20 °C and the extreme temperatures are 5 °C in January and 40 °C in July. Average annual rainfall is 1457 mm. The wetland consists of four cells consisting of a total land area of about 4 km2, and it is situated 100–150 m above sea level and is surrounded by rice fields. The total area of the wetland system is 96,622 m2. The system is enclosed by dam walls constructed of rock sand mine tailings. The water retention time is in the range of 5 to 7 days. This system could be divided into two parts: an upper portion that was a wetland dominated by cattail and lower portion a stabilization pond without physical separation. There were two entrances to the combined treatment system. Typha latifolia (cattail), Phragmites australis (common reed), and Cyperus malaccensis (Malacca Galingale) were the dominant plant species grown in the wetland area.
There were eight sampling sites in the inlet and outlet of each wetland cell (Fig. 1). Water samples were collected just below the surface water in the wetland using acid-cleaned polyethylene bottles. The samples were collected from the sampling points (Fig. 1) during the dry and wet seasons. There are a total of eight sampling sites × 2 types of water sample × 4 seasons × 5 replicates = 320 water samples. Surface water samples in the inlet of the wetland were taken daily in the whole year of study period. Water was immediately acidified with concentrated HNO3 and placed in an ice box and then brought to the laboratory and refrigerated.
Sediment samples were collected using a core sampler with a belowground level of 10, 20, and 30 cm. All the sediment samples were sealed in clean polyethylene bags and placed on ice during transport to the laboratory. There are a total of eight sampling sites × 3 layers × 4 seasons × 3 replicates = 288 sediment samples. All sediment samples were freeze-dried and then sieved through a 63-μm mesh to obtain the fine fractions, specifically silt and clay in which metals are often highly concentrated compared to coarse fractions (e.g., sand). The sieved sediments were used to determine total and diethylenetriaminepentaacetic acid (DTPA)-extracted concentrations of four metals (Pb, Zn, Cu, and Cd) according to USEPA method 3051A. DTPA is a widely used extraction approach to assess metal mobility in soil. DTPA is capable of releasing metals that are soluble, exchangeable, adsorbed, organically bound, and possibly some that are bound to oxides. Standard reference material National Institute for Standards and Technology (NIST) 8704 (Buffalo River sediment) was used to assess the accuracy of sample processing and analysis (Cheung et al. 2008), and metal determination and the mean recovery rates were 90 ± 10 %.
Plant samples including T. latifolia, P. australis, and C. malaccensis were collected in randomly selected areas of 1 m2 in each wetland cell. Five replicates of whole plant samples (shoot and root) were collected from the quadrats. For plant samples, there are a total of eight sampling sites × 3 plants species × 4 seasons × 5 replicates = 480 plant samples. All plant samples were placed in polyethylene Ziploc bags and transported to the laboratory for chemical analysis. All samples were rinsed with tap water and then with distilled water. Shoot and root tissues were separated, and the shoot samples were dried in an oven at 70 °C for 1 week. The dried tissues were weighed and ground to pass 2-mm standard mesh in preparation for chemical analysis according to USEPA method 3050B. Standard reference material of spinach leaves (NIST 1570a) was used to verify the accuracy of sample processing and metal analysis.
As the number of fishes was low in the contaminated wetland, fish living in the wetlands were collected as many as possible in the wetlands. Fish were transported in iceboxes immediately to the laboratory. The number, total length, and body weight of each specimen were recorded and measured according to the method described in Cheung et al. (2008), and the results are shown in Table 1. The fish samples were sealed in ziplock bags and frozen at −20 °C. After the fish were thawed, it was rinsed thoroughly with deionized water; each fish was dissected by a stainless steel scalpel to obtain the muscle tissues. After dissection, each muscle sample was freeze-dried. The dried fish muscle was then homogenized and stored in desiccators before metal analyses. Biota samples were digested by a mixture of concentrated HNO3 and HCl according to USEPA method 3050B. Standard reference material NIST 1566b oyster tissue and NRCC TORT-2 lobster hepatopancreas were used to verify the accuracy of sample processing and metal determination. The recovery rates were 90 ± 10 %.
All of the digests were filtered through Advantec Grade 5C filter paper and stored at 4 °C. Pb, Zn, Cu, and Cd in filtered digests were determined by inductively coupled plasma optical emission spectrometry (Optima 3000 DV; Perkin Elmer). Metal contents were expressed as milligrams per kilogram (dry weight) of all samples. The metal concentration of standard reference material was checked, and the recovery rates were within 95 ± 10 %.
Statistical analysis
Analysis of variance was performed on all experimental data, and means were compared using Duncan’s multiple range test with SPSS (Chicago, IL) software. The significant level was p < 0.05.
Principal component analysis of the wetland system
Variable input for PCA included Pb, Zn, Cu, and Cd content in each ecological compartment. A total of 1500 raw data were included, and the data were first examined by Kaiser–Meyer–Olkin (KMO) statistics and Bartlett’s test for suitability for PCA, before they were processed using the Primer 6 software. Those tests are measures of sampling adequacy that use the proportion of variance. The KMO value must be greater than 0.5, and the significance level of the Bartlett’s test must be less than 0.05. When the eigenvalue of a principal component is equal to, or greater than 1, the result of the PCA is considered significant. To minimize the variations among the variables for each factor, the factor axes were varimax-rotated. Rotating the principal components can produce a meaningful representation of the underlying factors by decreasing the contribution of variables with minor significance and increasing the contribution of those with more significance.
Health risk assessment
According to USEPA guideline (USEPA 2000), effective ingested dose of trace elements by humans is calculated as follows:
where
- Em:
-
Effective ingested dose of analyte m in the population of concern averaged over a 70-year lifetime (mg/kg/day).
- Cm :
-
Concentration of chemical m in the edible portion of the species of interest (mg/kg, dw).
- CR:
-
Mean daily consumption rate of the species of interest by the general population or subpopulation of concern averaged over a 70-year lifetime (kg/day) (i.e., 142.2 g/day, estimate of average consumption of fish and shellfish by people in south China).
- Xm :
-
Relative absorption coefficient or the ratio of human absorption efficiency to test animal absorption efficiency for chemical m (dimensionless). In most instances, relative absorption coefficients (X m ) are assumed to be 1.0.
- BW:
-
Mean body weight of the general population or subpopulation of concern (kg) (i.e., 70 kg, average adult body weight).
Then, calculated results were compared to reference dose (RfD) (USEPA 2000). RfD is an estimate of a daily exposure to the human population (including sensitive subpopulations) that is likely to be without appreciable risk of deleterious effects during a lifetime.
Results
Performance of wetland to purify wastewater
Table 2 shows the retention percentage of metals by wetland in the study period. The total metal budget (g/year) of Pb, Zn, Cu, and Cd in the effluent were 710,208, 1,945,294, 57,981, and 4302, respectively. The unloading of metals at the outlet showed that less than 1 % of the metal budget was found. On average, most of the metals removed by the wetland were retained in the sediment (99.9 %), mediated by the wetland plants. However, only a small amount of heavy metals were accumulated in the plants.
Metal levels in different ecological compartments
The range total and DTPA-extractable concentrations in sediment were the following: Pb 3570–45,233 and 1780–6087 mg/kg, dw; Zn 4154–40,005 and 306.4–672 mg/kg, dw; Cu 54.4–388.7 and 11.05–92.7 mg/kg, dw; and Cd 15.02–53.11 and 1.98–4.09 mg/kg, dw, respectively (Table 3). All metal levels in sediments were higher than the other compartments such as fishes and plants (p < 0.05). Sediment generally had higher Pb and Zn concentrations than the other heavy metals. All sediment samples exceeded the Dutch Intervention value (i.e., Pb = 85 mg/kg, dw; Zn = 140 mg/kg, dw; Cu = 36 mg/kg, dw; Cd = 0.8 mg/kg, dw) (Netherlands Ministry of Housing 1994).
Average concentrations of Pb, Zn, Cu, and Cd in plant shoot and roots varied considerably for each element during the study period (Table 4). Among the wetland plant species tested, the greatest concentration of Pb in shoots was found in C. malaccensis, following by T. latifolia and P. australis. However, T. latifolia accumulated the highest concentrations of Zn, Cu, and Cd in its shoots among the three species. In the root, the highest concentrations of Pb, Zn, and Cd were found in T. latifolia among the three species studied.
The ranges of metal concentration in fish muscle were the following: Pb 16.54–113.4 mg/kg; Zn 35.44–183.1 mg/kg; Cu 4.352–19.41 mg/kg; and Cd 0.347–0.846 mg/kg. Metal concentrations in N. normalis were significantly (p < 0.05) greater (Pb 113.4 mg/kg, Zn 183.1 mg/kg, Cu 19.41 mg/kg, and Cd 0.846 mg/kg) (dw) than the other five fish species (Table 5). In addition, Pb (0.1 mg/kg) and Cd (0.5 mg/kg) levels of all fishes analyzed were above the permitted concentration according to the China National Standards Management Department Standard (2001).
Health risk assessment
Table 6 shows the estimated daily intakes and reference dose value (Rfd) for trace elements recommended by USEPA. In general, the average value of Pb and Cd was close to the RfD values. Zn and Cu showed lower value of effective ingested dose than the RfD value. Thus, except N. normalis, other fishes may not cause immediate health risk to people who subsist in Shaoguan wetland by comparing with Rfd value. High Rfd value in N. normalis indicated that it may be threated to the health of consumers, especially subsistence fishermen.
Principal component analysis (PCA) in constructed wetland
The PCA is performed on the heavy metals in ecological compartments collected in the wetland (Fig. 2). Sites of greater similarities are plotted closer together, while sites of low similarity are further apart. The PCA showed two distinct groups according to their metal concentrations. There is one majority of grouped heavy metals concentration as similar in composition of ecological compartment, with the Cd concentration quite dissimilar.
Discussion
Comparison of heavy metal concentration in sediment with Dutch guideline
Levels of Pb, Zn, Cu, and Cd in sediments collected from wetland were above the value stated by the Dutch guidelines. Dutch Intervention Value, established by Ministry of Housing, Spatial Planning, and the Environment (VROM), is used by the authority to identify areas that are “Seriously Contaminated” and are only intended for use in evaluating pollution status and properties. According to Quintin et al. (2010), the DIVs for soil were developed for use in determining whether land that is “already contaminated” poses a serious threat to the public health. In addition, the DIVs are intended to be applied on a spatial scale, not for comparing to individual sample results. Therefore, Dutch Target Values, which are intended to protect sustainable soil quality and have an ecological health basis, are intended for use in evaluating “uncontaminated” land. Total Pb, Zn, Cu, and Cd sediments were approximately 532, 285, 11, and 66 times of those values. Increased metal concentrations reflected long-term pollution caused by human activities. Shaoguan wetland is situated in mining site, and smelting factories are located in this area, so high concentrations of metals that are indices of pollution in sediment samples can be explained. Also, disposal of municipal wastes, old machinery, and poor agricultural practices by local farmers could be the most important sources of heavy metals in the waterways in the area of study.
Apparently, high metal content was retained into all wetland cells, and the concentration ranges of total Pb, Zn, Cu, and Cd were 5202–45233, 5350–40005, 74.4–388.7, and 15.02–79.03 mg/kg, respectively. The direct of such purification system minimized the environmental health hazards and also lessened the deterioration of the natural environment and disruption of the ecological balance. The combined system would have the following mechanisms for capturing heavy metal from wastewater released from mining industry. In physical aspect, the primary role in suspended solids removal is to limit resuspension of settled particulate matter (DeBusk 1999). The surface water typically moved very slowly through wetlands due to the resistance provided by rooted plants. The low and nonturbulent flow velocity promoted sedimentation of suspended solids, resulting in accumulation of solids and associated contaminants on the wetland soil surface. In chemical aspect, the most important chemical removal process in wetland soils is absorption, which results in short-term retention or long-term immobilization of several classes of contaminants. The potential of removing heavy metals from solution by plant uptake is mainly due to chemical precipitation and sorption of sediment, aided by the marcophytes. Although the submerged portion of the shoot in wetland plants has less filtration and bacterial support potential than the roots, it has the advantages of extending through the entire water column. So, the plants play a critical role in metal retention via filtration, adsorption, and cation exchange, and through plant-induced chemical changes in rhizosphere (Dhir et al. 2009), although the concentration of heavy metal in the wetland plants was lower than in the sediment. In biological aspect, herbaceous plants, plant detritus, or litter were contributed to accumulate heavy metals at the soil/sediment surface. In most wetlands, since the rate of decomposition was substantially decreased under the anaerobic (oxygen-depleted) conditions in wetland soil, the rate of organic matter decomposition is lower than the rate of organic matter deposition on the soil, and formation of peat occurs (DeBusk 1999). As a result, some of the contaminants originally taken up the plants that can be trapped and stored as peat. Peat may accumulate to great depths in wetlands and can provide long-term storage for contaminants. Besides, the symbiotic relationship between plant and bacteria in root was induced to form iron oxyhydroxides (Fleming et al. 2014), and it can play a key role in the metal retention process in wetland. As the amount of mature plants was larger in cell 1 than in other cells and such adult plants were grown in the well-modified rhizosphere environment, as a result, the plants have much better developed aerenchymatous tissue and higher oxygen transport and radial oxygen loss capabilities (Pezeshki et al. 2012) and great ability to modify the rhizosphere and to immobilize metals in the rhizosphere. Therefore, high concentrations of heavy metals were found in wetland cell 1 than in the wetland cells 2, 3, and 4 that were subjected to the formation of such compound, resulting in higher metal immobilization in the rhizosphere of plants.
Other than the factors of wetlands, external factors such as climate, season, and nutrient availability (Olaniran et al. 2013) may affect the amount of metal retention in the wetland. The fact that the Fankou wetland systems were not linked to seasonal changes is very beneficial because it ensures that discharges are in compliance with regulatory limits throughout the year and achieved 99 % reduction of heavy metals in wastewater.
Heavy metal accumulations in wetland plants
The results showed high heavy metal concentrations in all plants. Macrophytes, typically classified as submerged, floating, or emergent plant species, are widely distributed in various aquatic environments from fresh to salt water, as well as the littoral through pelagic zones. They have several characteristics favorable for metal accumulation. First, in terms of biomass, aquatic and wetland macrophytes are the predominant organisms in the highly productive, littoral ecosystems, such as wetlands and seabeds (Brix and Schierup 1989). Second, leaves and epiphytes provide an expanded area to trap particulate matter, absorb metal ions, and accumulate and sequester pollutants (Ward 1987). Third, rooted species can absorb metals through their roots and rhizomes as well as through their leaves (Welsh 1980). Fourth, aquatic macrophytes are stationary and constantly exposed to (and absorbing) contaminants such as Pb, Zn, Cu, and Cd (Say et al. 1981). Thus, application of the wetland plants for remediating pollutants from industrial wastewaters and act as a sink for pollutants is common around the world.
By comparing heavy metal concentration in ecological compartment by PCA, a significant difference of Cd levels was found. It may pose hazard to human’s health as human contact with biota in wetland because Cd has been linked to foodborne illness, with ecological compartment likely serving as a vehicle. By observing the site nearby the wetland, rice field around the study site is also a critical factor for high concentration of Cd at the wetland. According to Wu et al. (1999), due to the unique genotype of rice in the affinity to Cd accumulation, more than a twofold difference was observed for Cd accumulation in rice grown in South China than the same species grown in other regions. Thus, bioavailable form of Cd may flow through the wetland and consequently accumulated by rice and hence absorbed and accumulated by fishes via ingestion and assimilation.
Heavy metal accumulations in fishes and risk assessment
Due to the availability of metals in different media, variation of the accumulation of trace metals in various tissues of fishes was noticeable. By comparing with the previous results (Chen 1990), substantial increase of metal concentration was found in the current study (Table 4). Besides, the accumulation of heavy metals in N. normalis was higher than other species. In fact, this fish species contains the highest lipid content, and thus, it may accumulate more bioavailable form of heavy metals due to its lipophilic nature, although factors such as size, age, and feeding habits will also affect the rates of their bioaccumulation (Wang and Fisher 1999). The major food source of most of the fish living in the wetland includes residues of crops and grasses and, to certain extent, contaminated sediment. The uncontrolled disposal of mine waste has contributed to the high levels of heavy metals detected in the plant tissues, which reached the wetland as a final sink. In addition, bioconcentrations from water via the gills, skin, and ingestion of contaminated sediment are possible routes for heavy metals to accumulate in fish tissues, and the rate of bioaccumulation depends mainly on their feeding preference, general behavior, and trophic level of fish (Fisher 1995; Lake et al. 1995). Fish living in wetland are benthic organisms and have frequent contact with the water–sediment interface, which can have higher metal concentrations than the water column and can be enriched with these compounds. In addition, according to our site observation, release of heavy metals during anthropogenic combustion of metal accumulating and hyperaccumulating plants by farmer in wetland will eventually cause the deposition of heavy metals on the remote sites. If these toxic particles settled on water surface, they can be incorporated onto larger fecal pellets or suspended matter and will then result in relatively rapid deposition into sediments and eventual uptake by fishes.
Daily intake of heavy metals was estimated on the basis of the concentrations measured in fish muscle and daily fish consumption rate. The present results showed that high consumption rate (142 g/day/person) of fish taken from the wetland might impose health hazards on the local population, especially to those who are more susceptible to Pb toxicity. Local people from poor urban areas in China where old paints still cover walls continue to be routinely contaminated by Pb ingestion, partly stimulated by the fact that the lead-bearing paint has a sweet taste. Furthermore, their children are more susceptible to neurotoxicants through breastfeeding. As reported by Charlet et al. (2012), lead produces encephalomyelopathy in newborn rats but not in adults, apparently because of differences in the stages of development of the blood–brain barrier.
Comparison of current results with other studies
By comparing with Lan et al. (1992), the current study was similar in which 4942 mg/kg of Pb was found in sediments, 350 mg/kg of Pb found in cattails, and 850 mg/g of Pb in Paspalum. Besides, bioaccumulation of heavy metal by T. latifolia was in line with the findings of Rai et al. (2009) in which the flows of mine drainage contributed metals to water column exchanging with porewaters in sediments and mobilized into substrate by the growth of cattails.
Conclusion
Sediments contaminated with heavy metals are of great concern in the wetland investigated in this study. In addition to direct metal contamination of wetlands, there were other problems associated with wetland health and conservation such as nonpoint source pollution, carbon storage loss, and biological invasion. Due to the increasing concern regarding chronic diseases caused by heavy metal in China recently, especially on the possible linkage between fish and crop consumption and their human loadings, it is necessary to increase transparency by improving and updating information on different heavy metal concentrations in the contaminated wetland in order to establish a framework for proper management and control by authorities. In relation to future prospect, phytoremediation technology for enhanced heavy metal accumulation by constructed wetland is still in early stage and needs more attention in gene manipulation area.
References
Alam MGM, Tanaka A, Allinson G, Laurenson LJB, Stagnitti F, Snow ET (2002) A comparison of trace element concentrations in cultured and wild carp (Cyprinus carpio) of Lake Kasumigaura, Japan. Ecotoxicol Environ Saf 53:348–354
Arcega-Cabrera F, Norena-Barroso E, Oceguera-Vargas I (2014) Lead from hunting activities and its potential environmental threat to wildlife in a protected wetland in Yucatan, Mexico. Ecotoxicol Environ Saf 100:251–257
Brix H, Schierup HH (1989) Use of aquatic macrophytes in water-pollution control. Ambio 18:100–107
Burger J, Gochfeld M (2005) Heavy metals in commercial fish in New Jersey. Environ Res 99:403–412
Canbek M, Demir TA, Uyanoglu M, Bayramoglu G, Emiroglu O, Arslan N, Koyuncu O (2007) Preliminary assessment of heavy metals in water and some Cyprinidae species from the Porsuk River, Turkey. J Appl Biol Sci 1:91–95
Charlet L, Chapron Y, Faller P, Kirscha R, Stoned A, Baveyee PC (2012) Neurodegenerative diseases and exposure to the environmental metals Mn, Pb, and Hg. Coord Chem Rev 256:2147–2163
Chen GZ, Ma MJ, Lan CY, Zeng SS, Li SH (1990) Investigations on a cattail plant purification pond ecosystem. Chin J Ecol 9:11–15
Cheung KC, Leung HM, Wong MH (2008) Metal concentrations of common freshwater and marine fish from the Pearl River Delta, South China. Arch Environ Contam Toxicol 54:705–715
China National Standards Management Department (2001) Safety qualification for agricultural product for non-environmental pollution aquatic products. GB 18406.4-2001. Beijing, China
DeBusk WF (1999) Wastewater treatment wetlands: contaminant removal processes. Soil and Water Science Department. Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida
Dhir B, Sharmila P, Saradhi PP (2009) Potential of aquatic macrophytes for removing contaminants from the environment. Crit Rev Environ Sci Technol 39:754–781
Fisher SW (1995) Mechanisms of bioaccumulation in aquatic systems. In: Ware GW (ed) Reviews of environmental contamination and toxicology, vol 142. Springer, New York, pp 87–118
Fleming EJ, Cetinić I, Chan CS, King DW, Emerson D (2014) Ecological succession among iron-oxidizing bacteria. ISME J 8:804–815
Lake JL, Mckinney R, Lake CA, Osterman FA, Heltshe J (1995) Comparisons of patterns of polychlorinated biphenyl congeners in water, sediment and indigenous organisms from New Bedford Harbour, Massachusetts. Arch Environ Contam Toxicol 29:207–220
Lan C, Chen G, Li L, Wong MH (1992) Purification of wastewater from a Pb/Zn mine using hydrophytes. In: Cooper PF, Findlater BC (eds) In constructed wetlands in water pollutuion control. Pergamon Press, Oxford, pp 419–428
Leung HM, Leung AOW, Wang HS, Ma KK, Liang Y, Ho KC, Cheung KC, Tohidi F, Yung KKL (2014) Assessment of heavy metals/metalloid (As, Pb, Cd, Ni, Zn, Cr, Cu, Mn) concentrations in edible fish species tissue in the Pearl River Delta (PRD), China. Mar Pollut Bull 78:235–245
Li JH, Dong F, Lu Y, Yan QY, Shim HJ (2014) Mechanisms controlling arsenic uptake in rice grown in mining impacted regions in South China. PLOS One 9, Article Number: e108300
Netherlands Ministry of Housing (1994) Dutch intervention values for soil remediation (Report HQ 94-021). Environmental Quality Objectives in the Netherlands, Ministry of Housing, The Hague
Olaniran AO, Balgobind A, Pillay B (2013) Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci 14:10197–10228
Pezeshki SR, DeLaune RD (2012) Soil Oxidation-Reduction in wetlands and its impact on plant functioning. Biol (Basel) 1:196–221
Quintin A, Fraiser L (2010) Comparison of international risk-based screening levels. Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy: Vol. 15, Article 24
Rai PK (2009) Heavy metals in water, sediments and wetland plants in an aquatic ecosystem of tropical industrial region, India. Environ Monit Assess 158:433–457
Say PJ, Harding JPC, Whitton BA (1981) Aquatic mosses as monitors of heavy metal contamination in the River Etherow, Great Britain. Environ Pollut B Chem Phys 2:295–307
USEPA (2000) Guidance for assessing chemical contaminant data for use in fish advisories, third edition, risk assessment and fish consumption limits, vol. 2, 2000
Wang WX, Fisher NS (1999) Assimilation efficiencies of chemical contaminants in aquatic invertebrates: a synthesis. Environ Toxicol Chem 18:2034–2045
Ward TJ (1987) Temporal variation of metals in the seagrass Posidonia australis and its potential as a sentinel accumulator near a lead smelter. Mar Biol 95:315–321
Welsh RPF, Denny P (1980) the uptake of lead and copper by submerged aquatic macrophytes in two English lakes. J Ecol 68:443–455
Wong et al. (2003) The water quality of influent and effluent of constucted wetland in constructed wetland. In: Environmental Pollution and Ecological Restoration Chapter 8, 236. The Science Publication [in Chinese]
Wu QT, Chen L, Wang GS (1999) Differences on Cd uptake and accumulation among rice cultivars and its mechanism. Acta Ecol Sin 19:104–107 [in Chinese]
Yilmaz F (2006) Bioaccumulation of heavy metals in water, sediment, aquatic plants and tissues of Cyprinus carpio from Kizilirmak, Turkey. Fresenius Environ Bull 15:360–369
Acknowledgments
Special thanks to Ms. Wendy Wong (HKBU) for field sampling at Shaoguan
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Leung, H.M., Duzgoren-Aydin, N.S., Au, C.K. et al. Monitoring and assessment of heavy metal contamination in a constructed wetland in Shaoguan (Guangdong Province, China): bioaccumulation of Pb, Zn, Cu and Cd in aquatic and terrestrial components. Environ Sci Pollut Res 24, 9079–9088 (2017). https://doi.org/10.1007/s11356-016-6756-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6756-4