Abstract
The immune system can be the target of many chemicals, with potentially severe adverse effects on the host’s health. In the literature, carbamate (CM) pesticides have been implicated in the increasing prevalence of diseases associated with alterations of the immune response, such as hypersensitivity reactions, some autoimmune diseases and cancers. CMs may initiate, facilitate, or exacerbate pathological immune processes, resulting in immunotoxicity by induction of mutations in genes coding for immunoregulatory factors and modifying immune tolerance. In the present study, direct immunotoxicity, endocrine disruption and inhibition of esterases activities have been introduced as the main mechanisms of CMs-induced immune dysregulation. Moreover, the evidence on the relationship between CM pesticide exposure, dysregulation of the immune system and predisposition to different types of cancers, allergies, autoimmune and infectious diseases is criticized. In addition, in this review, we will discuss the relationship between immunotoxicity and cancer, and the advances made toward understanding the basis of cancer immune evasion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The immune system of mammalian species is comprised of a highly complex network of interacting cells that are both antigen-nonspecific (innate) and antigen-specific (adaptive). The main role of the immune system is to protect all other organ systems from invading pathogens, which include infectious agents and malignant cells, and to eliminate senescent or phenotypically altered cells, such as cancer cells, that are not performing their appropriate functions.
The merger of immunology and toxicology formally took place during the mid-1970s. Toxicants can be chemical agents, for example, pesticides, respectively, which induce harmful effects. Depending on the dose, a pesticide may range from being essential (necessary for the maintenance of health) to being toxic. When this chemical is stated to be toxic, it does not necessarily mean that it induces the death of cells; a toxic effect may not result in cytotoxicity but alteration of cell function, leading to a detrimental outcome.
Among the most used worldwide pesticides, there are carbamate (CM) pesticides (Table 1). This compounds derived from carbamic acid are probably the insecticides with the widest range of biocide activities. The structure of the biologically active carbamates is displayed in Fig. 1. In these structures, X can be oxygen or sulphur, and R1 and R2 are usually organic radicals, but R1 or R2 may also be hydrogen. When R2 is hydrogen and R1 is methyl, the carbamate exhibits insecticide activity; if R1 is an aromatic group, the compounds are used as herbicides, whilst fungicide activity is present if R1 is a benzimidazole moiety. R3 is mostly an organic radical and sometimes a metal. CM pesticides are inhibitors of acetylcholinesterase (AChE) activity and therefore, are also able to reversibly inhibit neuropathy target esterase. However, carbamates are not able to “age” the inhibited enzyme, and therefore, they are not inducers of neuropathy (Lotti 1992). The main detoxification routes of carbamate insecticides are hydrolysis and oxidation, the hydrolysis by esterases being a most effective detoxification route. Although the role of esterases in the carbamate detoxification is well understood, there are no in-depth toxicological and biochemical studies in mammals on interactions between these enzymes and carbamate insecticides (Lotti 1992; Lotti and Moretto 1999).
Extensive research has focused on investigating carbamate toxicity. Indeed, in the literature, including PubMed, Scopus and Google Scholar were searched to identify authentic articles published in English language during the last 20 years. The main keywords of the search were “carbamate” in combination with each of the terms “immmunotoxic”, “immunotoxicity”, and “immune system”. Additional searches included the terms “insecticide”, “herbicide” and “fungicide” in combination with “immune system” with no limitation in the type or date of publication. Besides, additional articles were extracted from the reference lists of the found publications. All publications were growing evidence that indicate that carbamate pesticides also have deleterious effects on the immune system (Bernier et al. 1995; Cha et al. 2000; El-Bini Dhouib et al. 2014). Carbamates may potentially play a role in carcinogenesis of various tissues through reduced immune surveillance. However, the specific effects of CMs on immune function remain poorly understood. Therefore, we considered that further investigation of CM immunotoxicity is warranted and conducted a PubMed search of CMs exposure and cancer immune-related effects. Here, we summarize the known toxicological effects of carbamate pesticides on immune function in laboratory animals, in vitro models and in humans, and identify possible future research directions to help close the gaps in knowledge.
Mechanism of action of carbamate pesticides on immune cells
For a better understanding of immunotoxic risk, the great value is data addressed to basic mechanisms of action of carbamate pesticides on cells of the immune system. Recognition of cell structures and their components that can be affected may help to identify specific and sensitive markers of immunotoxic effects (Table 2). They interfere with metabolism, signal transduction pathways, structural tissue compartments and cellular structures which pose additional challenges for experimental as well as clinical studies.
Esterase inhibition
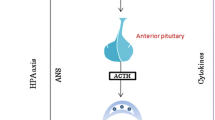
The understanding that carbamates insecticides share the same mechanism of action achieved by the inhibition of AChE, a serine hydrolase, and that the serine hydrolase activity is probably linked to several immune functions gave a clearer picture of the potential immunotoxicity of these compounds (Fukuto 1990) (Fig. 2).
Banks and Lein (2012) demonstrated that CMs can alter lymphocytic cholinergic signalling through inhibition of acetylcholinesterase (AChE). Indeed, in acute CM poisoning, overstimulation of cholinergic receptors by accumulated acetylcholine (Ach) can cause rapid and transient Ca2+ signalling, up-regulation of c-Fos expression and interleukin (IL)-2-induced signal transduction in T and B cells as well as triggering inflammatory responses in macrophages (Banks and Lein 2012). Moreover, CMs can alter esterases activities associated with the cell membrane of immunocytes (Banks and Lein 2012). This inhibition may lead to structural and functional changes in immunocyte populations. Modulation of signal transduction pathways, as the main functional consequence of esterase inhibition, can inhibit or stimulate activation, proliferation and subsequently effector function of immune cells (Chambers and Oppenheimer 2004). For example, impaired neutrophil function (Wang et al. 2010), cytolytic activity of natural killer cells (Luebke et al. 2001) and functional impairment of dendritic cells and macrophages (Dietert 2008) result in lead to a T helper 2 (Th2)-biased immunity and/or inappropriate chronic inflammatory-related disorders (Dietert 2008). These in turn may elevate risk of cancers, psychiatric disorders, chronic obstructive pulmonary disease, inflammatory bowel disease, rheumatoid arthritis, psoriasis and metabolic syndrome-related conditions.
Oxidative stress
In most cases, the metabolism of pesticides can cause the generation of toxic metabolites or reactive oxygen species (ROS) (Lasram et al. 2014) (Fig. 2). These reactive intermediates may induce cytolethality through damaging the components of the cell, including lipids, proteins and DNA. Further, CM-induced oxidative stress can disturb various parts of cellular signalling and oxidative homeostasis through mediating redox signalling and depletion of antioxidant reservoirs (Abdollahi et al. 2004; El-Bini Dhouib et al. 2014). Additionally, as an alternative mechanism, CM-induced oxidative stress may promote carcinogenic mutations via induction of DNA damage (Klaunig and Kamendulis 2004).
Induction of free radicals, lipid peroxidation and impaired antioxidant status by CMs has been widely studied in humans and animals (Gupta et al. 2001). The studies reported in manufacturing workers accidentally exposed to CM pesticides are also similar. Vidyasagar et al. (2004) reported an increase of MDA level and superoxide dismutase (SOD) activity in human cases accidentally poisoned by various CMs. Ranjbar et al. (2002) also reported an increase in MDA levels and reduced thiols and total antioxidant capacity among manufacturing workers exposed to different doses of CM pesticides. Thus, acute and chronic exposure of male rats to different doses of carbosulfan causes the same changes in the redox status in the liver, spleen and thymus (El-Bini Dhouib et al. 2014, 2015).
Oxidative stress was reflected by an increase of lipid peroxidation (LPO). Indeed, increase of LPO has been demonstrated after acute and/or chronic exposure to other carbamates like benomyl and thiram (Banks and Soliman 1997; Cereser et al. 2001). Yang and Dettbarn (1996) observed a strong correlation between the accumulation of acetylcholine and the extent of LPO. Also, butyrylcholinesterase (BChE), a cholinesterase enzyme, decreased in spleen of carbosulfan-treated rats (El-Bini Dhouib et al. 2014). BChE can be considered as an endogenous scavenger of anticholinesterase compounds and detoxifies them before they reach to AChE at physiologically important target sites. Some important compounds of carbamates and organophosphorous pesticides were detoxified by BChE (Adresi 2003). Increased oxidative stress by CMs might be a result of cholinergic hyperactivity or might be due to its direct effect on the production of reactive oxygen and nitrogen species. The mechanism involved in increased production of reactive oxygen species was the inhibition of cytochrome c oxidase (Gupta et al. 2001). It is also reported that CMs induce nitric oxide synthase, which is implicated in the overproduction of superoxide anions (Pou et al. 1992). End products of LPO are believed to be largely responsible for the cytotoxic effects observed in spleen (Pou et al. 1992; El-Bini Dhouib et al. 2014). Therefore, carbamates induced oxidative stress which may contribute to its immunotoxicity as previously reported (Caroleo et al. 1996; Gao et al. 2015).
Free radicals are known to attack unsaturated fatty acid side chains of phospholipids, causing a substantial decrease of these cell membrane components. This effect leads to reduced membrane fluidity (Gao et al. 2015). In physiological conditions, the process underlying lymphocyte interaction with antigens or with other cell subsets requires the integrity of cell membrane. It is possible that free oxygen radicals generated by CM pesticides could inhibit T cell function via membrane lipid peroxidation (Gao et al. 2015). El-Bini Dhouib et al. (2014) demonstrated that in the cell system, treatment of lymphocytes with carbosulfan induced a profound decrease in their total and reduced glutathione (GSH) content in rat spleen. Also, the susceptibility of T cells to lipid peroxidation may be the result of lower levels of intracellular antioxidants, such as reduced GSH, and it is important in lymphocyte activation and proliferation. Altogether, many data confirm the hypothesis of immunotoxicity induced by oxidative stress caused by CMs (Handy et al. 2002; El-Bini Dhouib et al. 2014; Singh et al. 2015). It is clear from these studies that the immune system may be a sensitive target for CM pesticides for which the exact molecular target or mechanism of immunotoxicity was oxidative stress. Hence, immunosuppression induced by carbosulfan and carbofuran may be a consequence of toxic chemical-induced cholinergic stimulation and its effects on immune cell function (Jeon et al. 2001). Moreover, CMs may also act directly or indirectly on lymphoidal cells, immunoglobulin metabolism, T/B cell macrophage cooperation and macromolecular biosynthesis responses. Such as, some dithiocarbamates compounds base their immunotoxic potential also on oxidative stress and on inflammation. Indeed, the expression of nuclear factor-κB (NF-κB) indicates that this can be the convergence point of the immune and neuroendocrine pathways.
Endocrine disruption
Endocrine disruption refers to mechanism of toxicity that impairs the ability of cells or the organs to communicate hormonally. Because immune system is strictly controlled by hormones released from various organs, endocrine disruptor pesticides, such as CM pesticides, can profoundly disturb both homeostasis and function of immune system (Mostafalou and Abdollahi 2013; Mokarizadeh et al. 2015). Since hypothalamic–pituitary–adrenal axis (HPA axis), a major part of the neuroendocrine system controls reaction to stress and regulates immune responses, CMs can disturb immune system through dysregulation of gonadotropin-releasing hormone (GnRH) biosynthesis in hypothalamus gland (Corsini et al. 2013). The alteration of immune system may occur directly through altering GnRH–receptor signalling in immune cells or indirectly by alteration in cortisol secretion from adrenal gland. In view of the fact that the adrenal gland induces production of a variety of cytokines, interactions between carbofuran (a carbamate pesticide) and HPA axis may result in change in the cytokine network (Blakley et al. 1999; Zhao et al. 2010; Mokarizadeh et al. 2015). Moreover, CM pesticide-mediated immunosuppression may partly attribute to dysregulation of pituitary gonadotropins (Tanriverdi et al. 2003). Several studies have demonstrated that activation of steroid and xenobiotic receptors in immune cells can inhibit the activity of nuclear factor-κB (NF-κB) as one of the major regulators of inflammation and immunity (Guo et al. 2006; Zhou et al. 2006). The knowledge that corticosteroids mediate part of their anti-inflammatory effect by inhibiting NF-κB activity through the induction of the rat endogenous inhibitor IκBα (Guo et al. 2006), and the finding of chemicals inducing cortico-steroids or corticosteroids-like molecule, as dithiocarbamates, suggests that they may elicit their effect through such a way (Mayne et al. 2004). With the increasing of molecular biology, a significant number of chemicals as some dithiocarbamates and propanil were found to interfere with signal transduction pathways altering the status and/or functionality of the immune system (Theus et al. 1993; Mayne et al. 2004). With the increasing understanding at the molecular level on immune system function, studies on the molecular mechanisms of action of potential immunotoxic compounds are mandatory and necessary for a proper risk assessment evaluation (Fig. 2).
Moreover, in the absence of specific IgE and allergen, estrogenic signalling of certain CM pesticides on mast cells can promote releasing of allergic mediators (Narita et al. 2007). Also, during initiation and development of allergy, such hyper-reactivity or hypo-reactivity of HPA axis may contribute to the allergic response (Buske-Kirschbaum 2009).
In the other hand, since the sex hormones play an important role in the modulation and development of immune responses, carbamates can disturb the normal function of the immune system via interference with secretion, metabolism and/or signalling of these hormones (Meeker 2010).
Experimental animal studies
Gene expression
Living organisms respond at the cellular level to unfavourable conditions, such as heat or other stressful situations of different origins, by a rapid, vigorous and transient acceleration in the rate of expression of a small number of specific heat shock/stress genes, resulting in the production of heat shock proteins (HSPs) after carbaryl exposure (Bierkens et al. 1998; Harboe and Quayle 1991). HSPs are believed to assist cells to adapt or survive by a rapid but transient reprogramming of cellular metabolic activity to protect cells from further oxidative and thermal stress in responsive tissues (Harboe and Quayle 1991). Potential mechanisms of protection from oxygen free radicals by HSPs include prevention of protein degradation, inhibition of membrane lipid peroxidation or calcium intrusion, maintenance of ATP levels and induction of classical scavengers such as SOD or GSH, which also plays a role in the induction of HSPs (Freeman and Meredith 1989; Bierkens et al. 1998). In various animal models, carbamate pesticides exposure is associated with altered expression of genes involved in immune response. In lungs of mice exposed to ethylcarbamate (900 mg/kg) for 14 days, significant changes were identified in transcripts encoding humoral immune response, antigen binding, TLRs, cytokines, cytokine receptors and genes involved in cell adhesion and migration (Fernando et al. 1996). Specifically, down-regulated expression of genes encoding TLR/IL1R signalling pathway was identified (Williams et al. 1998).
Based on the study by Sharova et al. (2002), injection of mice with ethylcarbamate altered the expression of numerous genes in spleen leukocytes. This was also true for immunostimulated mice, with comparative up-regulation of genes involved in cell cycle control. Immune stimulation of ethylcarbamate-treated animals shifted the coordinate gene expression in splenic leukocytes, as compared to mice receiving ethylcarbamate only, a reflection of the up-regulation of 22 genes. Up-regulation of TGFβ3 was associated with the highest individual contribution coefficient, suggesting the importance of altered expression of the gene for this growth factor between ethylcarbamate-only and ethylcarbamate + immunostimulation mice. This is an interesting similarity to an observation by Sharova et al. (2002) that maternal immunostimulation significantly increased TGFβ2 mRNA and reduced birth defects in cyclophosphamide-treated mice. In this regard, TGFβ2 and TGFβ3 are important regulators of tightly controlled cellular differentiation and proliferation events during development (Sharova et al. 2002).
Carcinogenesis induced by CMs
Experimental evidence indicates that CMs alter gene expression and down-regulation of the antioxidant defence system, cause adverse effects on the dopamine system and induce neurotoxicity and carcinogenesis (Bemis et al. 2015). In an old study conducted by Lawson and Pound (1978), CMs were shown to induce carcinogenesis in mice. Cheng and Conner (1982) showed that six different CMs had a striking similarity in relative potencies for sister chromatid exchange induction and known tumorigenic potencies.
Underlying mechanisms of CM pesticide-induced cancer may include DNA damage and oxidative stress. Carbamates, esters of N-methyl carbamic acid, act by inhibition of AChE. All carbamates contain ethylene thiourea and produced tumours in the thyroid and at other sites in rodent (Mahajan et al. 2007a, b). Carbamate derivatives such as carbaryl and N-nitrosocarbaryl are carcinogens in mice rodents (Bigot-Lasserre et al. 2003). In addition, CMs increase expression of P450 1A1, which catalyzes procarcinogen activation. It also promotes liver tumours via ROS formation. The increase in ROS is due to CM-induced regulation of glutathione reductase and other antioxidant enzymes. Also, CMs can interfere with DNA repair processes and can also cause direct induction of DNA damage, DNA–protein crosslinking and sister-chromatid exchanges, which contributes to its toxicity and carcinogenicity.
The mutagenic potential of CM pesticides such as propoxur and carbosulfan was demonstrated to significantly induce the formation of micronuclei in bone marrow cells of mice at different dose levels at 24 and 48 h by the intraperitoneal and oral routes (Giri et al. 2002). In the old study realized by Lawson and Pound (1978), the tumour initiating potency of three simple alkyl CMs and mono-N substituted ethyl CMs was examined in Hall strain mice. Ethyl CM was the most potent carcinogen for the epidermis, liver and lung, followed by its N-alkyl derivatives. This result was confirmed by Agrawal and Mehrotra in 1997. The authors showed that methyl CM was without effect, but n-propyl and n-butyl were possible carcinogens. The ethyl esters bound to a greater extent to DNA in liver and skin than did the methyl, n-propyl and n-butyl esters, and only this binding persisted (Prado-Ochoa et al. 2014a, b). In the recent study by Soloneski et al. (2015), sister chromatid exchange induction by pirimicarb and zineb, CM pesticides, was examined in alveolar macrophage, bone marrow and regenerating liver cells of mice. In general, alveolar macrophage and regenerating liver cells had higher responses, although not significantly, than bone marrow. Also, Cheng and Conner (1982) showed that vinyl CM produced significant increases in sister chromatid exchange frequencies over a dose range of 10 μmol/kg to 75 μmol/kg. At the highest dose, sister chromatid exchange frequencies in extrahepatic tissues of hepatectomized mice were significantly higher than those in intact mice and in hepatectomized mice, and alveolar macrophage and regenerating liver cell responses were greater than bone marrow responses. Vinyl CM was approximately 30 times as potent a sister chromatid exchange inducer as reported previously for ethyl CM (Cheng and Conner 1982).
The mutagenic potential of propoxur, a widely used dithiocarbamate pesticide, has also been studied. Since the restriction of the use of DDT in most industrialized countries, propoxur has been proposed as a replacement for DDT, resulting in a major increase in the use of propoxur during the past 10 years. However, studies indicate that nitrosopropoxur, the nitroso derivative of propoxur, is highly mutagenic in Salmonella typhimurium (Allinson et al. 2012), Escherichia coli (Dong et al. 2003), Saccharomyces cerevisiae (Azad et al. 2014) and human lymphocytes in vitro (Li et al. 2015). Propoxur has an effect on induced dominant lethal mutations in mice (Agrawal 1999) and produces sister chromatid exchange and micronuclei formation in human lymphocytes (Agrawal and Mehrotra 1997). A study by Agrawal and Mehrotra (1997) further demonstrated the mutagenic potential of propoxur to significantly induce the formation of micronuclei in bone marrow cells of mice. Also, single application of indole-3-carbinol, a glucobrassicin derivative present in cruciferous vegetables, significantly inhibited propoxur-induced micronuclei formation when it was given at a dose level of 500 mg/kg body weight 48 h before the single application of propoxur. Therefore, it seems that propoxur is mutagenic in these test systems and indole-3-carbinol significantly inhibits the mutagenicity of propoxur (Agrawal and Mehrotra 1997).
Carbosulfan is reported to be non-mutagenic in strains TA 97, 98, 100 and 102 of Salmonella typhimurium, but it induces mitotic aneuploidy in strain D61 M of Saccharomyces cerevisiae (Wiedenmann et al. 1990). In human lymphocytes, carbosulfan has been reported to decrease the replication index and mitotic index in a dose-dependent manner (Rencüzogullari and Topaktas 1996, 1998; Giri et al. 2002). So, these studies provide further evidence in support of a mutagenic potential of carbosulfan, and hence, exposure to carbosulfan should be restricted.
In addition, the mutagenic and genotoxic effect of carbosulfan was carried out in fish Channa punctatus using micronucleus (MN) test and comet assay (Nwani et al. 2010). In general, significant effects from both concentrations and time of exposure were observed in exposed fishes. The MN induction was highest on 96 h at all the concentrations in the peripheral blood. Similar trend was observed for the DNA damage measured in terms of the percentage of tail DNA in the erythrocyte and gill cells. This study confirmed that the comet and micronucleus assays are useful tools in determining potential genotoxicity of water pollutants and might be appropriate as a part of monitoring program (Nwani et al. 2010).
Lymphocyte subpopulations
Studies in rats (El-Bini Dhouib et al. 2014), mice (Jeong et al. 1996), rabbit (Capcarova et al. 2010) and catfish (Ibrahim and Harabawy 2014) show that carbamate pesticides can suppress the weight, index and/or cellularity of major immunocompetent organs, including spleen and thymus. In chronically exposed mice, reduced CD4+ T cell populations and CD4/CD8 ratio were evident, concurrent with observations in carbosulfan-exposed rats (Jeong et al. 1996; El-Bini Dhouib et al. 2014), as well as increased percentage of lymphocytes in splenic mononuclear cells (El-Bini Dhouib et al. 2014). In catfish, carbofuran increased atypical lymphocytes and depleted lymphoid and melano-macrophage populations in head kidney, a major immunocompetent organ (Monteiro et al. 2006; Ibrahim and Harabawy 2014). Interestingly, a single intra-tracheal exposure of mice to 0.075, 0.15 and 0.3 mg/kg of carbofuran markedly decreased peritoneal lymphocyte counts and splenic T cell, B cell and macrophage numbers by 58, 61 and 30 %, respectively, without affecting their proportions (Jeon et al. 2001).
Lymphocyte activation
Consistent with epidemiological observations, carbamate pesticides can inhibit lymphocytes production. Chronic carbaryl exposure (30 mg/kg) inhibits mitogen-stimulated proliferation of T splenic cells in rats (Jorsaraei et al. 2014) and in mice (Rodgers et al. 1986). In addition, subchronic exposure to carbosulfan alters T cell proliferation in rat spleen (El-Bini Dhouib et al. 2014). Consequently, decreases have been observed in secretion of IFN-γ, IL-2, IL-6 and IL-12 in mice (Rodgers et al. 1986) and in rats (El-Bini Dhouib et al. 2014; Jorsaraei et al. 2014) and “IL-4-like factors” from HK T cells in catfish (Pfeiffer et al. 1997). An important consideration regarding animal studies is that carbamate pesticides concentrations administered typically far exceed human exposures, which may account for differential effects observed.
Humoral and hypersensitivity responses
Carbamate pesticides can inhibit humoral immunity, as evidenced by suppressed in vitro primary and/or secondary antibody forming cell (AFC) responses of rodent splenocytes (Medina-De la Garza et al. 2012). IL-2 is a primary target of this inhibition in mice (Hajoui et al. 1992). Further, carbamate pesticides suppressed delayed-type hypersensitivity reaction, a response to cutaneous sensitization, in mice (Medina-De la Garza et al. 2012), rats (Seth et al. 2002) and chickens (Singhal et al. 2003).Compared to controls, carbendazim-exposed sensitized mice demonstrated reduced lymph node cell proliferation, ear swelling, activated Langerhans cells (LC) in cervical lymph nodes, peritoneal macrophages and circulating neutrophils (Singhal et al. 2003), suggesting that carbamate pesticides inhibits LC migration to lymph nodes and subsequent T cell activation (Medina-De la Garza et al. 2012).
Immune alterations in carbamates-exposed on humans
The majority of papers on the clinical immunotoxicity of carbamate pesticides described immune alterations in occupational exposed workers or farmers, and accidentally exposed humans. In some instances, these alterations were suggested to be associated with infectious complications.
In workers exposed to CMs, the killing ability of neutrophils has been shown to be significantly abrogated, probably via interference with myeloperoxidase activity (Corsini et al. 2013). A carefully designated epidemiological study conducted in 23 women chronically exposed to aldicarb-contaminated groundwater at level of 16.1 ppb showed persistent changes in the T cell subset count with a significant increase number of T-CD8+ cells and a decreased T4/T8 ratio compared to a matched control group of 27 unexposed women (Fiore et al. 1986). A significant negative correlation between average daily aldicarb ingestion and T4/T8 ratio values suggested a dose-dependent effect. In vitro lymphocyte stimulation to Candida antigen was increased in exposed women, again with a dose–response effect. However, the values were within the normal range of variability. In addition, the lack of similar response with other antigens or mitogens and the small number of tested patients strongly limit the significance of these results. Overall, it has been suggested that CMs modulate immune responses through a series of separate mechanisms including inhibition of serine hydrolases in immune cells, oxidative damage to immune organs and modulation of signal transduction pathways in women (Corsini et al. 2013) and in children (Jones et al. 2014).
In addition, numerous studies have been shown on the effects of CM pesticides on immune system (Vial 1996). Moreover, in vivo and in vitro studies have shown that CMs can affect immune responses including antibody production, IL-2 production and T cell proliferation, autoantibody production, changes in Th1 and Th2 cytokine production, NK, LAK and TC cells inhibition via different mechanisms (Li 2007, 2002). On the other hand, systemic poisoning with carbaryl can result immune suppression, increasing the risk of allergic responses against allergens such as dust mites coming from domestic waste due to inappropriate immune responses (Gholam et al. 2014).
Role of CMs-induced immunosuppression in relationship to carcinogenicity
The idea that the immune system might recognize and destroy tumour cells was conceived more than 100 years ago. An overwhelming amount of data from animal models, together with compelling data from human patients, indicates that a functional cancer immunosurveillance process exists that works to prevent outgrowth of many types of primary and transplanted tumours (Hanahan and Weinberg 2011). It has also become clear, however, that the immune system can also facilitate tumour progression. The recognition that immunity plays a dual role in the complex interactions between tumours and the host prompted a refinement of the cancer immunosurveillance hypothesis into one termed cancer immunoediting (Dunn et al. 2004). Chemical-induced immunotoxicity is comprised of three phases; this process is collectively denoted the three E’s of cancer immunoediting—elimination, equilibrium and escape:
-
1.
Elimination: cancer immune surveillance
-
2.
Equilibrium: a phase of tumour dormancy where tumour cells and immunity enter into a dynamic equilibrium that keeps tumour expansion in check
-
3.
Escape where tumour cells emerge that either display reduced immunogenicity or engage a large number of possible immunosuppressive mechanisms to attenuate antitumor immune responses leading to the appearance of progressively growing tumours. One can envisage that.
Factors that tumour exploit to avoid immune responses
Immunosuppression in the tumour induced by carbamate pesticides, mediated by CD4+CD25+ FoxP3+ regulatory T cells (Tregs), or other types of suppressive cells, seems to be a major mechanism of tumour immune escape and can be a crucial hurdle for tumour immunotherapy (Whalen et al. 2003).
It is well established that another fundamental mechanism by which tumours evade immune surveillance is by down-modulating antigen processing machinery affecting essentially the major histocompatibility complex (MHC) I pathway (Mahajan et al. 2007a, b). Thus, expression of tumour antigen is down-regulated, which can lead to enhanced tumour incidence and metastasis because cytotoxic T lymphocyte (CTL) can no longer recognize target antigens on the tumour cells (Corsini et al. 2013).
As alluded to above, tumours can evade immune surveillance by crippling CTL functionality after chronic exposure to carbofuran via production of several immune suppressive cytokines, either by the cancer cells or by the noncancerous cells present in the tumour microenvironment, especially including immune cells and epithelial cells. TGF-β is a chief mediator of this activity (Jeon et al. 2001). In addition, tumour necrosis factor (TNF)-α, IL-1, IL-6, colony-stimulating factor (CSF)-1, IL-8, IL-10 and IFN-δ can also significantly contribute to cancer growth (Lind et al. 2004).
On the other hand, tumours are also known to evade immune attack by shifting the balance from Th1 to Th2 (immune deviation) in a TGF-β and IL-10-dependent manner (Jeon et al. 2001).
In the end, apoptosis is another fundamental mechanism by which tumours evade immune surveillance. A number of studies have shown that cancer cells delete tumour-specific CTLs through apoptosis (Yoon et al. 2001).
The different factors governing tumour growth and immune evasion strategies are briefly outlined in Fig. 3.
Conclusion, recommendations and future directions
This review summarized the research studies about the immunotoxicity of carbamate pesticides:
-
1.
In vivo studies depict CMs as an immunomodulator that could render the host immunocompromised. Such immune alterations could help explain increased risk of infections and several cancers observed in chronically exposed human populations. CMs-mediated alterations of cellular and humoral immunity reported in animal and in vitro models generally agree with immunological outcomes in humans. However, more work is needed to close the gap between experimental data and risk of human immunotoxicity.
-
2.
CMs induce immune alteration through altering well-regulated immune responses to tumour antigens, allergens, self-antigens and microbial antigens that can contribute to predisposition to different types of cancers, allergies, autoimmune and infectious diseases.
-
3.
CMs induce endocrine-disrupting and can provide the stage for other factors to contribute to the development or exacerbation of autoimmune diseases. Nonetheless, despite the ability of several CM pesticides to trigger the development of autoimmune responses, as far as the authors are concerned, few studies have been conducted to evaluate the possible role of CMs as risk factors for autoimmune diseases.
-
4.
CMs may contribute to tumorigenesis either through interference with immune surveillance or by innate immune dysfunction leading to chronic inflammation. Nonetheless, since CM pesticides can cause cancer through a number of non-immunologic pathways (such as: genetic damage), the responsibility of immune dysregulation in onset of cancers linked to CM pesticides is not absolute.
-
5.
Oxidative stress is the main mechanism by which CM pesticides can increase susceptibility to immunotoxicity and infectious diseases.
-
6.
In spite of many ongoing studies, the exact immune toxicity of most pesticides is still unclear. Additionally, more epidemiological and experimental studies should be conducted to reveal the exact relationship between the level of exposure and toxic effect.
-
7.
Moreover, inconsistencies in epidemiological findings, possibly due to differences in dose, sampling, genetic background and environmental/nutritional factors indicate need for larger participant numbers and diverse ethnic populations. Due to differential effects of exposures, populations having low, intermediate and high exposure should be evaluated to better understand dose-dependent relationships. For example, in most immunotoxicity studies, animals are exposed to a single pesticide, whilst in real-life conditions, individuals are contacted with a series of different chemicals and/or pesticides. Due to the probable synergies or antagonistic effects of chemicals when they are combined together, the toxicity and the resulting hazards may be over or differ from their single effects.
References
Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A (2004) Pesticides and oxidative stress: a review. Med Sci Monit 10(6):141–147
Adresi Y (2003) Butyrylcholinesterase: structure and physiological importance. Turk J Biochem 28:54–61
Agrawal RC (1999) Induction of chromosomal aberrations by propoxur in mouse bone marrow cells. Biomed Environ Sci 12(4):292–5
Agrawal RC, Mehrotra NK (1997) Assessment of mutagenic potential of propoxur and its modulation by indole-3-carbinol. Food Chem Toxicol 35(10–11):1081–4
Allinson M, Kageyama S, Nakajima D, Kamata R, Shiraishi F, Goto S, Salzman SA, Allinson G (2012) A pilot survey of 39 Victorian WWTP effluents using a high speed luminescent umu test in conjunction with a novel GC-MS-database technique for automatic identification of micropollutants. Water Sci Technol 66(4):768–74
Azad GK, Singh V, Tomar RS (2014) Assessment of the biological pathways targeted by isocyanate using N-succinimidyl N-methylcarbamate in budding yeast Saccharomyces cerevisiae. PLoS One 9(3):e92993
Banks CN, Lein JA (2012) Review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 33:575–584
Banks D, Soliman MR (1997) Protective effects of antioxidants against benomyl-induced lipid peroxidation and glutathione depletion in rats. Toxicology 116:177–81
Bemis J C, Labash C, Avlasevich S L, Carlson K, Berg A, Torous D K, Barragato M, James T, Gregor M, Dertinger S D (2015) Rat Pig-a mutation assay responds to the genotoxic carcinogen ethyl carbamate but not the non-genotoxic carcinogen methyl carbamate. Mutagenesis. In press.
Bernier J, Girard D, Krzystyniak K, Chevalier G, Trottier B, Nadeau D, Rola-Pleszczynski M, Fournier M (1995) Immunotoxicity of aminocarb. III. Exposure route-dependent immunomodulation by aminocarb in mice. Toxicology 99(1–2):135–146
Bierkens J, Maes J, Plaetse FV (1998) Dose-dependent induction of heat shock protein 70 synthesis in Raphidocelis subcapitata following exposure to different classes of environmental pollutants. Environ Pollut 101(1):91–97
Bigot-Lasserre D, Chuzel F, Debruyne ELM, Bars R, Carmichael NG (2003) Tumorigenic potential of carbaryl in the heterozygous p53 knockout mouse model. Food Chem Toxicol 41(2003):99–106
Blakley B, Brousseau P, Fournier M, Voccia I (1999) Immunotoxicity of pesticides: a review. Toxicol Indust Health 15:119–132
Buske-Kirschbaum A (2009) Cortisol responses to stress in allergic children: interaction with the immune response. Neuroimmunomodulation 16:325–32
Capcarova M, Petrovova E, Flesarova S, Dankova M, Massanyi P, Danko J (2010) Bendiocarbamate induced alterations in selected parameters of rabbit homeostasis after experimental peroral administration. Pest Biochem Physiol 98:213–218
Caroleo MC, Rispoli V, Arbitrio M et al (1996) Chronic administration of paraquat produces immunosuppression of T lymphocytes and astrocytosis in rats. Toxic Subst Mech 15:183–94
Cereser C, Boget S, Parvaz P, Revol A (2001) Thiram-induced cytotoxicity is accompanied by a rapid and drastic oxidation of reduced glutathione with consecutive lipid peroxidation and cell death. Toxicology 63:153–62
Cha SW, Gu HK, Lee KP, Lee MH, Han SS, Jeong TC (2000) Immunotoxicity of ethyl carbamate in female BALB/c mice: role of esterase and cytochrome P450. Toxicol Lett 115(3):173–81
Chambers J, Oppenheimer SF (2004) Organophosphates, serine esterase inhibition, and modeling of organophosphate toxicity. Toxicol Sci 77:185–187
Cheng M, Conner MK (1982) Comparison of sister chromatid exchange induction and known carcinogenic activities of vinyl and allyl carbamates. Cancer Res 42(6):2165–7
Corsini E, Sokooti M, Galli CL, Moretto A, Colosio C (2013) Pesticide induced immunotoxicity in humans: a comprehensive review of the existing evidence. Toxicology 307:123–35
Dietert RR (2008) Developmental immunotoxicity (DIT) in drug safety testing: matching DIT testing to adverse outcomes and childhood disease risk. Curr Drug Saf 3:216–226
Dong J, Lu X, Wei Y, Luo L, Dunaway-Mariano D, Carey PR (2003) The strength of dehalogenase-substrate hydrogen bonding correlates with the rate of Meisenheimer intermediate formation. Biochemistry 42(31):9482–90
Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immunoediting. Annu Rev Immunol 22:329–60
El-Bini Dhouib I, Lasram MM, Abdeladhim M, Gharbi N, Ben Ahmed M, El-Fazaa S (2014) Immunosuppression and oxidative stress induced by subchronic exposure to carbosulfan in rat spleen; Immunomodulatory and antioxidant role of N-acetylcysteine. Toxicol Mech Methods 24(6):417–27
El-Bini Dhouib I, Annabi A, Jrad A, El-Golli N, Gharbi N, Lasram MM, El-Fazaa S (2015) Carbosulfan-induced oxidative damage following subchronic exposure and the protective effects of N-acetylcysteine in rats. Gen Physiol Biophys 34(3):249–6
Femando RC, Nair J, Barbin A, Miller JA, Bartsch H (1996) Detection of 1 rA/6 -ethenodeoxyadenosine and S^-elhenodeoxycytidine by immunoaffinity/32P-postlabelling in liver and lung DNA of mice treated with ethyl carbamate (urethane) or its metabolites. Carcinogenesis 17:1711–1718
Fiore MC, Anderson HA, Hong R, Gilubjatnokov R, Seiser JE, Nordstrom D, Hanrahan L, Belluk D (1986) Chronic exposure to aldicarb-contaminated groundwater and human immune function. Environ Res 41:633–645
Freeman ML, Meredith MJ (1989) Measurement of Protein Thiols after Heat Shock Using 3-(N-Maleimido-Propionyl) Biocytin Labeled Proteins Separated by SDS-PAGE and Electroluted onto Nitrocellulose: Thiol Blotting. Radiat Res 117(2):326–333
Fukuto TR (1990) Mechanism of Action of Organophosphorus and Carbamate Insecticides. Environ Health Perspect 87:245–254
Fukuyama T, Tajima Y, Ueda H, Hayashi K, Shutoh Y, Harada T, Kosaka T (2009) Allergic reaction induced by dermal and/or respiratory exposure to low-dose phenoxyacetic acid, organophosphorus, and carbamate pesticides. Toxicology 261(3):152–161
Gao H, Wang D, Zhang S, Xu M, Yang W, Yan P, Liu Y, Luo X, Wu H, Yao P, Yan H, Liu L (2015) Roles of ROS mediated oxidative stress and DNA damage in 3-methyl- 2-quinoxalin benzenevinylketo-1, 4-dioxide-induced immunotoxicity of SpragueeDawley rats. Regulatory Toxicology and Pharmacology. Article in press.
Gholam S, Jorsaraei A, Maliji G, Azadmehr A, Akbar A, Moghamddamnia A, Faraji AA (2014) Immunotoxicity effects of Carbaryl In Vivo and In Vitro. Environ Toxicol Pharmacol 38(3):838–844
Giri S, Giri A, Sharma GD, Prasad SB (2002) Mutagenic effects of carbosulfan, a carbamate pesticide. Mutat Res 519:75–82
Guo TL, Chi RP, Zhang XL (2006) Modulation of immune response following dietary genistein exposure in F0 and F1 generations of C57BL/6 mice: evidence of thymic regulation. Food Chem Toxicol 44:316–25
Gupta RC, Milatovic D, Dettbarn WD (2001) Nitric oxide modulates high-energy phosphates in brain regions of rats intoxicated with diisopropylphosphorofluoridate or carbofuran: prevention by N-tert-butyl-alpha-phenylnitrone or vitamin E. Arch Toxicol 75:346–56
Hajoui O, Flipo D, Mansour S, Fournier M, Krzystyniak K (1992) Immunotoxicity of subchronic versus chronic exposure to aldicarb in mice. Int J Immunopharmacol 14(7):1203–11
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–74
Handy RD, Abd-El Samei HA, Bayomy MFF, Mahran AM, Abdeen AM (2002) Chronic diazinon exposure: pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse. Toxicology 172(5):13–34
Harboe M, Quayle AJ (1991) Heat shock proteins: friend and foe? Clin Exp Immunol 86:2–5
Ibrahim AA, Harabawy ASA (2014) Sublethal toxicity of carbofuran on the African catfish Clarias gariepinus: hormonal, enzymatic and antioxidant responses. Ecotoxicol Environ Saf 106:33–39
Jeon SD, Lim JS, Moon CK (2001) Carbofuran suppresses T-cell-mediated immune responses by the suppression of T-cell responsiveness, the differential inhibition of cytokine production, and NO production in macrophages. Toxicol Lett 119:143–155
Jeong TC, Kim HJ, Cha SW, Park JI, Shin HC, Kim DH, Han SS, Roh JK (1996) Effects of ethyl carbamate and its metabolites on the antibody response in splenocyte cultures from female BALB/c mice. Immunopharmacol Immunotoxicol 18:91–103
Jones K, Everard M, Harding A-H (2014) Investigation of gastrointestinal effects of organophosphate and carbamate pesticide residues on young children. Int J Hyg Environ Health 217(2–3):392–398
Jorsaraei SGA, Maliji G, Azadmehr A, Moghadamnia AA, Faraji AA (2014) Immunotoxicity effects of carbaryl in vivo and in vitro. Environ Toxicol Pharmacol 38(3):838–844
Klaunig JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 44:239–67
Lasram MM, Bini Dhouib I, Bouzid K, Jrad Lamine A, Annabi A, Belhadjhmida N, Ben Ahmed M, El Fazaa S, Abdelmoula J, Gharbi N (2014) Association of inflammatory response and oxidative injury in the pathogenesis of liver steatosis and insulin resistance following subchronic exposure to malathion in rats. ETAP 38:542–53
Lawson T, Pound A (1978) Phosphate ester formation by alkyl carbamate in vivo. Proc Am Assoc Cancer Res 19:184
Li Q (2007) New mechanism of organophosphorus pesticideinduced immunotoxicity. J Nippon Med Sch 2:92–105
Li Q, Kobayashi M, Kawada T (2015) Carbamate pesticide-induced apoptosis in human T lymphocytes. Int J Environ Res Public Health 12(4):3633–45
Li Q, Nagahara N, Takahashi H, Takeda K, Okumura K, Minami M (2002) Organophosphorus pesticides markedly inhibit the activities of natural killer, cytotoxic T lymphocyte and lymphokine-activated killer: a proposed inhibiting mechanism via granzyme inhibition. Toxicology 172:181–190
Lind MH, Rozell B, Wallin RP, van Hogerlinden M, Ljunggren HG, Toftgård R (2004) Tumor necrosis factor receptor 1-mediated signaling is required for skin cancer development induced by NF-kappa B inhibition. Proc Natl Acad Sci 101:4972–7
Lotti M, Moretto A (1999) Promotion of organophosphateinduced delayed polyneurophathy by certain esterase inhibitors. Chem Biol Interact 120:519–524
Lotti M (1992) The pathogenesis of organophosphate polyneuropathy. Crit Rev Toxicol 21:465–487
Luebke RW, Copeland CB, Daniels M, Lambert AL, Gilmour MI (2001) Suppression of Allergic Immune Responses to House Dust Mite (HDM) in Rats Exposed to 2,3,7,8-TCDD. Toxicol Sci 62(1):71–79
Mahajan R, Blair A, Coble J, Lynch CF, Hoppin JA, Sandler DP, Alavanja MC (2007a) Carbaryl exposure and incident cancer in the Agricultural Health Study. Int J Cancer 121(8):1799–1805
Mahajan R, Blair A, Coble J, Lynch CF, Hoppin JA, Sandler DP, Alavanja MCR (2007b) Carbaryl exposure and incident cancer in the Agricultural Health Study, Int. J. Cancer 121:1799–1805
Mayne GJ, Martin PA, Bishop CA, Boermans HJ (2004) Stress and immune responses of nestling tree swallows (Tachycinet bicolor) and eastern bluebirds (Sialia sialis) exposed to nonpersistent pesticides and p, p′-dichlorodiphenyldichloroethylene in apple orchards of southern ontario, Canada. Environ Toxicol Chem 23(12):2930–2940
Medina-De la Garza CE, Guerrero-Ramírez G, García-Hernández M, Castro-Corona MA, Torres-López E, Brattig NW, Salinas-Carmona MC (2012) Immunomodulatory activity of diethylcarbamazine on humoral, cellular cytokine response and respiratory burst in BALB/c mice. Immunopharmacol Immunotoxicol 34(3):477–83
Meeker JD (2010) Exposure to environmental endocrine disrupting compounds and men’s health. Maturitas 66:236–241
Mokarizadeh A, Faryabi MR, Rezvanfar MA, Abdollahi M (2015) A comprehensive review of pesticides and the immune dysregulation: mechanisms, evidence and consequences. Toxicol Mech Methods 25(4):258–78
Monteiro M, Quintaneiro C, Pastorinho M, Pereira ML, Morgado F, Guilhermino L, Soares AMVM (2006) Acute effects of 3,4-dichloroaniline on biomarkers and spleen histology of the common goby Pomatoschistus microps. Chemosphere 62:1333–1339
Mostafalou S, Abdollahi M (2013) Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol 268:157–77
Narita S, Goldblum RM, Watson CS (2007) Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators. Environ Health Perspect 115:48–52
Nwani CD, Lakra WS, Nagpure NS, Kumar R, Kushwaha B, Srivastava SK (2010) Mutagenic and genotoxic effects of carbosulfan in freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Food Chem Toxicol 48(1):202–8
Pfeiffer CJ, Qiu B, Cho CH (1997) Electron microscopic perspectives of gill pathology induced by 1-naphthyl-Nmethylcarbamate in the goldfish (Carassius auratus Linnaeus). Histol Histopathol 12(3):645–53
Pou S, Pou WS, Bredt DS et al (1992) Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem 267:24173–6
Prado-Ochoa MG, Abrego-Reyes VH, Velázquez-Sánchez AM, Muñoz-Guzmán MA, Ramírez-Noguera P, Angeles E, Alba-Hurtado F (2014a) Subchronic toxicity study in rats of two new ethyl-carbamates with ixodicidal activity. Biomed Res Int 2014:467105
Prado-Ochoa MG, Gutiérrez-Amezquita RA, Abrego-Reyes VH, Velázquez-Sánchez AM, Muñoz-Guzmán MA, Ramírez-Noguera P, Angeles E, Alba-Hurtado F (2014b) Assessment of acute oral and dermal toxicity of 2 ethyl-carbamates with activity against Rhipicephalus microplus in rats. Biomed Res Int 2014:956456
Ranjbar A, Pasalar P, Abdollahi M (2002) Induction of oxidative stress and acetylcholinesterase inhibition in organophosphorous pesti-cide manufacturing workers. Hum Exp Toxicol 21:179–182
Rencüzogullari E, Topaktas M (1996) The effects of Marshal and its effective ingredient carbosulfan on SCE, MI and RI in cultured human lymphocytes. Turk J Biol 20:1–12
Rencüzogullari E, Topaktas M (1998) Sister chromatid exchange in cultured human lymphocytes treated with carbosulfan, ethyl carbamate, ethyl methanosulfonate separately and in mixtures. Turk J Biol 22:369–387
Rodgers KE, Leung N, Imamura T, Devens BH (1986) Rapid in Vitro Screening Assay for lmmunotoxic Effects of Organophosphorus and Carbamate Insecticides on the Generation Cytotoxic T-Lymphocyte Responses’ Pesticide biochemistry and physiology 26: 292–301 Pfeiffer CJ, Qiu B, Cho CH (1997) Electron microscopic perspectives of gill pathology induced by 1-naphthyl-N-methylcarbamate in the goldfish (Carassius auratus Linnaeus). Histol Histopathol 12(3):645–53
Seth V, Banerjee BD, Chakraborty AK, Institoris L, Desi I (2002) Effect of propoxur on humoral and cell-mediated immune responses in albino rats. Bull Environ Contam Toxicol 68(3):369–76
Sharova LV, Gogal RM, Sharov AA, Chrisman MV, Holladay SD (2002) Immune stimulation in urethane-exposed pregnant mice increases expression level of spleen leukocyte genes for TGFβ3 GM-CSF and other cytokines that may play a role in reduced chemical-induced birth defects. Int Immunopharmacol 2(10):1477–1489
Singh SK, Bano F, Mohanty B (2015) Vitamin E pretreatment prevents the immunotoxicity of dithiocarbamate pesticide mancozeb in vitro: a comparative age-related assessment in mice and chick. Pestic Biochem Physiol. In press
Singhal LK, Bagga S, Kumar R, Chauhan RS (2003) Down regulation of humoral immunity in chickens due to carbendazim. Toxicol In Vitro 17(5–6):687–92
Soloneski S, Kujawski M, Scuto A, Larramendy ML (2015) Carbamates: a study on genotoxic, cytotoxic, and apoptotic effects 4 induced in Chinese hamster ovary (CHO-K1) cells. Toxicol In Vitro. In press
Song X, Tian H, Bressler J, Pruett S, Pope C (2002) Acute and Repeated Restraint Stress Have Little Effect on Pyridostigmine Toxicity or Brain Regional Cholinesterase Inhibition in Rats. Toxicol Sci 69(1):157–164
Tanriverdi F, Silveira LF, MacColl GS, Bouloux PM (2003) The hypothalamic-pituitary-gonadal axis: immune function and autoimmunity. J Endocrinol 176:293–304
Theus SA, Tabor DR, Gand JY, Barnett JB (1993) Alteration of Macrophage Cytotoxicity through Endogenous Interferon and Tumor Necrosis Factor α Induction by Propanil. Toxicol Appl 118:46–52
Vial T, Nicolasa B, Descotesa J (1996) Clinical immunotoxicity of pesticides. J Toxicol Environ Health 48:215–229
Vidyasagar J, Karunakar N, Reddy MS, Rajnarayana K, Surender T, Krishna DR (2004) Oxidative stress and antioxidant status in acute organophosphorous insecticide poisoning. Indian J Pharmacol 36:76–79
Wang Z, Wilhelmsson C, Hyrsl P, Loof TG, Dobes P, Klupp M, Loseva O, Mörgelin M, Iklé J, Cripps RM, Herwald H, Theopold U (2010) Pathogen entrapment by transglutaminase — a conserved early innate immune mechanism. PLoS Pathogens 6(2):e1000763
Whalen MM, Loganathan BG, Yamashita N, Saito T (2003) Immunomodulation of human natural killer cell cytotoxic function by triazine and carbamate pesticides. Chem Biol Interact 145:311–319
Wiedenmann D, Stehrerer-Schmide P, Wolf HU (1990) Mutagenic effects of carbosulfan and furathiocarb in the Ames test and Yeast assay, in: Proceedings of the 31st Spring Meeting of the Deutsche Gesellschaft für Pharmakologische Toxicologie (German Society for Pharmacology and Toxicology), Mainz, West Germany, 13–16 March, Naunyn Schmiedeberg’s Arch. Pharmacol. Suppl. 341 R 29
Williams CV, Fletcher K, Tinwell H, Ashby J (1998) Mutagenicity of ethyl carbamate to lacZ- transgenic mice. Mutagenesis 13:133–137
Yang ZP, Dettbarn WD (1996) Diisopropylphosphorofluoridate induced cholinergic hyperactivity and lipid peroxidation. Toxicol Appl Pharmacol 138:48–53
Yoon JY, Oh SH, Yoo SM, Lee SJ, Lee HS, Choi SJ, Moon CK, Lee BH (2001) N-Nitrosocarbofuran, but not Carbofuran, induces apoptosis and cell cycle arrest in CHL cell. Toxicology 169(2):153–61
Zhao M, Chen F, Wang C, Zhang Q, Gan J, Liu W (2010) Integrative assessment of enantio selectivity in endocrine disruption and immunotoxicity of synthetic pyrethroids. Environ Pollut 158:1968–1973
Zhou C, Tabb MM, Nelson EL (2006) Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest 116:2280
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Dhouib, I., Jallouli, M., Annabi, A. et al. From immunotoxicity to carcinogenicity: the effects of carbamate pesticides on the immune system. Environ Sci Pollut Res 23, 9448–9458 (2016). https://doi.org/10.1007/s11356-016-6418-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6418-6