Abstract
The objective of this work was the immobilization of soluble manganese (Mn) and ammonium nitrogen (NH4 +-N) leached from electrolytic manganese residue (EMR). Immobilization of Mn was investigated via carbonation using carbon dioxide (CO2) and alkaline additives. NH4 +-N immobilization was evaluated via struvite precipitation using magnesium and phosphate sources. Results indicated that the immobilization efficiency of Mn using CO2 and quicklime (CaO) was higher than using CO2 and sodium hydroxide (NaOH). This higher efficiency was likely due to the slower release of OH− during CaO hydrolysis. The immobilization efficiency of Mn was >99.99 % at the CaO:EMR mass ratio of 0.05:1 for 20-min reaction time. The struvite precipitation of NH4 +-N was conducted in the carbonated EMR slurry and the immobilization efficiency was 89 % using MgCl2 · 6H2O + Na3PO4 · 12H2O at the Mg:P:N molar ratio of 1.5:1.5:1 for 90-min reaction time. A leaching test showed that the concentrations of Mn and NH4 +-N in the filtrate of the treated EMR were 0.2 and 9 mg/L, respectively. The combined immobilization of Mn and NH4 +-N was an effective pretreatment method in the harmless treatment of the EMR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrolytic manganese metal (EMM), widely used in metallurgy, aerospace, chemical processing, etc., is an important basic material. It is reported that China is the world’s largest country of production, consumption, and export of EMM (Du et al. 2014). With the increase of Mn demand in the global market, the development of EMM industry has been promoted recently. The quick development results in various environment problems, especially the contamination from electrolytic manganese residue (EMR) (Duan et al. 2010). EMR, a by-product of the electrolytic manganese metal process, is produced by the acid leaching, neutralization, and pressure filtration treatment of manganese carbonate powder. The EMR contains high concentrations of soluble Mn and NH4 +-N (Chen et al. 2015). At present, ∼10–12 t of EMR are discharged into the environment during the production of 1 t of EMM (Zhou et al. 2013). In China, about 10 × 106 t of EMR are discharged into the environment each year and the accumulated amount during the past many years is about 50 × 106 t (Duan et al. 2010; Zhou et al. 2014). Currently, EMR is primarily dumped into the environment without pretreatment in China. Such a large amount of EMR poses a serious threat to the surrounding environment and the population. Therefore, the development of EMR disposal technologies is urgently required.
Studies have reported about the disposal and utilization of the EMR. Feng et al. (2006) used EMR as a cement setting retarder. Liu et al. (2012) used EMR as supplementary cementitious materials. Li et al. (2007) used burned EMR and fly ash as complex additives for cement. Applications of EMR as soil amendment and roadbed backfill were also investigated (Lan 2006; Xu 2001). Nevertheless, these applications could not be generalized in practice due to the low quantity of added EMR and the leaching of Mn and NH4 +-N. The extraction of metals from the EMR was also investigated. Ouyang et al. (2007) reported that the extraction efficiency of Mn reached 57.3 % using citric acid as the leaching reagent assisted with ultrasound. Yao et al. (2003) added glucose and saccharose into a sulfuric acid solvent to extract Mn, resulting in an extraction efficiency of 85 %. Xin et al. (2011) obtained a 93 % extraction efficiency of Mn from the EMR using sulfur-oxidizing bacteria. Owing to the high cost and complicated procedures, these technologies could not be applied in practice. Currently, the landfill treatment of EMR is still a primary choice for EMM industries. It is significantly necessary that the EMR is pretreated to immobilize soluble Mn and NH4 +-N before the landfill treatment.
In view of high efficiency and low cost, the stabilization/solidification technology is extensively applied to the harmless disposal of various pollutants (Bednarik et al. 2005). The contaminants can be immobilized in the solid waste materials. The strength of the waste materials can also be enhanced. This technology is favorable to the application of these waste materials to landfill, building materials, and roadbeds. CaO and NaOH are typical additives used for decreasing metal mobility and leachability. Several studies reported that CaO and NaOH were effective additives to stabilize heavy metals (Zhou et al. 2013; Guo et al. 2006), because these additives were easily soluble and available for reactions. One drawback of these additives is the unacceptable increase of pH (>10) (Zhou et al. 2013), because a high proportion is required to add to waste materials. Additionally, previous studies reported the removal of NH4 +-N using magnesium and phosphate sources in landfill leachate and wastewater (Huang et al. 2014; Stolzenburg et al. 2015).
The objective of this work was to immobilize (1) soluble Mn from EMR by CO2 with alkaline additives and (2) NH4 +-N via struvite precipitation using magnesium and phosphate sources. In addition, mechanisms of the Mn immobilization via carbonation and the struvite precipitation of NH4 +-N were characterized and the optimal conditions were determined. The advantages of this study were the utilization of greenhouse gas (CO2) by converting soluble Mn into Mn carbonate that can be recovered further by flotation (Zhou et al. 2015) and direct immobilization of NH4 +-N via struvite precipitation in the EMR slurry.
Materials and methods
Materials
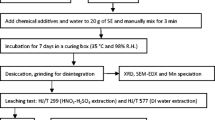
The EMR was obtained from a residue storage facility at an electrolytic manganese plant in Chongqing, China. After being thoroughly mixed, the residue was dried to constant weight at 105 °C. The dried residue was then ground to a powder with a ball mill and sieved through an 80-mesh screen (180 μm) for testing. Analytical grade CaO (≥98 %) and NaOH (≥96 %) were used as alkaline additives. CO2 was collected from the ore leaching process of electrolytic manganese production and used to immobilize Mn. Analytical grade MgCl2 · 6H2O and MgO were used as magnesium sources, and Na3PO4 · 12H2O was used as a phosphate source.
Immobilization of Mn via carbonation
The experiments were conducted at room temperature with a jar test apparatus. For each experiment, 20-g ground EMR, 40-mL distilled water, and different amount of alkaline additives were mixed into a 250-mL beaker with 0.8-L/min CO2 flow rate. Each experiment was run in triplicate for statistical accuracy, and mean values were reported. To determine the effect of different alkaline additives (CaO and NaOH):EMR mass ratios on the immobilization of Mn, comparative tests were carried out by changing alkaline additives:EMR mass ratios in the range of 0.01:1–0.075:1. Additionally, the effect of reaction time on the immobilization of Mn was measured under different alkaline additives. The optimized experimental conditions, including an alkaline additive, the alkaline additive:EMR mass ratio, and reaction time, were determined based on the experimental results.
Immobilization of NH4 +-N via struvite precipitation
After the immobilization of Mn from the EMR at the optimized conditions, immobilization experiments of NH4 +-N were carried out using MgCl2 · 6H2O + Na3PO4 · 12H2O and MgO + Na3PO4 · 12H2O, respectively. The immobilization efficiency of NH4 +-N was determined at the different Mg:P:N molar ratios. In addition, the effects of reaction time on the immobilization of NH4 +-N, concentration of P, and stability of Mn carbonate formed by CO2 with the alkaline additive were investigated. After the Mn and NH4 +-N immobilization under optimum conditions, a leaching experiment was conducted under the conditions of a water:treated EMR mass ratio of 10:1, 8-h vibration time, and 16-h resting time according to the leaching standard designed by Chinese government (GB 5085.3-2007).
Calculation of immobilization efficiency
After each experiment, the treated sample was discharged from the reactor. Air pump filtration was performed to collect the filtrate in a 0.2-L flask for analyses. The immobilization efficiency of Mn (ζ) and the immobilization efficiency of NH4 +-N (η) were defined by Eqs. (1) and (2), respectively.
where n and n t were the Mn mass (mg) in the raw EMR and the filtrate from the treated EMR, respectively, and m and m t were the NH4 +-N mass (mg) in the raw EMR and the filtrate, respectively.
Characterization
The chemical components of the raw EMR were analyzed using an X-ray fluorescence (XRF) spectrometer (XRF-1800, Shimadzu, Japan). The pH of the slurry was measured using a pH meter (pHS-25, INESA, China). The EMR slurry after treatment was filtered and the solid phase was dried at 35 °C for 48 h. X-ray diffractometer (XRD) technique (X’Pert PRO, Panalytical, Holland), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy system (EDS; ∑IGMA + X-Max20, Zeiss, Germany) were used to characterize the treated EMR. Mn concentration in the filtrate was determined using a flame atomic absorption spectrophotometer (180-80, Hitachi, Japan). The concentrations of NH4 +-N and P were determined using a UV-Vis spectrometer (UV-8000S, Shanghai Metash, China) at wavelengths of 420 and 700 nm, respectively.
Results and discussion
Characterization of raw EMR
The major chemical components of the raw EMR determined by XRF analysis were presented in Table 1. The contents of Mn and NH4 +-N, respectively, accounted for 2.07 and 0.55 %. Crystalline phases of the raw EMR primarily consisted of MnSO4 · H2O, (NH4)2SO4, (NH4)2Mn(SO4)2 · 6H2O, (NH4)2Mg(SO4)2 · 6H2O, CaSO4 · 2H2O, and SiO2 (Fig. 1a). Regular cylindrical particles and particles in irregular shape overlapped randomly and loosely (Fig. 2a). A small amount of irregular floccule residues were observed in the spaces formed by the particles. A leaching experiment of the raw EMR was conducted under the conditions of a water:EMR mass ratio of 2:1, 8-h mixing time. The results indicated that Mn (7135 mg/L) and NH4 +-N (2768 mg/L) were the major contaminants in the filtrate. The production of these contaminants was attributed to the incomplete pressure filtration of Mn2+ and NH4 +-N that was added to the ore slurry to adjust the pH during the electrolytic manganese production (Xu et al. 2014). A small fraction of Mn2+ and most NH4 +-N were leached into the EMR after the pressure filtration and formed MnSO4 · H2O, (NH4)2SO4, (NH4)2Mn(SO4)2 · 6H2O, (NH4)2Mg(SO4)2 · 6H2O, etc. (Fig. 1a). These compounds could be dissolved in water, and the dissolved Mn2+ and NH4 +-N posed serious threats to the environment (Li et al. 2014a). In addition, the observation of MnSO4 · H2O, (NH4)2Mn(SO4)2 · 6H2O, (NH4)2Mg(SO4)2 · 6H2O, and (NH4)2SO4 indicated that Mn and NH4 +-N were in crystalline forms in EMR, which was inconsistent with previous studies (Du et al. 2015; Zhou et al. 2013).
Immobilization of Mn
Figure 3 shows the results of Mn immobilization by CO2 with alkaline additives (CaO and NaOH) under different alkaline additives:EMR mass ratios, 30-min reaction time, and 0.8-L/min CO2 flow rate. The immobilization efficiency of Mn was only 33.1 % when CO2 was bubbled into the EMR slurry without alkaline additives. The immobilization efficiency of Mn increased when the mass ratios of alkaline additives:EMR increased. The immobilization efficiency of Mn using CO2 + CaO was higher than using CO2 + NaOH. The immobilization efficiency reached 99.99 % when the CaO:EMR mass ratio was greater than 0.05:1. The immobilization efficiency of Mn using CO2 + NaOH was 99.99 % when the ratio of NaOH:EMR was 0.075:1. For the immobilization of Mn in same mass, more mass of NaOH is required than CaO.
More NH4 +-N was volatized using NaOH than using CaO at the same dosage during the Mn immobilization (Fig. 3a). This observation was due to the higher initial pH of the EMR slurry mixed using NaOH than using CaO (Fig. 3b). NH4 + could be converted to NH3 which evaporated under a higher pH (Bonmati and Flotats 2003; Gustin and Marinsek-Logar 2011). The release of OH− from the hydrolysis of CaO was slower and resulted in a higher pH of the EMR slurry after 7-min reaction time (Fig. 4b), which was the main reason that the Mn immobilization efficiency using CO2 + CaO was higher than using CO2 + NaOH.
Figure 1b presents the diffractogram of the carbonated sample under the conditions of the CaO:EMR mass ratio of 0.05:1, 30-min reaction time, and 0.8-L/min CO2 flow rate. The loss of X-ray reflections for (NH4)2Mn(SO4)2 · 6H2O, (NH4)2Mg(SO4)2 · 6H2O, MnSO4 · H2O, and (NH4)2SO4 was observed in the carbonated sample. The observation indicated that the minerals from the raw EMR reacted with CO2 and CaO to form MnCO3 (Fig. 2b) and CaMg(CO3)2, of which the X-ray reflections were identified in the carbonated sample. The occurrence reactions were described by Eqs. (3)–(7). The peaks of CaSO4 · 2H2O and SiO2 existing in the raw EMR were also detected, which demonstrated that these phases were resistant to the carbonation. In addition, the slight increase of intensity of X-ray peaks for CaSO4 · 2H2O was attributed to the formation of additional CaSO4 · 2H2O (Eqs. (4)–(6)). (NH4)2SO4, which was observed partly in the raw EMR and formed partly by carbonation process, was almost dissolved in the slurry, except the volatilization of a fraction of NH4 +-N (Fig. 4a).
Figure 1c displays the diffractogram of the carbonated sample under the conditions of the NaOH:EMR mass ratio of 0.075:1, 30-min reaction time, and 0.8-L/min CO2 flow rate. Similar to the diffractogram in Fig. 1b, the X-ray reflections for (NH4)2Mn(SO4)2 · 6H2O, (NH4)2Mg(SO4)2 · 6H2O, MnSO4 · H2O, and (NH4)2SO4 disappeared in the carbonated sample, and the peaks of MnCO3 (Fig. 2c) and CaMg(CO3)2 were identified in the carbonated sample. The generation of MnCO3 resulted from CO2 and NaOH reacting with (NH4)2Mn(SO4)2 · 6H2O and MnSO4 · H2O, as shown by Eqs. (8) and (9). Additionally, the formation of CaMg(CO3)2 was related with a certain amount of CaSO4 · 2H2O subjected to desulfating by NaOH to form Ca(OH)2 (Eq. (10)), which further reacted with CO2 and (NH4)2Mg(SO4)2 · 6H2O (Eq. (11)) at the beginning of the reaction where the EMR slurry had a higher pH (Bang et al. 2014).
Figure 4a demonstrates the effect of time on the immobilization of Mn from the EMR at 0.8-L/min CO2 flow rate with the CaO:EMR mass ratio of 0.05:1 and the NaOH:EMR mass ratio of 0.075:1, respectively. The immobilization efficiency of Mn using CO2 + CaO was higher than using CO2 + NaOH within 30-min reaction time. Twenty-minute reaction time was adequate to immobilize 99.99 % Mn using CO2 + CaO. As shown in Fig. 4b, the pH of the EMR slurry was almost constant after 20-min reaction time, although the pH of the slurry using CO2 + CaO was higher than using CO2 + NaOH after 7-min reaction time due to the slower release of OH− from the hydrolysis of CaO. In addition, The NH4 +-N reduction efficiency of the EMR slurry using CO2 + NaOH was higher than using CO2 + CaO, implying NaOH resulting in more NH4 +-N volatilization which led to more hazard for environment. In view of the higher Mn immobilization efficiency and the lower cost, CaO was more suitable as the alkaline additive for immobilizing Mn. The optimized experimental conditions were the CaO:EMR mass ratio of 0.05:1, 20-min reaction time, and 0.8-L/min CO2 flow rate.
Immobilization of NH4 +-N
Prior to immobilization of NH4 +-N from the EMR slurry, soluble Mn was immobilized by CO2 + CaO under the optimized experimental conditions. The efficiencies and characteristics of two ways of immobilizing NH4 +-N in the EMR slurry were compared directly using MgCl2 · 6H2O + Na3PO4 · 12H2O and MgO + Na3PO4 · 12H2O, respectively. Figure 5 presents the results of NH4 +-N immobilization directly using MgCl2 · 6H2O + Na3PO4 · 12H2O at different Mg:P:N molar ratios with 90-min reaction time. The immobilization efficiency of NH4 +-N for the P:N molar ratios of 1.5:1 and 2:1 were similar when the molar ratio of Mg:N ranged from 1:1 to 4:1. The lowest efficiency was observed when the molar ratio of P:N was 1:1. The highest efficiency of NH4 +-N immobilization was 89 % when the molar ratio of Mg:P:N was 1.5:1.5:1. When the molar ratio of Mg:P:N was greater than 1.5:1.5:1, the immobilization efficiency of NH4 +-N showed little change, but the remaining P concentration increased with the increase of P:N molar ratio (Fig. 5a). The product of NH4 +-N immobilization was the struvite (MgNH4PO4 · 6H2O) as determined by laboratory tests (Figs. 6a and 7a), suggesting the reaction shown by Eq. (12). The Ca2+ from CaSO4 · 2H2O in the EMR likely competed for PO4 3− by forming Ca3(PO4)2 precipitation (Fig. 6a). This competing process could explain the lower NH4 +-N immobilization efficiency when the molar ratio of P:N was 1:1 (Huang et al. 2014; Le Corre et al. 2005). Additionally, the pH of the EMR slurry (Fig. 5b) with the Mg:P:N molar ratio of 1.5:1.5:1 was 8.6, which favored the stabilization of struvite precipitation. Similarly, Li et al. (1999) reported that the optimum pH for struvite precipitation was in the range of 8.5–9.0 in landfill leachate.
Figure 8 displays the results of NH4 +-N immobilization directly using MgO + Na3PO4 · 12H2O at different Mg:P:N molar ratios with 90-min reaction time. The immobilization efficiency of NH4 +-N using MgO + Na3PO4 · 12H2O was lower than using MgCl2 · 6H2O + Na3PO4 · 12H2O when the molar ratios of P:N were 1:1, 1.5:1, and 2:1, respectively. This observation could be attributed to pH > 10 (Fig. 8b), because the higher pH could increase the solubility of struvite (Nelson et al. 2003) and even give rise to the stripping of part NH4 +-N (Zhou et al. 2013). Additionally, extra Na3PO4 could react with added MgO to form Mg3(PO4)2 at the higher pH (Fig. 6b), which lowered the concentration of P (<3 mg/L; Fig. 8a). The product of NH4 +-N immobilization using MgO + Na3PO4 · 12H2O was the struvite (MgNH4PO4 · 6H2O) determined by XRD and SEM-EDS analyses (Figs. 6b and 7b).
Figure 9 presents the effects of time on the NH4 +-N immobilization and P concentration using MgCl2 · 6H2O + Na3PO4 · 12H2O and MgO + Na3PO4 · 12H2O, respectively. The stabilization of NH4 +-N using MgCl2 · 6H2O + Na3PO4 · 12H2O started at 60 min and the efficiency was 89 %, while P concentration was stable at 20 mg/L in the EMR slurry at 90 min (Fig. 9a). The NH4 +-N immobilization efficiency using MgO + Na3PO4 · 12H2O (Fig. 9b) was stable at ∼73 % after 60 min. Additionally, for NH4 +-N immobilization using two ways, undetectable Mn concentrations in the filtrates indicated that newly formed Mn carbonate was not decomposed. Thus, the optimized conditions for NH4 +-N immobilization were using MgCl2 · 6H2O + Na3PO4 · 12H2O, 90-min reaction time, and the Mg:P:N molar ratio of 1.5:1.5:1.
Leaching test
After the immobilization of Mn and NH4 +-N under the optimized conditions, the concentrations of Mn and NH4 +-N in the filtrate decreased from 7135 to 0.4 mg/L and from 2768 to 302 mg/L, respectively. The lowered concentration of NH4 +-N in the filtrate could further be removed by electrochemical methods (Li et al. 2009; Lei et al. 2009). In order to test the leached toxic substance concentrations from the treated EMR which was dumped subsequently in landfill site, a leaching experiment was conducted according to the leaching standard designed by Chinese government (GB 5085.3-2007). The results showed that the concentrations of leached Mn, NH4 +-N, and P in the leached fluid were only 0.2, 9, and 0.4 mg/L, respectively. This study could immobilize both soluble Mn and NH4 +-N in the EMR slurry in comparison with other EMR harmless studies shown in Table 2.
Mechanism analysis
Although CaO could reduce the leaching of Mn (Du et al. 2015), CO2 further decreased the leachability of Mn and nudged the transformation of Mn compounds to Mn carbonate. After the combined treatment of Mn and NH4 +-N via immobilization, the pH of the EMR slurry was ∼8.6, and this value could stabilize the structures of Mn carbonate and struvite precipitation (Li et al. 1999). In addition, the contaminant of NH4 +-N from the EMR was seldom investigated, although it led to the environment pollution. The combination treatment of Mn carbonation and NH4 +-N precipitation was an effective exploration for the immobilization of contaminants from the EMR. The mechanisms could be divided into three steps (Fig. 10). Firstly, Mn2+ and NH4 +-N of MnSO4 · H2O, (NH4)2SO4, (NH4)2Mn(SO4)2 · 6H2O, and (NH4)2Mg(SO4)2 · 6H2O in the EMR were released into the slurry (the process of A → B). Secondly, Mn2+ reacted with CO2 and CaO to form Mn carbonate (the process of B → C). Thirdly, the struvite precipitation (MgNH4PO4 · 6H2O) of NH4 +-N was formed after adding MgCl2 · 6H2O + Na3PO4 · 12H2O (the process of B → D).
Economic analysis
An economic evaluation of the immobilization of contaminants in the EMR was performed. In the assessment, seeing that CO2 was collected from the ore leaching process of electrolytic manganese production and need not be purchased, the cost of other chemicals used in the EMR treatment was considered. The market prices of the used chemicals were obtained from the trading platform of Alibaba, and the results were shown in Table 3. The cost of the chemicals for the Mn immobilization using CO2 + CaO was calculated as $ 0.031/kg EMR. This value was less than using CO2 + NaOH. The cost of the chemicals for NH4 +-N immobilization using MgCl2 · 6H2O + Na3PO4 · 12H2O was $ 0.204/kg EMR, which was slightly less than using MgO + Na3PO4 · 12H2O. The cost of the immobilization of Mn and NH4 +-N using CO2 + CaO and MgCl2 · 6H2O + Na3PO4 · 12H2O was $ 0.235/kg EMR, which is less than other chemical combinations.
Conclusions
A large amount of soluble Mn and NH4 +-N in the EMR pose serious threats to the environment. This study showed an effective method for the immobilization of contaminants from the EMR. The Mn immobilization was conducted by using greenhouse gas (CO2) with the alkaline additives. The immobilization efficiency of Mn using CO2 + CaO was higher than using CO2 + NaOH. The efficiency was >99.99 % at the CaO:EMR mass ratio of 0.05:1 and 20-min reaction time. The NH4 +-N immobilization was conducted by magnesium and phosphate sources. The immobilization efficiency of NH4 +-N using MgCl2 · 6H2O + Na3PO4 · 12H2O was higher than using MgO + Na3PO4 · 12H2O. The immobilization efficiency was 89 % under the optimized conditions, which were the Mg:P:N molar ratio of 1.5:1.5:1 and 90-min reaction time. The Mn was immobilized to form Mn carbonate and the NH4 +-N was immobilized to form struvite. An economic evaluation shows that the cost of the immobilization of Mn and NH4 +-N in the EMR using CO2 + CaO and MgCl2 · 6H2O + Na3PO4 · 12H2O was less than other chemical combinations.
References
Bang JH, Kim W, Song KS, Jeon CW, Chae SC, Cho HJ, Jang YN, Park SJ (2014) Effect of experimental parameters on the carbonate mineralization with CaSO4 · 2H2O using CO2 microbubbles. Chem Eng J 244:282–287
Bednarik V, Vondruska M, Koutny M (2005) Stabilization/solidification of galvanic sludges by asphalt emulsions. J Hazard Mater 122:139–145
Bonmati A, Flotats X (2003) Air stripping of ammonia from pig slurry: characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion. Waste Manag 23:261–272
Chen HL, Liu RL, Shu JC, Li WS (2015) Simultaneous stripping recovery of ammonia-nitrogen and precipitation of manganese from electrolytic manganese residue by air under calcium oxide assist. J Environ Sci Health A 50:1282–1290
Du B, Zhou CB, Dan ZG, Luan ZK, Duan N (2014) Preparation and characteristics of steam-autoclaved bricks produced from electrolytic manganese solid waste. Constr Build Mater 50:291–299
Du B, Hou DY, Duan N, Zhou CB, Wang J, Dan ZG (2015) Immobilization of high concentrations of soluble Mn(II) from electrolytic manganese solid waste using inorganic chemicals. Environ Sci Pollut Res 22:7782–7793
Duan N, Wang F, Zhou CB, Zhu CL, Yu HB (2010) Analysis of pollution materials generated from electrolytic manganese industries in China. Resour Conserv Recycl 54:506–511
Feng Y, Liu F, Bao XC (2006) Possibility of using manganese slag as one of cement setting retarder to replace part of gypsum. Cement 2:22–24
Guo GL, Zhou QX, Ma LQ (2006) Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: a review. Environ Monit Assess 116:513–528
Gustin S, Marinsek-Logar R (2011) Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf Environ 89:61–66
Huang H, Xiao D, Zhang QR, Ding L (2014) Removal of ammonia from landfill leachate by struvite precipitation with the use of low-cost phosphate and magnesium sources. J Environ Manag 145:191–198
Lan JQ (2006) A test to corn production with fertilizer of Mn-dregs. China’s Manganese Ind 2:43–44
Le Corre KS, Valsami-Jones E, Hobbs P, Parsons SA (2005) Impact of calcium on struvite crystal size, shape and purity. J Cryst Growth 283:514–522
Lei XH, Li M, Zhang ZY, Feng CP, Bai W, Sugiura N (2009) Electrochemical regeneration of zeolites and the removal of ammonia. J Hazard Mater 169:746–750
Li XZ, Zhao QL, Hao XD (1999) Ammonium removal from landfill leachate by chemical precipitation. Waste Manag 19:409–415
Li TP, Xie HL, He XM, Zhou XZ (2007) Experimental study of calcined electrolysis manganese residue and fly ash complex admixture. Bull Chin Ceram Soc 3:567–571
Li M, Feng CP, Zhang ZY, Zhao R, Lei XH, Chen RZ, Sugiura N (2009) Application of an electrochemical-ion exchange reactor for ammonia removal. Electrochim Acta 55:159–164
Li CX, Zhong H, Wang S, Xue JR (2014a) Leaching behavior and risk assessment of heavy metals in a landfill of electrolytic manganese residue in Western Hunan, China. Hum Ecol Risk Assess 20:1249–1263
Li CX, Zhong H, Wang S, Xue JR, Zhang ZY (2014b) A solidification technology for heavy metals in EMM residue. China’s Manganese Ind 4:23–26
Liu RJ, Ding QJ, Chen P, Yang GY (2012) Durability of concrete made with manganese slag as supplementary cementitious materials. J Shanghai Jiaotong Univ (Sci) 3:345–349
Nelson NO, Mikkelsen RL, Hesterberg DL (2003) Struvite precipitation in anaerobic swine lagoon liquid: effect of pH and Mg:P ratio and determination of rate constant. Bioresour Technol 89:229–236
Ouyang YZ, Peng XW, Cao JB, Li ZP, Deng XD (2007) Ultrasonic leaching of electrolytic manganese residue with additive. Environ Prot Chem Ind 3:257–259
Stolzenburg P, Capdevielle A, Teychene S, Biscans B (2015) Struvite precipitation with MgO as a precursor: application to wastewater treatment. Chem Eng Sci 133:9–15
Xin BP, Chen B, Duan N, Zhou CB (2011) Extraction of manganese from electrolytic manganese residue by bioleaching. Bioresour Technol 102:1683–1687
Xu FG (2001) Experimental research on application of Mn-slag to roadbed backfill. China’s Manganese Ind 4:1–3
Xu FY, Jiang LH, Dan ZG, Gao XJ, Duan N, Han GM, Zhu H (2014) Water balance analysis and wastewater recycling investigation in electrolytic manganese industry of China—a case study. Hydrometallurgy 149:12–22
Yang C, Lv XX, Tian XK, Wang YX, Komarneni S (2014) An investigation on the use of electrolytic manganese residue as filler in sulfur concrete. Constr Build Mater 73:305–310
Yao J, Chen S, Xiao SH, Xiao Y, Tuo Y (2003) Influence of lixiviate rate of manganese in rhodochrosite by adding addition agent. J Jishou Univ Na Sci Ed 1:43–45
Zhou CB, Wang JW, Wang NF (2013) Treating electrolytic manganese residue with alkaline additives for stabilizing manganese and removing ammonia. Korean J Chem Eng 30:2037–2042
Zhou CB, Du B, Wang NF, Chen Z (2014) Preparation and strength property of autoclaved bricks from electrolytic manganese residue. J Clean Prod 84:707–714
Zhou F, Chen T, Yan CJ, Liang H, Chen T, Li D, Wang QY (2015) The flotation of low-grade manganese ore using a novel linoleate hydroxamic acid. Colloids Surf A 466:1–9
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51274261) and the Natural Science Research Project of the Education Department of Guizhou Province, China (No. Qianjiaohe KY[2013]202).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Chen, H., Liu, R., Liu, Z. et al. Immobilization of Mn and NH4 +-N from electrolytic manganese residue waste. Environ Sci Pollut Res 23, 12352–12361 (2016). https://doi.org/10.1007/s11356-016-6446-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6446-2