Abstract
Pioneer native plant species from weathered oil spill-affected sites were selected to study their potential for phytoremediation on the basis of their ecological and phenological changes during the phytoremediation process. Experiments were conducted in field and in greenhouse. In field, native plants from aged oil spill-impacted sites with up 400 g of weathered petroleum hydrocarbons per kilogram soil were selected. In the impacted sites, the principal dominant plant species with potential for hydrocarbons removal were Cyperus laxus, Cyperus esculentus, and Ludwigia peploides. In greenhouse, the phenology of the selected plant species was drastically affected by the hydrocarbons level above 325 g total petroleum hydrocarbons (TPH) per kilogram soil after 2 years of phytoremediation of soils from the aged oil spill-impacted sites. From the phytoremediation treatments, a mix-culture of C. laxus, C. esculentus, and L. peploides in soil containing 325 g TPH/kg soil, from which 20.3 % were polyaromatic hydrocarbons (PAH) and 34.2 % were asphaltenes (ASF), was able to remove up 93 % of the TPH, while in unvegetated soil the TPH removal was 12.6 %. Furthermore, evaluation of the biodiversity and life forms of plant species in the impacted sites showed that phytoremediation with C. esculentus, alone or in a mix-culture with C. laxus and L. peploides, reduces the TPH to such extent that the native plant community was progressively reestablished by replacing the cultivated species resulting in the ecological recovery of the affected soil. These results demonstrate that native Cyperus species from weathered oil spill-affected sites, specifically C. esculentus and C. laxus, alone or in a mix-culture, have particular potential for phytoremediation of soils from tropical wetlands contaminated with weathered oil hydrocarbons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination of wetlands by oil spills is a significant environmental problem in most oil production areas affecting the aquatic and terrestrial ecosystems (Ke et al. 2002; Lin et al. 2002a). Such affectation may produce critical ecological changes, as those observed in the wetlands at the eastern tropical zone of Tabasco, Mexico, which has been dramatically affected for several years by chronic oil spills and is facing strong necessity to recover the impacted areas (Vazquez et al. 2002; Molina et al. 2004; Ponce-Velez et al. 2006). In these wetlands, several aged contaminated sites contain more than 400,000 mg total petroleum hydrocarbons (TPH) per kilogram soil, resulting in drastic ecological changes in the groundwater, the soil quality, and the soil fauna and flora (Martínez et al. 2000; Gallegos et al. 2000). Although the hydromorphic pedofeatures and biological activities of soils from these sites have been studied regarding to the behavior of the hydrocarbons accumulation (Gutierrez and Zavala, 2002), there are few reports over the ecological recovering of the contaminated sites (Molina et al. 2004; Escalante-Espinoza et al. 2005). It has been reported that several plant species in similar tropical oil-contaminated sites containing up 5 % (w/w) of a heavy-crude-oil are killed upon the contact with the hydrocarbons (Merkl et al. 2004, Merkl and Schultze-Kraft 2005). However, owing to the natural dynamic of an ecosystem, revegetation of the sites by emergence of invasive putative oil-tolerant plant species occurs, which has been reported to be a result of the phenophases and ecological adjustment to the hydrocarbons presence (Mendelssohn et al. 2002; Lin et al. 2005a). Nevertheless, although more investigation is needed to understand the dynamic and dramatic phenological and ecological changes of these oil-tolerant plants species, they can be useful for remediation of similarly contaminated sites thorough phytoremediation (Palmroth et al. 2006; Sudová et al. 2007). Indeed, some plant species tolerant to the presence of oil hydrocarbons have been applied successfully for the recovering of oil-impacted sites (Tischer and Hübner, 2002; Liste and Felgentreu, 2006). However, only few studies have used plant species indigenous from the impacted sites (Brown and Nadeau, 2002; Gallegos et al. 2000), and in most cases where the applied plants were alien to the contaminated zone the phytoremediation strategies have failed (Kulakow et al. 2000; Gallegos et al. 2000). In addition, studies performed ex situ using synthetic supports or spiked uncontaminated soil instead of real contaminated soils (Escalante-Espinoza et al. 2005; Palmroth et al. 2002) ignore the significance of plant-microorganism interactions in the rhizosphere between native species, fundamental in phytoremediation processes (Handa and Jefferies, 2000; Merkl et al. 2005; Abedi-Koupai et al. 2007). That is, native microflora is preferred by native plants since they are adapted each other at the contaminated conditions (Rivera-Cruz et al. 2004; Escalante-Espinoza et al. 2005; Liste and Felgentreu, 2006). For example, in a previous study to investigate the type of microflora associated with plant species cultivated in highly hydrocarbon-contaminated soils, several nitrogen-fixing bacteria showing specific hydrocarbon degradation were found to promote both the plant growth and the hydrocarbon removal (Pérez-Vargas et al. 2000; 2001).
The objectives of this work were as follows: (1) in field, to select oil-tolerant plant species from aged oil spill-affected sites on the bases of the ecological changes (density, dominance, frequency, importance value) of the native plant community regarding to uncontaminated surrounding areas, and (2) in greenhouse, to investigate the phenological changes and the TPH removal capability of the selected plants when cultivated in soils from similar aged oil spill-contaminated sites with high content of weathered oil hydrocarbons evaluated by the content of polyaromatic hydrocarbons (PAH) and asphaltenes (ASF) as indicators of the weathering effect on the petroleum hydrocarbons (Merkl et al. 2004, 2005; Merkl and Schultze-Kraft 2005). The results from this work with native plant species from these weathered oil spill-affected sites might have particular potential for the phytoremediation of tropical wetlands contaminated with oil hydrocarbons.
Material and methods
Two experiments were conducted: in field and in greenhouse. In field, the ecological changes of oil-tolerant plant species found in oil spill-impacted sites with high amounts of weathered petroleum hydrocarbons, measured as PAH and ASF, were studied. Three long term (several years: S1, S5, S6) and five recent (6–12 months: S2, S4, S7, S8, S9) oil spills-impacted sites with high amounts of weathered petroleum hydrocarbons with a site impacted in 1980 (S3) but ecologically recovered and unimpacted since then (Table 1) were explored for this work. On the bases of their availability along the study, biodiversity and TPH level, the long-term S1 site, and the recent S2 and S4 sites, were used for this work. The ecologically recovered and unimpacted site S3, which resembled the plant biodiversity of the surrounding areas, was used as control. In greenhouse, two studies were conducted: the phenology of putative oil-tolerant plant species sampled at the long-term oil-impacted site S6 or grown from their seeds and their hydrocarbon removal capability from soils collected from the selected sites.
Selection of the sites of study and soil characterization
Nine environmentally aged affected sites (Table 1) from the oil extraction and exploration zone of Petroleos Mexicanos (PEMEX) affected for several years by chronic oil spills (Vazquez et al. 2002; Molina et al. 2004; Ponce-Velez et al. 2006) and with high content of weathered oil hydrocarbons were inspected and selected for the study in the tropical wetlands of Tabasco, Mexico (Fig. 1), between the coordinates at 18° 00″ N to 18°15″ N and long 93° 43″ W to 94° 01″ W (Table 1). The soil was air-dried, mixed, and sieved through a No. 10 mesh (2 mm sieve) and then classified on the bases of the texture evaluated by its sand-silt-clay composition measured by the hydrometer method (Ashworth et al. 2001) . The sites S1 and S2, showing drastic ecological changes in the groundwater, the soil quality, and the soil fauna and flora were chosen for the ecological and phenological studies. The selection of the putative oil-tolerant plant species was performed on the bases of the ecological changes (density, dominance, frequency, and importance value) observed in the plant community present in aged oil spill-impacted sites containing up 400,000 mg total petroleum hydrocarbons (TPH) per kilogram soil after a field study coordinated by the Interdisciplinary Commission of Environmental and Social Development of Tabasco (CIMADES). To monitor the natural progression of revegetation in an unvegetated and clean site, the site S3 was used because by the time of this study PEMEX replaced the contaminated native soil of this site with new soil from a surrounding uncontaminated area.
Ecology of species
Census of vegetation in eight randomly selected quadrants (1.0 × 1.0 m) from each site was performed as reported by Müeller-Dumbois and Ellemberg (1974). Plant community composition and main ecological attributes (density, dominance, frequency, and importance value) were analyzed according to Noble and Slayter (1980), Cox (1981), and Lambers et al. (1998). Taxonomic identification and herbal study were performed with support of the Mexican National Herbarium, UNAM (MEXU), as reported by Fidalgo and Bononi (1984), and Lot and Chiang (1986), respectively.
Phenology
Phenological characteristics to evaluate the phenology were germination, survival, and development. Seeds from Cyperus laxus, Cyperus esculentus, and Ludwigia peploides found in a 20-year-old contaminated site containing 280,000 mg TPH/kg soil (S6, Table 1) were collected, sowed, germinated, and cultivated by triplicate in greenhouse in pots without drainage and with soils from the sites S1, S2, and S3 in two types of pot systems. In the first system, pots of 215 × 215 × 194 mm (w × l × h) containing 11 kg of soil were sowed with hundred seeds of individual species. In the second system, pots of 215 × 596 × 194 mm (w × l × h) containing 32 kg of soil were sowed with hundred seeds from each of the three plant species (mix 1), or a mixture of hundred seeds of L. peploides and C. laxus (mix 2), or C. esculentu and, C. laxus (mix 3). A set of each pot system was not sowed (unvegetated) and used as reference to investigate the natural bank of seeds and TPH removal and in each site as reported by Primack and Kand (1989). The pots were incubated in a greenhouse at 28 °C, 45–50 % relative humidity, and kept partially flooded by adding distillate water as required to mimic the field conditions. Germination, survival, and development of individual plants were monitored by using a combination of the Baskin and Baskin (1988), and Rathcke and Lacey (1985) methods. The germination frequency was determined by the radicle and cotyledon emergence as reported by Amadi et al. (1993) and Maila and Cloete (2002). Development, monitored by morphometric evaluations, and survival were performed as reported by Mückschel and Otte (2003).
TPH assay
Analysis of TPH was performed using the US-EPA Method 8015B (1986). At least three independent samples of 3 g of soil were extracted three times with 5 mL of dichloromethane by stirring 2 h in conical tubes and then centrifuged at 8000g for 10 min. The supernatant was pooled and concentrated in a rotary evaporator at 30 °C. The remnant was resuspended in 1 mL of hexane and GC analyzed for the TPH content as reported previously (Pérez-Vargas et al. 2000). For the analysis, 2 μL of the hexane solution was injected in a Perkin Elmer (Auto System) GC equipped with a flame ionization detector and a CP-Sil 8CV (25 m × 0.32 mm ID) column, using nitrogen at pressure of 6 psi. The temperature was set up at 250 °C for the injector and 280 °C for the detector. The program for the column oven was from 60 °C/10 min to 240 °C/0 min changing at 15 °C/min; increasing at 5 °C/min up to 300 °C/16 min. The results were corrected for the TPH basal content and background-extractable material by comparison with soil from the site S3.
PAH and ASF assay
Extraction of polyaromatic (PAH) and asphaltenes (ASF) was performed using the US-EPA Method 3550B (1996) with acetone/hexane (1:1, v/v) and analyzed by HPLC as previously described (Rivera Casado et al. 2008). Three independent samples of 3 g of soil were extracted by 10 min sonication with 5 mL of the extraction mixture in centrifugal screw top glass tubes and then centrifuged at 8000g for 10 min. Separation of PAH and ASF was achieved using a Prodigy ODS2 5 μm column (250 mm × 4.6 mm i. d.) from Phenomenex (Cheshire, UK). Elution was performed using a gradient of 1 mM trifluoroacetic acid (TFA) and acetonitrile (ACN), as follows [time (min), 1 mM TFA (%)]: 0–5, 90; 5–25, 80; 25–45, 35; 45–60, 20; 60–70, 5; 70–85, 10. Under these conditions, using the Supelco-CRM48905 Polynuclear Aromatic Hydrocarbons Mix, the detection limit for PAH was between 5 to 80 μM pmol/injection (20 μL).
Phytoremediation effect on TPH removal
It was evaluated after 2 years of phytoremediation treatment using the above pot systems described to evaluate the phenological changes. The TPH content was evaluated in both systems cultivated and uncultivated (no plants) as indicated in Table 4. A set of pots containing 11 kg of soil from sites S1–S4 were cultured with young vigorous plants of Echinocloa polystachya, C. esculentus, L. peploides, and Carex crus-corvis collected from the site S6. Another set of pots were cultured with plants grown from seeds collected from plants fund at the same oil spill-affected site S6.
Statistical analyses
The SPSS V 15 statistical package (SPSS Inc, 2006) and Microsoft Excel 2002 software were used. The TPH effect over the TPH removal in the phytoremediation treatments was evaluated by the ANOVA of the residual TPH. For post hoc analysis to evaluate statistical differences between treatments, the Duncan’s test at the 0.10 probability level was used. All data represent averages of at least three independent samples.
Results
Experiment I
Characterization of the selected sites of the study and soil classification
The area of study was located 20 m above sea level in the tropical wetlands of Tabasco, Mexico (Fig. 1), with warm-humid climate, average temperature of 27 °C, and rains in the summer, annual average precipitation of 2159 mm (INEGI 2001), and subjected to chronic oil spills (CIMADES 2000). The soil in this area could be classified in two types: well-drained, sandy soils and badly drained with clay composition soils suffering flooding periods. The soil was mostly Gleysol type, and on the bases of the clay (41.7 ± 3.9 %), silt (17.8 ± 6.1 %), and sand (40.4 ± 7.6 %) composition, its texture was clay or sandy-clay showing slow internal drainage (Table 1). Consequently, the contaminated sites selected for the ecological studies and sampling of soil and plants, were mostly flooded with formation of bodies of water (Fig. 2c, e), and as shown in Table 1, with high content of THP (145–400 g/kg soil) and weathered hydrocarbons; from which 14–20 % of the THP were PAH and 20–49 % were ASF (Table 1). Historical data from CIMADES (2000) revealed that the sites S1, S5, and S6, were long-term impacted sites (first impacted in 1950 and the other two in1980), while S2, S4, S7, S8, and S9 were recent impacted sites (in 1999 or 2000). Afterwards, all these sites suffered chronic contamination by further oil spills and by the normal hydrodynamic movement of the water in the flooded sites during the 6 months of rains. Site S2, as S7 with sandy-clay soil, usually remains flooded for about 6 months per year, maintaining a constant flow of water. Hence, the spreading rate of the hydrocarbon plume in this site is huge. Thus, by spring of 2002, from the data at Table 1, it can be estimated that the long-term impacted site S1 showed the highest content of total weathered hydrocarbons (WTH = 177.1 ± 43.6 g/kg soil), i.e., the PAH plus the ASF fractions, followed by the site S6 with 108 ± 9.6, of g WTH/kg soil, which was similar to the WTH content at recent impacted sites (76.8 ± 13 to 103.2 ± 8.6 g WTH/kg soil). Therefore, S1 and S2 were selected as representatives of the long-term and recent impacted sites, respectively, for the phytoremediation treatments. In addition, the site S3 was first affected in 1980; however, its content of TPH (16 ± 2 g/kg soil) and WTH (5.8 ± 0.7 g/kg soil) was similar to the uncontaminated zone because it was restored by PEMEX in 2000 by substituting 1.5 m depth of the contaminated soil by another from a nearby uncontaminated area. Thus, in this S3 site, the natural revegetation processes was monitored, and its TPH content was considered as the basal background-extractable material from soils of uncontaminated sites.
Seedlings of Cyperus esculentus and Ludwigia peploides (a), plants of Cyperus laxus (b, c), C. esculentus (d, e), and L. peploides (f, g) growing under greenhouse conditions in pots containing contaminated soils from the site S1 (325,000 ppm TPH) (b, d, g), site S2 (141, 000 ppm TPH) (a, f), and in the affected site S1 (c, e). L. peploides malformed plants after cultivation for 2 years in the contaminated soil from site S1 (g)
Ecology of the plant community in the impacted sites regarding to uncontaminated area
In uncontaminated areas surrounding the sites of the study, 36 plant species were identified (Table 2). As can be observed, the plant species are from the herbaceous, aquatic, and subaquatic types (Table 2), but mostly from the marshland or popal, and the swampland or tular communities (Table 3). From these plants, Cyperus papyrus, Pontederia cordata, Thalia geniculata, and Typha latifolia covered 60–90 % of the surface area. The secondary vegetation with high growth rate in the uncontaminated area was mostly from the acahual community, such as Cecropia obtusifolia, Pithecellobium lanceolatum, Muntigia calabura, and Bursera simaruba.
In contrast to the uncontaminated area, in the impacted sites, the community and diversity of plants was very reduced in the species number (Table 2). Typically, they belonged to the marshland and swampland communities, showing few dynamic changes from 1 year to another. During the exploration of the recently impacted sites (2, 4, 7, 8, and 9), it was observed that after 4–6 months of the oil spill, the plant biodiversity was drastically affected resulting in a complete disappearance of the native vegetation. These early contaminated sites, represented by S2, remained as wastelands for 3–4 months and then started a gradual revegetation by settlement of diverse pioneer invasive plant species (Table 3). Typically, in the marshlands of the affected sites, the new plant community was mostly from the grass type, with C. laxus and C. esculentus as the pioneer and dominant species (Fig. 2c and e), but at the border of the swamplands L. peploides was the dominant. These pioneer and putative hydrocarbons tolerant plant species in the affected sites emerged once the native vegetation disappears and remain as dominant for long time. For example, in aged contaminated sites (1, 5, 6), represented by site S1, in the first year of study, seven plant species, six monocotyledonous (Liliopsidae), and one pteridophyte (Phylicopsidae), from six genus and five families were identified (Table 2). As shown at data in Table 2, by the second year of study, the number of species in this site changed from seven to fourteen, showing twelve genus and ten families. Of those species, the monocots population showed the most drastic changes: from six to nine. Interestingly, in weathered impacted sites (S1), four new dicotyledonous species (Magnoliopsidae) appeared in the second year (Table 3). Similarly, in the same period, in an early contaminated site (S2) and the treated site S3, the plant species increased from 4 to 7 and from 3 to 4, respectively (Table 2). Overall, after 2 years, the number of species increased by 100 % in the site S1 and by 60 % and 25 % in the sites S2 and S3 respectively. Interestingly, by the end of the second year, in both the contaminated and the uncontaminated sites, the dominant species were mostly monocotyledonous of the popal community (Table 2). The botanical family with the biggest taxa was the Cyperaceae with 2 genus (Carex and Cyperus), and 5 species (Table 3). As can be appreciated the best genus represented during the whole study was Cyperus with 3 species. Thus, in the first year, in site S1, C. esculentus showed an importance value (Iv) of 104.9, with C. papyrus as the co-dominant species (Iv = 63.5). However, in the second year the dominant was C. laxus (Iv = 84.9), whereas C. esculentus (Iv = 55.7) was the co-dominant species. In the sandy-clay site S2, the first year C. laxus was the dominant and T. latifolia (Iv = 54.2) the co-dominant, but it was shortly succeeded by Eragrostis reptans (Michx.) Nees, and a Poaceae with undetermined genus. In the uncontaminated site S3, the dominant species in the first year was C. esculentus (Iv = 138.6), but in the second year it was succeeded by T. latifolia showing the highest Iv (204.9). Thus, although C. esculentus and C. laxus showed the best adaptive capacity; their survival and permanence in the contaminated sites depended upon the gradual settlement of the native dominant species of the uncontaminated surrounding areas. The last phenomena of population succession were also observed in the uncontaminated site S3, where Cyperus and Ludwigia were succeeded by Typha, the dominant species in the zone of study (Table 3). In addition, according to the Sörensen similarity index (SIs) to evaluate the overall ecological similarities on the plant composition between the impacted and uncontaminated sites (Müller-Dumbois and Ellemberg, 1974), S1 and S3 presented the best similarity (SIs = 54.5 %) during the first year, but in the second year the best similarity was between Sites S2 and S3 (SIs = 50 %).
Experiment II
To evaluate the three phenophases (germination, growth, and survival), and the hydrocarbons removal in the greenhouse experiments, from the above results three putative oil-tolerant plant species with phytoremediation capabilities were selected from the plant community native of the aged oil spills impacted site S6 containing highly weathered petroleum (108 ± 9.6, of g WTH/kg soil): C. laxus and C. esculentus found in the marshlands, and L. peploides found at the border of swamplands (Fig. 2) were selected. These three plant species showed a representative abundance in the long-term contaminated sites (Table 3).
Phenology of selected plant species
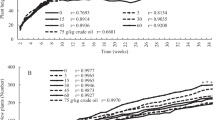
Germination frequency of Cyperus was affected by the hydrocarbons levels (Fig. 3). Both Cyperus species were very sensitive; however, Ludwigia was affected only at high TPH concentrations, showing similar germination frequency in the less contaminated S2 and S3 soils (Fig. 3). In S3, Cyperus species reached the maximum germination frequency of 31 % after 14 days, while in S1 and S2 the maximum was c.a. 20 % after 16 days. For Ludwigia, the highest germination frequency occurred in S3 (82 % by the 18th day), while in the contaminated soils S1 and S2 was 77 % by the 18th day and 62 % by the 19th day, respectively. Similar results were observed in pots cultivated with a mix culture of Ludwigia and both Cyperus. In addition, spontaneous germination of other plant species from the natural bank of seeds was observed after 6 weeks of culture. In pots with S3, five additional species were identified: Mimosa pigra, T. latifolia, Phlebodium decumanun, Clibadium erosum, and C. crus-corvi. Of these plants, P. decumanum and C. crus-corvi showed vegetative development for 85 weeks, M. pigra survived 8 weeks without producing seeds, Clibadium survived for 85 weeks producing so great quantity of seeds that formed a seedling mantle with diverse degrees of survival. T. latifolia produced vegetative shoots every 6 weeks and survived for 28 weeks. Similarly, in pots containing soil S1, plantlets of C. crus-corvi and other unidentified Cyperus were observed. Carex survived for 80 weeks without showing maturity features, but with vegetative reproduction with 1 or 2 shoots every 40 weeks. In pots with soil from S2, only appearance of C. crus-corvi was observed. It survived in vegetative state until the week 85 without production of asexual shoots. With exception of Clibadium, the species that spontaneously emerged in the pots cultivated in the greenhouse were also observed in areas close to the sites of study (Table 3).
Cumulative germination frequency of Cyperus laxus (a), Cyperus esculentus (b), and Ludwigia peploides (c) sowed in rectangular pots without drain system and containing 11 kg of soil. The pots were incubated in a greenhouse without temperature or humidity controls. Error bars represent the standard deviation of five experimental units sowed with hundred seeds each
Survival and growth of the three plant species were also drastically affected by the presence of hydrocarbons, reaching a maximal survival in any soil after 4 weeks (Fig. 4). However, the Cyperus survival was successfully stabilized after 13 15 weeks, while Ludwigia resulted almost intolerant to the presence of hydrocarbons and the most of the individuals died after 9–10 weeks in pots containing contaminated soils S1 and S2, and after 12–13 weeks in the uncontaminated soil S3 (Fig. 4e). Flowering and fructification processes of Cyperus started after 19 weeks of culture, and their ordinary vital cycle finished after 20 weeks by a gradual deceasing of the individuals. By the weeks 12 and 14, some of the Cyperus plants produced 1 to 3 vegetative shoots that regenerated into a new generation of individuals maintained under the same experimental conditions. Seeds from these died plants were collected and used to induce a second generation of individuals under the same experimental conditions to further phenological studies. The procedure was repeated until the fourth generation of plants, which died after 85 weeks, showing phenological features as the first generation. On the other hand, survival of L. peploides was similar in any soil for 4 weeks, but afterwards it declined drastically in the contaminated soils, and was stabilized after 15 weeks in S3, and after 21 weeks in the most of the contaminated soils. With the time, in pots containing contaminated soils, a gradual population displacement of Ludwigia by Cyperus was evident, resulting in a final survival ratio of c.a. 1 % after 85 weeks. Similarly, in pots with soil S3, new plant species different of Cyperus, but commonly observed in uncontaminated areas nearby to the sites of study, emerged, and gradually succeeded L. peploides. Thus, hydrocarbons affected the survival of the studied species in the following increasing order: L. peploides > C. esculentus > C. laxus.
Survival and growth of plants of Cyperus laxus (a, b), Cyperus esculentus (c, d), and Ludwigia peploides (e, f) grown in a greenhouse as pointed out in Fig. 2. Error bars represent the standard deviation of five experimental units sowed with hundred seeds each
Although growth rates for the three species in any soil started to increase after 5–6 weeks, growth was considerably less in the contaminated soils (Fig. 4b, d, f). Both Cyperus species reached their maximum growth after 17 weeks and died by the week 21. However, L. peploides reached an average height of 1.6 cm high after 10 weeks and 4.4 cm after 21 weeks in soils from the contaminated sites S1 and S2, respectively, and 43.3 cm after 21 weeks and 135 cm after 85 weeks, when cultivated in soil from the uncontaminated soil S3 (Fig. 4f). Although most of the Ludwigia plants developed in contaminated soils S1 and S2 died after 11 and 21 weeks, respectively, some individuals grown in the contaminated soil S1 reached almost 180 cm after 2 years, but showing serious morphological alterations, putative variation symptoms regarding to healthy plants (Fig. 2g). These altered plants showed thick and rigid steam, and great ramification of several order and dimensions, characteristic of the sympodial growth of shrubs.
Phytoremediation effect on TPH removal
Soil type, hydrocarbons content, and plant species affected the TPH removal efficiency (Table 4). Although most treatments with collected plants were lost because they died after few months of culture in the greenhouse, some individuals of C. esculentus, C. laxus, E. polystachya, and C. crus-corvis survived after 2 years and therefore they were included in the analysis to evaluate their effect on the TPH removal efficiency (Table 4). Both the plant species and the initial TPH content affected significantly (df = 8, F = 2.80, p = 0.012 and df = 3, F = 17.58, p < 0.0001, respectively) the removal efficiency of hydrocarbons from the contaminated soils cultivated by 2 years with plants established either from seeds or from transplanted plants. Duncan’s post hoc means comparison test revealed three groups of means (a, b, c) for the final TPH content regarding to both plant species and the initial TPH content. Overall, residual TPH content was not significantly different between the control treatments without plants and treatments with L. peploides, C. laxus, and C. crus-corvi. In contrast, in treatments where C. esculentus was present, single or in combination with L. peploides and/or C. laxus, the final estimated nominal TPH content was significantly lower than in unvegetated treatments independently of the soil type. In fact, the best TPH removal efficiency was with a mix of C. esculentus, L. peploides, and C. laxus (92 % from the most contaminated soil S1) and with C. esculentus alone in soil S4 (85 %). In addition, treatments with E. polystachya were significantly different between S3 and S4 (Table 4); however, this plant survived only in the uncontaminated soil S3 and in the lower long-term contaminated soil S4, but was unable to survive in the highly contaminated soil S1 and in the Fluvisol gley-eutric soil S2. On the other hand, the differences in the results between S1 and S4 (Table 4) suggest that high levels of weathered hydrocarbons might prevent lightly the hydrocarbons removal capability, at least for C. esculentus. Thus, at the end of the experiment, Duncan’s test showed that soil S4, either treated with C. esculentus or with E. polystachya achieved contents of TPH similar to the uncontaminated soil S3. Thus, the best TPH removal levels in S1 was achieved with plants of C. esculentus (70 %) and a mix culture of C. esculentus, L. peploides, and C. laxus (92 %), which was also efficient to remove the TPH from the Fluvisol gley-eutric soil S2, where the mix culture of L. peploides with C. laxus was the most efficient.
Discussion
The diversity of plants found through the time in the contaminated sites represents good biological indicator for the recovery status of the site, as has been suggested for other contaminated ecosystems (Palmroth et al. 2002; Vitalino et al. 2002; Wolters et al. 2000). Therefore, the dynamic regeneration of sites to their natural conditions should be consequence of the gradual succession of pioneer species. For example, the presence of T. latifolia in the second year in the uncontaminated site S3, supplied with new soil, points out that the environmental conditions of this site soon will achieve the natural community of plant species as the surrounding area. Similar results have been reported for floristic composition disturbance (Collins et al. 1995; Mackey and Curie, 2001) and woody plant control studies to maintain the diversity of species in local sites (Fulbgriht, 1996). Beside the profile of plant diversity, dynamic succession of species was notable in the sites of study, but it was impressive in the site added with new soil S3, where the dominant and co-dominant species were changing continuously. The frequent emergence of new plant species at very low densities in soils from contaminated sites suggested an ordered and dynamic succession, perhaps controlled by the current specific plant populations.
As other reports for similar hydrocarbon-contaminated sites (Gentry, 1988; Lin et al. 2005a; Lot, 2004; Mendelssohn et al. 2002), the plant community in the oil spill-impacted sites was eliminated by the presence of hydrocarbons. This observation suggests that appearance of invasive pioneer species in the impacted sites is an initial ecological signal of the process leading to the native plant community regeneration. For example, in intact wetlands of Louisiana (USA), Spartina alternifolia dominated the salt marsh, while Spartina patens and Distichlis spicata co-dominated brackish marsh, and Saggitaria lancifolia dominated freshwater marsh (Mendelssohn et al. 2002; Lin et al. 2005a). However, in oil-contaminated wetlands after in-situ burning, the marshland was revegetated by S. lancifolia and other invasive species, such as Eleocharis fallax, Alternanthera philoxeroides, and Echinochloa crus-galli (Lin et al. 2005b). Similarly, in our results, after disappearance of the native vegetation dominated by T. geniculata, C. papyrus, P. cordata, and T. latifolia (Rivera-Cruz et al. 2004), new invasive pioneer species were established, from which the Cyperus genus showed the best capacity to grow and survive in the contaminated sites, which to our knowledge has not been reported before. Furthermore, although C. esculentus and C. laxus were the marshlands dominant and co-dominant species respectively, and L. peploides was the border of swamplands dominant, after 2 years, the general tendency of the plant community showed that the local dominant species would be finally succeed these invader pioneer plants leading to the ecological recovery of the contaminated sites (Table 3). This observation agrees with those reported by Gallegos et al. (2000) and Escalante-Espinoza et al. (2005), who conclude that C. laxus was one of the pioneer invasive species in similar hydrocarbon-contaminated sites, but it was finally succeeded by the natural dominant species. In consequence, if these invasive pioneer species were cultivated in the contaminated sites for phytoremediation purposes, as we reported recently for ex situ phytoremediation of soils from such sites at greenhouse level (Rivera Casado et al. 2015), they would induce the necessary ecological changes to catalyze the natural restoration process of the contaminated sites to the original ecological conditions.
The above reasoning was supported also by the results from the studies performed in the greenhouse, which showed that the best removal efficiency of TPH (93.2 % from a 325 g TPH/kg soil) after 2 years was in S1 with a mix culture of C. esculentus, L. peploides, and C. laxus (Table 4). That is, in spite of the high TPH content in the soils (Table 1), the above plant community was able to grow and survive in such adverse conditions. Therefore, this mix culture might be the best for the ecological recovery of similar contaminated sites. Furthermore, even in the sandy soil S2, which is nutritionally poorer than the Gleysolic types (Gentry, 1988; Zavala, 1988; Ke et al. 2002), the final TPH residual content in those treatments with this mix culture (22 ± 2 g TPH/kg soil) was similar to the uncontaminated soil S3 (15 ± 4 g TPH/kg soil). The differences in the results between S1 and S4 (Table 4) point out that high levels of weathered hydrocarbons might prevent lightly the hydrocarbons removal capability of C. esculentus. On the other hand, the emergence of native plant species in the pots incubated in the greenhouse, which gradually succeeded the cultivated plants, pointed out not only that an important bank of seeds is contained in the contaminated sites but it might also be a signal of the dropping of the TPH levels and the restoring of the soil. Similar reflection have been reported for studies performed with species like mangrove (Ke et al. 2002) and the salt marsh grass Spartina alterniflora (Lin et al. 2002b).
Conclusions
Oil-tolerant plant species, native at aged sites affected by oil spills, were selected on the bases of their ecological changes regarding with unimpacted areas. After disappearance of the native vegetation by the hydrocarbons’ presence, new pioneer plant species were established in the impacted sites. Although C. esculentus and C. laxus showed the best adaptive capacity, their survival and permanence in the contaminated sites depended upon the gradual settlement of the native dominant species of the surrounding areas because of dynamic succession of species in the sites of study. Therefore, these invasive pioneer plants would be naturally replaced by the dominant species of the zone. On the other hand, the phenological changes and the hydrocarbon removal capability of the selected plants cultivated at greenhouse level in soils from impacted sites showed that C. esculentus, alone or in a mix culture with C. laxus and L. peploides, was the most tolerant to the hydrocarbons presence and yielded the best TPH removal level, although high levels of weathered hydrocarbons might prevent lightly the hydrocarbon removal capability of C. esculentus. Accordingly, the results from this work might have particular potential for the phytoremediation of tropical wetlands contaminated with oil hydrocarbons with these plant species to catalyze the natural restoration process of the impacted sites to the original ecological conditions.
References
Abedi-Koupai J, Ezzatian R, Vossoughi-Shavari M, Yaghmaei S, Borghei M (2007) The effects of microbial population on phytoremediation of petroleum contaminated soils using tall fescue. Int J Agr Biol 9:242–246
Amadi A, Dickson AA, Maate GO (1993) Remediation of oil polluted soils: 1. Effect of organic and inorganic nutrient supplements on the performance of maize (Zea mays L). Water Air Soil Pollut 66:59–76
Ashworth J, Keyes D, Kirk R, Lessard R (2001) Standard procedure in the hydrometer method for particle size analysis. Commun Soil Sci Plant Anal 32(5-6):633–642
Baskin CC, Baskin JM (1988) Germination ecophysiology of herbaceous plant species in a temperate region. Am J Bot 75:286–305
Brown JL, Nadeau RJ (2002) Restoration of petroleum-contaminated soil using phased bioremediation. Biorem J 6:315–319
CIMADES (Interdisciplinary Commission of Environmental and Social Development) (2000) Descripción de la infraestructura petrolera y del entorno ambiental del Activo Cinco Presidentes. Gobierno del estado de Tabasco, México, p 42
Collins SL, Glenn SM, Gibson DJ (1995) Experimental analysis of intermediate disturbance and initial floristic composition: decoupling cause and effect. Ecology 76:486–492
Cox GW (1981) Laboratory manual of general ecology. W.C. Brown Publishers-San Diego State University, U.S.A., p 237
Escalante-Espinoza E, Gallegos-Martínez ME, Favela-Torres E, Gutierrez-Rojas M (2005) Improvement of the hydrocarbon phytoremediation rate by Cyperus laxus Lam. inoculated with a microbial consortium in a model system. Chemosphere 59:405–413
Fidalgo O, Bononi VLR (1984) Técnicas de colecta, preservaçao e herborizaçao de material botánico. Instituto de Botánica-U, Sao Paulo, p 106
Fulbgriht TE (1996) Viewpoint: a theoretical basis for planning woody plant control to maintain species diversity. J Range Management 49:554–559
Gallegos-Martínez M, Gómez-Santos A, González-Cruz L, Montes de Oca-García A, Yáñez-Trujillo L, Zermeño-Eguía Lis J, Gutierrez-Rojas M (2000) Diagnostic and resulting approaches to restore petroleum-contaminated soil in a Mexican tropical swamp. Water Sci Technol 42:377–384
Gentry AH (1988) Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann Mo Bot Garden 75:1–34
Gutierrez CMC, Zavala CJ (2002) Hydromorphic pedofeatures in hydrocarbon polluted tropical soils. Terra Latinoamericana 20:101–111
Handa IT, Jefferies RL (2000) Assisted revegetation trials in degraded salt-marshes. J Appl Ecol 37:944–958
INEGI (Instituto Nacional de Estadística Geografía e Informática) (2001) Anuario estadístico del estado de Tabasco. Gobierno del estado de Tabasco, México, p 480
Ke L, Wong TWY, Tam NFY (2002) Fate of polycyclic aromatic hydrocarbon (PAH) contamination in a mangrove swamp in Hong Kong following an oil spill. Marine Pollut Bull 45:339–347
Kulakow PA, Schwab AP, Banks MK (2000) Screening plant species for growth on weathered, petroleum hydrocarbon-contaminated sediments. Int J Phytoremediat 2:297–317
Lambers H, Chapin FS, Pons TL (1998) Plant physiological ecology. Springer, New York, p 289
Lin Q, Mendelssohn IA, Carney K, Bryner NP, Walton WD (2002a) Salt marsh recovery and oil spill remediation after in-situ burning: effect of water depth and burn duration. Environ Sci Technol 36:578–581
Lin Q, Mendelssohn IA, Siudan MT, Lee K, Venosa AD (2002b) The dose-response relationship between no. 2 fuel oil and the growth of salt marsh grass, Spartina alterniflora. Marine Pollut Bull 44:879–902
Lin Q, Mendelssohn IA, Bryner NP, Walton WD (2005a) In-situ burning of oil in coastal marshes. 1: vegetation recovery and soil temperature as a function of water depth, oil type and marsh type. Environ Sci Technol 39:1848–1854
Lin Q, Mendelssohn IA, Carney K, Miles S, Bryner NP, Walton WD (2005b) In-situ burning of oil in coastal marshes. 2. Oil spill cleanup efficiency as a function of oil type, marsh type, and water depth. Environ Sci Technol 39:1855–1860
Liste H-H, Felgentreu D (2006) Crop growth, culturable bacteria, and degradation of petrol hydrocarbons (PHCs) in a long-term contaminated field soil. Appl Soil Ecol 31:43–52
Lot A (2004) Flora y vegetación de los humedales de agua dulce en la zona costera del Golfo de México. In: Caso M, Pisanty I, Ezcurra E (eds) Diagnóstico ambiental del Golfo de México. Instituto Nacional de Ecología, México, pp 521–553
Lot A, Chiang F (1986) Manual de herbario, administración y manejo de colecciones, técnicas de recolección y preparación de ejemplares botánicos. Consejo Nacional de la Flora de México, México, D.F, p 143
Mackey RL, Currie DJ (2001) The diversity-disturbance relationship: is it strong and peaked? Ecology 82:3479–3492
Maila MP, Cloete TE (2002) Germination of Lepidium sativum as a method to evaluate polycyclic aromatic hydrocarbons (PAHs) removal from contaminated soil. Internat Biodeterior Biodegrad 50:107–113
Martínez MG, Santos AG, Cruz LG, de Oca García MM, Trujillo LY, Lis JZE, Gutierrez-Rojas M (2000) Diagnostic and resulting approaches to restore petroleum-contaminated soil in a Mexican tropical swamp. Water Sci Technol 42(5-6):377–384
Mendelssohn IA, Lin Q, Bryner NP, Walton WD, Twilley WH, Mullin JV (2002) In-situ oil burning in the marshland environment-recovery and regrowth of Spartina alterniflora, Spartina patens, and Sagittaria lancifolia plants. Proceedings of the twenty-fifth artic and marine oil spill program technical seminar. Environment Canada, In, pp 785–802, http://fire.nist.gov/bfrlpubs/fire02/PDF/f02068.pdf
Merkl N., Schultze-Kraft R. 2005. Phytoremediation of petroleum-contaminated soils in the tropics. In: Deutscher Tropentag. The Global Food & Product Chain - Dynamics, Innovations, Conflicts, Strategies. University of Hohenheim, Oct. 11–13. Book of Abstract. http://www.tropentag.de/2005/abstracts/links/Merkl_BJt2Mok2.php
Merkl N, Schultze-Kraft R, Infante C (2004) Phytoremediation of petroleum-contaminated soils in the tropics—Preliminary assessment of the potential of species from eastern Venezuela. J Appl Bot Food Qual 78:185–192
Merkl N, Schultze-Kraft R, Infante C (2005) Assessment of tropical grasses and legumes for phytoremediation of petroleum-contaminated soils. Water Air Soil Pollut 165:195–209
Molina-Barahona L, Rodriguez-Vazquez R, Hernandez-Velasco M, Vega-Jarquin C, Zapata-Perez O, Mendoza-Cantu A, Albores A (2004) Diesel removal from contaminated soils by biostimulation and supplementation with crop residues. Appl Soil Ecol 27:165–175
Mückschel C, Otte A (2003) Morphometric parameters: an approach for the indication of environmental conditions on calcareous grassland. Agric Ecosyst Environm 98:213–225
Müller-Dumbois D, Ellemberg H (1974) Aims and methods of vegetation ecology. John Wiley and sons, New York, p 547
Noble IR, Slayter RO (1980) The use of vital attributes to predict successional changes in plant communities subject to recurrent disturbances. Vegetatio 43:5–21
Palmroth MRT, Pitchel J, Puhakka JA (2002) Phytoremediation of subartic soil contaminated with diesel fuel. Biores Technol 84:221–228
Palmroth MRT, Koskinen PEP, Pichtel J, Vaajasaari K, Joutti A, Tuhkanen TA, Puhakka JA (2006) Field-scale assessment of phytotreatment of soil contaminated with weathered hydrocarbons and heavy metals. J Soils Sediments 6:128–136
Pérez-Vargas J, Poggi-Varaldo HM, Calva-Calva G, Ríos-Leal E, Rodríguez-Vázquez R, Ferrera-Cerrato R, Esparza-García F (2000) Nitrogen fixing bacteria capable of using kerosene hydrocarbons as a sole carbon source. Water Sci Technol 42:407–410
Perez-Vargas J, Poggi-Varaldo HM, Calva-Calva G, Albores RA, Rodriguez-Vazquez R, Esparza-Garcia F, Ferrera-Cerrato R (2001) Azomonas: an NFB capable of using kerosene as a carbon source. In: Magar VS, Von Fahnestock FM, Leeson A (eds) Ex situ biological treatment technologies, vol 6(6). Columbus, Battelle Press, pp 219–226
Ponce-Velez G, Botello AV, Diaz-Gonzalez G (2006) Organic and inorganic pollutants in marine sediments from northern and southern continental shelf of the Gulf of Mexico. Int J Environment and Pollution 26:295–311
Primack RB, Kang H (1989) Measuring fitness and natural selection in wild plant populations. Ann Rev Ecol Syst 20:367–396
Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Ann Rev Ecol Syst 16:179–214
Rivera Casado NA, Rodríguez Vázquez R, Montes Horcasitas M del C, Pérez Vargas J, Gómez Guzmán O, Calva Calva G (2008). Hidrocarburos aromáticos y fenilpropanoides presentes en la rizósfera de plantas de Cyperus laxus crecido en suelos contaminados con hidrocarburos. Tecnocultura 7(20): 4-15. http://difusion.tese.edu.mx/tese2010/loader.aspx?n = NFKNRAQG
Rivera Casado NA, Montes Horcasitasa Mdel C, Rodríguez Vázquez R, Esparza García FJ, Pérez Vargas J, Ariza Castolo A, Ferrera-Cerrato R, Gómez Guzmán O, Calva Calva G (2015) The Fatty acid profile analysis of Cyperus laxus used for phytoremediation of soils from aged oil spill-impacted sites revealed that this is a C18:3 plant species. PLoS One 10(10):e0140103. doi:10.1371/journal.pone.0140103.eCollection2015
Rivera-Cruz MD, Ferrera-Cerrato R, Sánchez-García P, Volke-Haller V, Fernández-Linares L, Rodríguez-Vázquez R (2004) Decontamination of soils polluted with crude petroleum using indigenous microorganisms and aleman grass [Echinochloa polystachya (H.B.K.) Hitchc.]. Agrociencia 38:1–12
SPSS Inc., 2006. SPSS, v. 15.0, Chicago, IL.
Sudová R, Pavlíková D, Macek T, Vosátka M (2007) The effect of EDDS chelate and inoculation with the arbuscular mycorrhizal fungus Glomus intraradices on the efficacy of lead phytoextraction by two tobacco clones. Appl Soil Ecol 35:163–173
Tischer S, Hübner T (2002) Model trials for phytoremediation of hydrocarbon-contaminated sites by the use of different plant species. Intern J Phytorem 4:187–203
US-EPA (U.S. Environmental Protection Agency) 1986. Method 8015B. Nonhalogenated organics using GC/FID. SW-846 Ch 4.3.1. http://www.caslab.com/EPA-Methods/PDF/8015b.pdf
US-EPA (U.S. Environmental Protection Agency) 1996. Method 3550B. SW-846 Ch 4.2.1. Ultrasonic extraction—organics. http://www.trincoll.edu/~henderso/textfi~1/416%20notes/3550b.pdf
Vazquez FG, Sharma VK, Perez-Cruz L (2002) Concentrations of elements and metals in sediments of the southeastern Gulf of Mexico. Environ Geol 42:41–46
Vitaliano JJ, Reid RN, Frame AB, Packer DB, Arlen L, Sacco JN (2002) Comparison of benthic invertebrate assemblages at Spartina alterniflora marshes reestablished after an oil spill and existing marshes in the Arthur Kill (NY/NJ). Mar Pollut Bull 44:1100–1108
Wolters V, Silver WL, Bignell DE, Coleman DC, Lavelle P, van Der Putten WH, De Ruiter P, Rusek J, Wall DH, Wardle DA, Brussaard L, Dangerfield JM, Brown VK, Giller KE, Hooper DU, Sala O, Tiedje J, van Veen JA (2000) Effects of global changes on above and belowground biodiversity in terrestrial ecosystems: implications for ecosystems functioning. Bioscience 50:1089–1098
Zavala CJ (1988) Regionalización natural de la zona petrolera de Tabasco. Casos de estudio. Instituto Nacional de Investigación sobre Recursos Bióticos. División Regional. Gobierno del estado de Tabasco, México, p 183
Acknowledgments
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT México: 211085-5-29307B). Special acknowledgment is given to CIMADES for the technical assistance during the field studies thorough the extraction and production oil zone in Tabasco, México. Felipe de J. Palma Cruz acknowledges a doctoral fellowship (83286) from CONACyT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Palma-Cruz, F.d.J., Pérez-Vargas, J., Rivera Casado, N.A. et al. Phytoremediation potential and ecological and phenological changes of native pioneer plants from weathered oil spill-impacted sites at tropical wetlands. Environ Sci Pollut Res 23, 16359–16371 (2016). https://doi.org/10.1007/s11356-016-6675-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6675-4