Abstract
Atmospheric nitrogen (N) deposition plays an important role in the global N cycle. Data for dry and wet N deposition in agricultural ecosystem of Shaanxi in China is still imperfect; in this study, we continuously measured concentrations and fluxes of dry N deposition from 2010 to 2013 in Yangling district of Shaanxi province and wet N deposition from 2010 to 2012. The average annual concentrations of NH3, NO2, HNO3, particulate ammonium, and nitrate (pNH4 + and pNO3 −) varied among 3.9–9.1, 6.6–8.0, 1.2–1.4, 3.1–4.3, and 3.3–4.8 μg N m−3, respectively, with mean values of 6.0, 7.2, 1.3, 3.8, and 4.1 μg N m−3, respectively, during the entire monitoring period. The annual NH4 +-N and NO3 −-N concentrations in precipitation ranged 3.9-4.3 and 2.8-3.4 mg N L−1 with the mean values of 4.1 and 3.3 mg N L−1. The NH4 +-N/NO3 −-N ratio in rainfall averaged 1.2. Dry N deposition flux was determined to be 19.2 kg N ha−1 year−1 and the wet N deposition flux was 27.2 kg N ha−1 year−1. The amount of total atmospheric N deposition (dry plus wet) reached 46.4 kg N ha−1 year−1, in which dry deposition accounted 41 %. Gaseous N deposition comprised over 75 % of the dry deposition, and the proportion of oxidized N in dry deposition was equal to the reduced N. Therefore, the results suggest that more stringent regional air pollution control policies are required in the target area and that N deposition is an important nutrient resource from the atmosphere that must be taken into consideration in nutrient management planning of agricultural ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Emissions of reactive nitrogen (Nr) have increased gradually since the start of the industrial revolution due to increasing population size and economic development (Galloway et al. 2008). The concomitant changes in the N cycle have led to large increases in atmospheric N deposition and have then affected the productivity and stability of terrestrial and aquatic ecosystems (Galloway et al. 2004; Dentener et al. 2006; Liu et al. 2011). Although atmospheric N deposition is an important nutrient source that can provide at least some of the N that is necessary for plant growth (Wang et al. 2008a), excessive N deposition will also lead to a series of environmental problems such as soil acidification (Fang et al. 2011), eutrophication (Erisman et al. 1994; Bouwman et al. 2002), mineralization of organic matter (Xu et al. 2004; Scheuner and Makeschin 2005), loss of biodiversity (Stevens et al. 2004; Phoenix et al. 2006; Clark and Tilman 2008; Song et al. 2012), and disruption of the climate system of the Earth (Erisman et al. 2011). Moreover, some previous reports indicated that human likely passed the global tipping point for anthropogenic N-fixation and that we are entering an era of unknown knock-on effects due to this increased mobilization of reactive nitrogen (Rockström et al. 2009).
The modeling studies by Dentener et al. (2006) and Galloway et al. (2008) indicate that China has the third largest rate of regional N deposition after North America and Europe. Liu et al. (2013) recently reported that the average annual bulk N deposition increased by approximately 8 kg N ha−1 between the 1980s and the 2000s, based on meta-analysis of historical published studies. Nitrogen deposition monitoring studies also recently showed very high N deposition rates on the North China Plain (54–117 kg N ha−1 year−1) (Zhang et al. 2008b; Shen et al. 2009; Pan et al. 2012; Luo et al. 2013), in central and western China (5–46 kg N ha−1 year−1) (Larssen et al. 2011; Li et al. 2013), in southeast China (63–105 kg N ha−1) (Wang et al. 2008a; Cui et al. 2010, 2014), and in the coastal region of the Yellow Sea (2–21 kg N ha−1 year−1) (Zhang et al 2007; Zhang et al. 2010; Qi et al. 2013). However, the majority of these studies have focused on the quantification of atmospheric N deposition in natural ecosystems (e.g., forests and aquatic ecosystems) worldwide (Fang et al. 2005, 2008; Wang et al. 2008b; Guo et al. 2010; Tian et al. 2013; Dong et al. 2014; Du et al. 2014), but data for agricultural ecosystems are poorly represented, especially dry deposition (Cui et al. 2010; Liu et al. 2011).
Shaanxi province is located at the junction between an eastern humid area and a western drought area occupying 4.8 million ha of arable land. The excessive use of N fertilizers is common in this area particularly over the last few decades in a similar agricultural practice on the North China Plain. Li and Li (1999) showed that the amount of wet N deposition was about 6.3–26.6 kg N ha−1 year−1 on the Guanzhong Plain in Shaanxi from 1990 to 1994. An N input to agricultural land via precipitation and dust deposition was 12.7–20.6 kg N ha−1 year−1 in the loess area of Shaanxi in 2006 (Wang et al. 2008c) and an atmospheric N deposition flux was 17.0–22.2 kg N ha−1 year−1 in typical agricultural areas of northern Shaanxi (Wei et al. 2010a). Unfortunately, many studies conducted in Shaanxi were done mainly on atmospheric wet deposition (Li et al. 1993; Wei et al. 2010b; Liang et al. 2014a, 2014b). Atmospheric dry deposition from gases and aerosols may contribute up to or more than half of the total N deposition (Flechard et al. 2011; Pan et al. 2012; Luo et al. 2013) and was ignored in this previous work. Therefore, a long-term in situ observation experiment was conducted at Yangling, Shaanxi, in order to generate more information about atmospheric dry deposition. The objectives of this study were (1) to quantify the atmospheric Nr concentrations and deposition fluxes and (2) to provide essential information for nutrient balance calculations in the local agricultural systems.
Materials and methods
Sampling sites
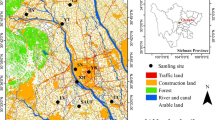
Sampling was carried out from April 2010 to December 2013 for dry deposition and Janurary 2010 to December 2012 for wet deposition at Wuquan Agricultural Experimental Base (34° 17′ N, 108° 0′ E, 520 m a.s.l.) in Yangling, Shaanxi province (Fig. 1). Yangling was located in the Guanzhong Plain of Shaanxi province (80 km west of Xi’an and 90 km east of Baoji) with a population of 200,000. Arable land accounts for 47 % of the total land area (135 km2) in Yangling, and maize, wheat, and kiwifruit are the main crops. The annual average temperature is 12.9 °C, and the annual mean precipitation is 632 mm, with most rainfall events received from July to September. The monitoring site is about 10 km northwest of the downtown area, surrounded by agricultural land and small villages. The local cropping system is a winter wheat–summer maize rotation. Winter wheat is usually sown in October, and summer maize is sown in June of the following year. The study site may be polluted by emissions from motor vehicles, agricultural fields, and burning of coal and straw for domestic heating in winter. This area experiences four seasons: spring (March to May), summer (June to August), autumn (September to November), and winter (December to February).
Collection and analysis of dry deposition
NH3, HNO3, pNH4 +, and pNO3 −
Atmospheric NH3, HNO3, particulate NH4 +, and NO3 − (pNH4 + and pNO3 −) were collected using (DEnuder for Long-Term Atmospheric sampling (DELTA) system, which is an active sampling equipment designed by the Centre for Ecology and Hydrology, Edinburgh, UK. The DELTA system has been used widely in the European N deposition monitoring network (Flechard et al. 2011). The sampling height was 2 m above the ground, and the sampling time was usually 1 month (28–31 days). Detailed information on the DELTA system has been described in Xu et al. (2015) and Shen et al. (2013). The sampling train consists of two potassium carbonate plus glycerol (1 % m/v K2CO3 + 1 % m/v glycerol in methanol) coated denuders in series for the simultaneous collection of HNO3, followed by two citric acid (5 % m/v citric acid in methanol) coated denuders for NH3 and finally by a filter-pack assembly with a first K2CO3/glycerol impregnated filter to capture particle phase anions (NO3 −, SO4 2−, Cl−) and cations (NH4 +, Na+, Mg2+, Ca2+), and a second filter coated with citric acid to collect any volatilized particulate NH4 +. The empirically determined effective size cutoff for particle sampling is 4.5 μm. The air was drawn through the sampling train at a rate of 0.2–0.4 L min−1 and directly into the first denuder with no inlet line to avoid sampling losses. The total sampled air volume of the DELTA system was recorded by the gas meter which was checked every month for data reading, performance, and maintenance. After sampling, the samples were immediately stored under refrigeration at 4 °C and analyzed within 1 month of collection. The HNO3 denuders and alkaline-coated filters capturing pNO3 − were extracted with 10 ml of 0.05 % H2O2 solution in aqueous solution. The NH3 denuders and acid-coated filters capturing pNH4 + were extracted with 10 ml high purity water. The extracted solutions were measured using an AA3 continuous-flow analyzer (Bran + Luebbe GmbH, Norderstedt, Germany). The detection limits were 0.01 mg N L−1 for NH4 + and NO3 −. It should be noted that NO3 − was converted to NO2 − during the chemical analysis. Therefore, NO2 − here was included in the analysis and NO3 − is equal to the sum of NO2 − and NO3 −.
NO2
Atmospheric NO2 was collected using Gradko diffusion tubes (Gradko International Limited, UK). They are passive samplers, and more information was described in detail in Luo et al. (2013) and Xu et al. (2015). Each sampler consisted of one acrylic tube (71.0 mm long × 11.0 mm internal diameter) with colored and white thermoplastic rubber caps and stainless steel mesh disks. Before sampling, two disks were placed within the colored cap, and then 30 μl of a 20 % m/v triethanolamine aqueous solution was coated onto the disks to absorb NO2. At each sampling point, three samplers were suspended 2 m above the ground and exposed to the air for 1 month. A prestudy of NO2 capture showed large capacity for the passive samplers to still absorb NO2 after 1-month sampling, suggesting that monthly sampling frequency for NO2 is reasonable. The samplers were stored at 4 °C until analysis within 1 month. The exposed disks were extracted with a solution containing sulfanilamide, H3PO4, and N−1-naphthylethylene-diamine, and the NO2 − content in the extract was determined using a colorimetric method by absorbance at a wavelength of 542 nm. The detection limit for NO2 − was 0.01 mg N L−1.
Collection of wet deposition
An automatic precipitation and dust sampler (APS-2A, Wuhan Tianhong Inc., China) controlled by sensors was installed beside the DELTA systems. The system collected rainwater samples only while the rainfall was occurring based on detection by rain sensors. After each precipitation event, the rainwater samples were thoroughly mixed and immediately stored in clean polyethylene bottles (50 ml), and then, the rainwater-collecting bottle was rinsed with deionized water to eliminate cross contamination. All samples were filtered with a 0.45 mm syringe filter (Tengda Inc., Tianjin, China), then filtrates were frozen in a refrigerator at −17 °C until an analysis of inorganic N (NH4 +-N and NO3 −-N) by a continuous flow analyzer within 2 months.
Estimation of deposition flux
Dry deposition fluxes of NH3, NO2, HNO3, pNH4 +, and pNO3 − were estimated using the inferential method which combines the measured concentration and a modeled dry deposition velocity (Vd) (Schwede et al. 2011; Pan et al. 2012; Luo et al. 2013). The concentrations of Nr species were measured as described in “Collection and analysis of dry deposition”. The monthly average Vd was calculated by the Goddard Earth Observing System (GEOS)-Chem chemical transport model (CTM) (Bey et al. 2001); more information is described in Xu et al. (2015). The model calculation of dry deposition of Nr species follows a standard big-leaf resistance-in-series model (Wesely 1989; Zhang et al. 2001). Vd is calculated as the function Vd = (Ra + Rb + Rc)−1 determined by local meteorological conditions and surface type as described in Zhang et al. (2012a) and Luo et al. (2013). Here, Ra is the aerodynamic resistance to turbulent transfer from the lowest model layer (70 m above the surface) to the roughness height. Rb is the boundary layer resistance to molecular diffusion, and Rc is the canopy or surface uptake resistance. In this study, Vd was obtained from the model calculation of dry deposition velocities for the whole of 2012 and archived hourly values for both gases and aerosols over the model domain (Xu et al. 2015). Monthly Vd was averaged based on the hourly dataset for further estimation of dry deposition flux of each Nr species during the observations.
Wet deposition fluxes of NH4 +-N and NO3 −-N were calculated based on the N concentrations and the amount of precipitation for each event, using the following equations (Liu et al. 2006; Li et al. 2013):
where F i is the N deposition flux per event (g N ha−1), C i is the concentration of NH4 +-N or NO3 −-N in precipitation (mg N L−1), and P i is the rainfall amount (mm).
where F m is the N deposition flux per month or year (kg N ha−1).
Statistical analysis
One-way analysis of variance was performed using the SAS version 8.1 software package to examine differences in the variables investigated among the four years. Statistically significant differences were set at probability values (P) <0.05.
Results
Atmospheric concentrations and annual variations of Nr species
Monthly and annual mean concentrations of atmospheric Nr species are shown in Fig. 2. The concentration of NH3, NO2, HNO3, pNH4 +, and pNO3 − during the 4-year study were in the range of 1.9–17.8, 4.3–11.7, 0.4–2.7, 0.6–9.7, and 1.2–11.8 μg N m−3, respectively. The concentrations of Nr species varied greatly throughout the monitoring period. The annual NH3 concentration varied from 3.9 μg N m−3 in 2011 to 9.10 in 2013 μg N m−3. There was a significant difference (P < 0.05) in annual NH3 concentration between 2013 and the other three years. Annual NO2 concentrations showed a decreasing trend from 2010 to 2012 (with 8.0, 7.4, and 6.6 μg N m−3, respectively), while the concentration of NO2 in 2013 (6.8 μg N m−3) increased slightly. However, there was no notable difference (P < 0.05) among the four years. Compared with other Nr species, annual HNO3 concentrations were relatively low, ranging from 1.2 μg N m−3 in 2010 to 1.4 μg N m−3 in 2011. The annual concentrations of pNH4 + and pNO3 − were in the ranges of 3.1–4.3 and 3.0–4.8 μg N m−3. Annual pNH4 + concentration showed an increasing trend in 2012 (4.29 μg N m−3), but it was not significantly different from other three years. Annual pNO3 − concentration showed a similar variation as pNH4 +. Overall, the average concentrations of NH3, NO2, HNO3, pNH4 +, and pNO3 − from 2010 to 2013 were 6.0, 7.2, 1.3, 3.8, and 4.1 μg N m−3, respectively.
Seasonal variation in atmospheric Nr concentrations
Seasonal variations in atmospheric Nr concentrations over a 4-year cycle (from 2010 to 2013) are shown in Fig. 3. There was a significant difference in seasonal mean NH3 concentration of each year. Seasonal NH3 concentration was higher in the summer of 2013 and the autumn of 2012. The seasonal NH3 concentration varied from 5.1 to 6.6 μg N m−3 in this study by the order of autumn > summer > spring > winter. Seasonal mean NO2 concentrations in this study were always higher in autumn and winter than in spring and summer, and their average values were 8.4 (autumn), 8.1 (winter), 6.3 (spring), and 5.9 (summer) μg N m−3. Similarly, the overall average values of seasonal mean HNO3 concentrations in winter and autumn were higher than in summer and spring from 2010 to 2013. The mean pNO3 − concentration was 6.1 μg N m−3 in winter followed by autumn, and it was generally consistent with the trends in NO2 and HNO3 concentrations. The pNH4 + concentrations did not show the same trend as other atmospheric Nr species. The highest value of seasonal pNH4 + concentration was 5.3 μg N m−3 in summer.
NH4 +-N and NO3 −-N concentrations in precipitation
The annual concentrations of NH4 +-N and NO3 −-N in precipitation (2010–2012) are shown in Fig. 4. The precipitation reached 503 to 902 mm in the three monitoring years. The annual concentrations of NH4 +-N and NO3 −-N were 3.9-4.3 and 2.8-3.4 mg N L−1, with average values of 4.1 and 3.3 mg N L−1, respectively. The mean value of NH4 +-N/NO3 −-N ratio was 1.2 during the monitoring period.
Dry and wet deposition of inorganic N
The ranges of the deposition velocities of NH3, NO2, HNO3, pNH4 +, and pNO3 − were 0.29-0.51, 0.04-0.34, 0.28-1.63, 0.17-0.32 and 0.17-0.32 cm s−1 (Fig. 5). Dry deposition fluxes of NH3, NO2, HNO3, pNH4 +, and pNO3 − were calculated as the product of the measured Nr concentrations and their deposition velocities. The deposition fluxes of from 2010 to 2013 were varying among 4.0–11.5, 3.4–4.0, 2.5–3.4, 1.9–2.7, and 1.5–2.7 kg N ha−1 year−1 for NH3, NO2, HNO3, pNH4 +, and pNO3 −, respectively. The annual dry deposition flux at the sampling site thus reached 19.2 kg N ha−1 year−1 (Table 1).
The average precipitation of the three years (2010–2012) was 668 mm (Fig. 4). The total inorganic nitrogen (TIN) deposition in precipitation ranged from 16.3 to 35.2 kg N ha−1 year−1, with the average value of 27.2 kg N ha−1 year−1. The average value of NH4 +-N deposition fluxes was 16.4 kg N ha-1 yr-1, contributing 1.5 times that of NO3 --N. The NH4 +-N was the dominant form of N deposition in precipitation and accounted for 60 % of wet deposition.
Discussion
Nr concentrations variation in the air and precipitation
The comparison of atmospheric Nr concentrations in different regions of China is shown in Table 2. It is noteworthy that the NH3 and pNH4 + concentration in Quzhou, Hebei province (a rural area) were almost three times higher than the values in the present study, and the NO2 and pNO3 - concentrations were slightly higher. The present study showed that the monthly mean NH3 concentrations have large variations over the whole monitoring period, and it is important to note the increasing trend (Fig. 2). This is most likely due to high NH3 emissions from fertilizer N applications. Statistics showed that the amount of N fertilizer application (pure N) in Shaanxi increased from 48.6 × 104 t in 1990 to 98.7 × 104 t in 2013, especially an 8 % increase in 2012; this trend was consistent with our results (Statistical Yearbook of Shaanxi, 2010–2014). Fertilizer N application rates up to 480–520 kg N ha−1 year−1 are applied in Yangling local area (Zhang et al. 2011) to achieve high yield of maize and wheat, and is applied mainly in the form of urea, ammonium bicarbonate, and diammonium phosphate. However, less than 20–30 % of the applied fertilizer N will be taken up by crops, and a large amount of the fertilizer N (20–30 %) is lost by NH3 emission (Cai et al. 2002; Zhang et al. 2008a; Ju et al. 2009); the remaining fertilizer N is residued in the soil (about 30 %) and lost by soil leaching and surface runoff (about 10 %) (Zhu 2008). Because the population and livestock husbandry had no obvious growth in recent years, the N fertilizer application is likely the main source of the NH3 emission (Statistical Yearbook of Shaanxi, 2010–2014; Rural Statistical Yearbook of China, 2010–2014). In the present study, the highest NH3 concentration occurred in autumn while the peak was usually found in summer in the north and northwest China (Shen et al. 2009, 2011; Li et al. 2013). This may be due to October being the local time for planting winter wheat and farmers usually applying a large number of chemical or organic fertilizers as basal fertilizers or single basal application of the whole rate to wheat. The NH3 concentration in summer was slightly low perhaps due to the effect of rainfall scavenging. Precipitation occurs mainly from July to September and usually accounts for more than 60 % of the annual rainfall (Liang et al. 2014b). Although the high temperatures in summer favor ammonia volatilization, the concentrated and frequent precipitation events will scavenge more NH3 and other Nr species (e.g., HNO3 and particulate N) from the air (Hong et al. 2002).
The highest NO2 concentration was found in 2010 and then tended to decrease in the following 3 years. These trends are consistent with annually increasing traffic flows, leading to increasing NOx emissions in motor vehicle exhausts and a peak value of atmospheric NOx concentration in 2009 (Xie 2012). The amount of NOx emission shows a decreasing pattern of more than twofold from 2011 to 2013, especially decreasing from 175 t in 2011 to 75 t in 2013 in Yangling area (Statistical Yearbook of Shaanxi, 2010–2014). Such phenomenon is most likely caused by the less NOx emitting in vehicle use, such as through the presence of catalytic converters on exhaust systems, improvements in fuel efficiency, lower NOx emissions per mass of fuel burned, and changing fuel types. The main sources of NO2 usually include the combustion of fossil fuels, vehicle emissions, and biomass burning (Richter et al. 2005; Martins et al. 2012). Moreover, temperature inversion, low wind speeds, and dry weather are not favorable for the transformation and diffusion of atmospheric pollution, leading to NOx accumulation in the air (Li et al. 2013). The higher concentration of NO2 in winter might be explained by increased fossil fuel consumption for heating. As an important source of NOx, the exhaust from fossil fuel combustion reached 206 millions N m−3 in 2013 at Yangling area (Statistical Yearbook of Shaanxi, 2010–2014). The lowest concentration of NO2 in summer is likely due to photochemical reactions and wet deposition (Atkins and Lee 1995; Hong et al. 2002). As previously noted, the higher precipitation in summer can increase the removal of atmospheric pollution.
HNO3 is the main component of photochemical smog and can be easily removed from the air by dry deposition because of its high sorption and water solubility. The annual variation of HNO3 concentrations is not evident in this study. The common assumption is that HNO3 is higher in warm seasons (summer) because it is generated by secondary photochemical reactions (Khoder 2002). However, the seasonal variation in HNO3 concentrations in this study shows higher values in winter and autumn than in summer and spring, consistent with the seasonal variation in NO2. This may be explained by the higher NO2 concentrations in winter and autumn, which provide more precursor gas for HNO3. Research on Beijing agricultural ecosystems indicates that most of the pNH4 + is likely in the form of (NH4)2SO4 (or NH4HSO4) and NH4NO3 (Shen et al. 2011). The transport distance between NH3 emitted from agriculture and NO2 and SO2 emitted from fossil fuel combustion has been shortened by a rapid urbanization. These pollutions have higher concentrations in summer, and winter would react more rapidly to form aerosols (e.g., PM2.5), leading to the high concentrations of pNH4 + and pNO3 − (Shen et al. 2009; Xu et al. 2015). In addition, the conversion of particulate NH4NO3 to gaseous NH3 and HNO3 could be reduced by decreasing temperature and increasing relative humidity in winter (Stelson and Seinfeld 1982). The seasonal variation in pNO3 − was higher in winter and autumn. This may be derived from coal combustion in winter, indicating that increasingly anthropogenic induced aerosols are present in Yangling. For example, Song et al. (2015) demonstrated that the replacement of coal by natural gas as the winter heating fuel in Urumqi city (in northwest China) led to a decrease of more than 70 % in PM2.5 and pNO3 − concentrations in the month of January from 2011 to 2014.
The annual concentrations of NH4 +-N and NO3 −-N in precipitation monitored in Yangling were equal to three to five times the annual concentrations of N in precipitation in the 1990s in China (Wang and Wang 1995), and they were approximately five to eight and three to four times those in Europe, North America, and Japan (Hara et al. 1995; Fahey et al. 1999; Whitall and Paerl 2001). Compared with these, the N concentration in the Yangling area found in the present study was higher and the NH4 +-N/NO3 −-N ratio was lower, and this might be related to economic development and changes in of industrial structure. The precursor NH4 +-N is NH3 in the air, which is mainly produced from nitrogen fertilizer application and livestock and poultry raising, while the precursor of NO3 −-N is NOx in the air, which is mainly produced from industrial activities and vehicle exhaust emissions (Song et al. 2005). Yangling is located in the Weihe valley agricultural ecosystem with the most highly developed agricultural systems in Shaanxi province (Wei et al. 2010b). Hence, the high N concentrations in the deposition are likely mainly derived from the increasing use of fertilizer emitting NH3, which increasing living standards and regulation of industrial waste gases and vehicle emissions have reduced NOx.
Fluxes of dry and wet deposition of inorganic N
High concentrations of atmospheric Nr species in Yangling indicate that dry deposition will be elevated. The mean deposition fluxes of NH3, NO2, HNO3, pNH4 +, and pNO3 − were all lower than the mean values reported on the North China Plain, where there is a high population density, highly intensive agricultural production, and a rapidly developing economy. Liu et al. (2011, 2013) pointed out that Chinese NH3 and NOx emissions increase continuously on a national scale with rapid economic development. However, the differences in N emissions in different regions are due to large differences in population density, economic function, land use type, and weather conditions (Luo et al. 2014). Compared with other regions of China (Table 2), the atmospheric pollution level in Shaanxi is intermediate but it is still necessary to take measures to control air pollution. Particulate N species (pNH4 + and pNO3 −) found in this study contributed approximately 25 % to total dry flux, which was much higher than in north China (10 %) (Pan et al. 2012), implying that local urban development has made a large contribution to particulate matter (PM) pollution, and the conditions of particulate pollution are even more problematic in the city of Yangling. On the other hand, oxidized species (9.3 kg N ha−1 year−1) accounted for 49 % of the total dry deposition flux and reduced species (9.8 kg N ha−1 year−1) were equal to this, implying that the local site is polluted by agriculture (e.g., fertilizers and livestock manures) and urbanization (e.g., transportation and winter heating). This is in contrast to an agricultural ecosystem in the southeast and an agricultural catchment in central China where reduced N is the main contributor to dry N deposition (Cui et al. 2010; Shen et al. 2013). It is clear that human activities significantly influence the environment and control technologies must be taken into consideration at the same time as economic development.
Wet deposition fluxes (27.2 kg N ha−1 year−1) were similar to the annual average wet deposition input (27.0 kg N ha−1 year−1) at Changshu ecological station (Wang et al. 2004) and the annual wet deposition flux (28.1 kg N ha−1 year−1) on the North China Plain (Zhang et al. 2006). Compared with previous studies, the local atmospheric wet deposition has increased and the proportion consisting of NO3 −-N has continued to increase (Li and Li 1999; Wang et al. 2008c; Wei et al. 2010a). In recent years, extensive use of fossil fuels has produced increasing emissions of NOx with industrialization, leading to a continuing increase in the magnitude and effects of atmospheric wet deposition.
Overall total atmospheric N deposition (dry and wet) fluxes were about 46.4 kg N ha−1 year−1 in this study, of which the percentage of Nr dry deposition was up to 41 %. In order to evaluate the role of dry deposition, several researchers have demonstrated that it can account for 30–80 % of the total pollutant deposition (Gromov et al. 2001). A recent report at the national scale showed that dry N deposition rates contribute almost half of total inorganic N deposition (Xu et al. 2015). Consistent with these conclusions, our findings indicate that Nr dry deposition plays an important role in atmospheric N deposition and it should be taken into consideration for N deposition quantification. Such high N deposition amounts seem to be an ignored N nutrient source, which is equivalent to 98.1 kg ha−1 of applied fertilizer urea and accounts for 18–21 % of the conventional fertilizer N input (Zhang et al. 2011). Additionally, the results of another investigation indicated that the rate of excessive N fertilizer application of wheat and maize in Guanzhong Plain reached 56 and 72 %, respectively (Wang 2014). Excessive fertilization not only causes fertilizer waste and environmental pollution but also leads to increasing N deposition flux by increasing the Nr concentration in the atmosphere. Therefore, atmospheric N deposition should be controlled by reducing the application rates of synthetic fertilizers or by using knowledge-based optimum fertilization strategies with the aim of avoiding excessive application of fertilizer N and reducing the severity of the damage to ecosystems due to low N use efficiency and higher losses of Nr. At the same time, it is necessary to take strict control measures to reduce air pollution during industrial development and urbanization.
It should be noted that there still remain some uncertainties in the estimation of the N dry and wet deposition fluxes. The annual deposition velocities of N compounds on different land use are summarized in Table 3. The dry deposition of gases and aerosols is generally treated as a flux of particles from the atmosphere caused by a direct air–surface interaction. The intensity of dry deposition depends on certain surface characteristics (roughness, type of vegetation, humidity, etc.), pollutant properties, and atmospheric stability (Ouyang et al. 2003; Zhang et al. 2004). These will strongly affect the deposition velocity and the absorbability of the ground surface to each Nr species (Loubet et al. 2008). In addition, there was uncertainty in the deposition fluxes for pNH4 + and pNO3 − in this study because of the difference between the cutoff sizes of particles in the samplers and those defined in the modeled Vd, which was calculated for atmospheric PM2.5 in GEOS-Chem. The cutoff sizes of the samples can also collect coarse NO3 − particles but should have little effect on NH4 + particles (mainly in the fine scale <1 μm) (Tang et al. 2009; Xu et al. 2015), resulting in an underestimation of pNO3 − deposition from the coarse mode. On the other hand, the total dry deposition flux in this study may be underestimated due to omission of the dry deposited organic N species. Even though it has been reported that the contribution of atmospheric organic N species to total Nr inputs is minor, it has been shown to be an important part of N dry deposition (Flechard et al. 2011; Turnipseed et al. 2006). Moreover, a large number of previous studies in local and abroad report that dissolved organic N accounts for approximately 30 % of the total deposition in rainwater (Cornell 2011; Zheng et al. 2007; Zhang et al. 2012b), but organic N in wet deposition was not considered in the present study. In order to obtain an accurate estimate of total atmospheric N deposition and reduce the uncertainty in this area, the organic N content of wet deposition and dry deposition velocities need to be addressed in future studies.
Conclusions and summary
-
1.
Annual average concentrations of NH3, NO2, HNO3, pNH4 +, and pNO3 − were 6.0, 7.2, 1.3, 3.8, and 4.1 μg N m−3, respectively, during the four years from 2010 to 2013 across Yangling, Shaanxi province, and the total atmospheric concentrations of the various Nr species amounted to 22.4 μg N m−3, reflecting severe air pollution by reactive N species. Higher concentrations of NO2, HNO3, and pNO3 − occurred in winter and autumn with a peak concentration of NH3 in autumn and the pNH4 + concentration was highest in summer, indicating that human activities (especially agricultural activities) had a significant impact on the atmospheric environment.
-
2.
Average annual NH4 +-N and NO3 −-N concentrations in precipitation of the years 2010 to 2012 were 4.1 and 3.3 mg N L−1, respectively, with a NH4 +-N/NO3 −-N ratio of 1.7.
-
3.
Dry deposition fluxes of NH3, NO2, HNO3, pNH4 +, and pNO3 − averaged 7.4, 3.8, 3.2, 2.5, and 2.3 kg N ha−1 year−1, respectively; hence, the total dry deposition flux was 19.2 kg N ha−1 year−1. The amount of wet deposition flux was 27.2 kg N ha−1 year−1, of which the fluxes of NH4 +-N and NO3 −-N were 16.4 and 10.8 kg N ha−1 year−1. Total atmospheric N deposition (dry and wet deposition) reached on average 46.4 kg N ha−1 year−1, of which dry deposition accounted for 41 %. Deposition of gaseous N (14.4 kg N ha−1 year−1) was higher and comprised over 75 % of the dry deposition, while the proportion of oxidized N in dry deposition was equal to the reduced N. Given the high N deposition fluxes, it is necessary to take measures to reduce the emissions of Nr into the environment and the N inputs from the atmosphere must be taken into account in fertilizer N management planning in local agricultural ecosystems.
References
Atkins DHF, Lee DS (1995) Spatial and temporal variation of rural nitrogen dioxide concentrations across the United Kingdom. Atmos Environ 29:223–239
Bey I, Jacob DJ, Yantosca RM, Logan JA, Field BD, Fiore AM, Li QB, Liu HY, Mickley LJ, Schultz MG (2001) Global modeling of tropospheric chemistry with assimilated meteorology: model description and evaluation. J Geophys Res 106:23073–23095
Bouwman AF, Van Vuuren DP, Derwent RG, Posch M (2002) A global analysis of acidification and eutrophication of terrestrial ecosystems. Water Air Soil Pollut 141:349–382
Cai GX, Chen DL, Ding H, Pacholski A, Fan XH, Zhu ZL (2002) Nitrogen losses from fertilizers applied to maize, wheat and rice in the North China Plain. Nutr Cycl Agroecosyst 63:187–195
Clark CM, Tilman D (2008) Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451:712–715
Cornell SE (2011) Atmospheric nitrogen deposition: revisiting the question of the importance of the organic component. Environ Pollut 159:2214–2222
Cui J, Zhou J, Yang H (2010) Atmospheric inorganic nitrogen in dry deposition to a typical red soil agro-ecosystem in southeastern China. J Environ Monit 12:1287–1294
Cui J, Zhou J, Peng Y, He YQ, Yang H, Mao JD (2014) Atmospheric wet deposition of nitrogen and sulfur to a typical red soil agroecosystem in Southeast China during the ten-year monsoon seasons (2003–2012). Atmos Environ 82:121–129
Dentener F, Drevet J, Lamarque JF, Bey I, Eickhout B, Fiore AM, Hauglustaine D, Hotowitz LW, Krol M, Kulshrestha UC, Lawrence M, Galy-Lacaux C, Rast S, Shindell D, Stevenson D, Van Noije T, Atherton C, Bell N, Bergman D, Butler T, Cofala J, Collins B, Doherty R, Ellingsen K, Galloway J, Gauss M, Montanaro V, Müller JF, Pitari G, Rodriguez J, Sanderson M, Solmon F, Strahan S, Schultz M, Sudo K, Szopa S, Wild O (2006) Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem Cy 20
Dong XY, Li J, Fu JS, Gao Y, Huang K, Zhuang GS (2014) Inorganic aerosols responses to emission changes in Yangtze River Delta, China. Sci Total Environ 481:522–532
Du EZ, Jiang Y, Fang JY, de Vries W (2014) Inorganic nitrogen deposition in China’s forests: status and characteristics. Atmos Environ 98:474–482
Erisman JW, Van Pul A, Wyers P (1994) Parametrization of surface resistance for the quantification of atmospheric deposition of acidifying pollutants and ozone. Atmos Environ 28:2595–2607
Erisman JW, Galloway J, Seitzinger S, Bleeker A, Butterbach-Bahl K (2011) Reactive nitrogen in the environment and its effect on climate change. Curr Opin Environ Sustain 3:281–290
Fahey TJ, Williams CJ, Rooney-Varga JN, Cleveland C, Postek KM, Smith SD, Bouldin DR (1999) Nitrogen deposition in and around an intensive agricultural district in central New York. J Environ Qual 28:1585–1600
Fang YT, Zhu WX, Mo JM, Zhou GY, Gundersen P (2005) Dynamics of soil inorganic nitrogen and their responses to nitrogen additions in three subtropical forests, south China. J Environ Sci (China) 18:752–759
Fang YT, Gundersen P, Mo JM, Zhu WX (2008) Input and output of dissolved organic and inorganic nitrogen in subtropical forests of South China under high air pollution. Biogeosciences 5:339–352
Fang YT, Yoh M, Koba K, Zhu WX, Takebayashi Y, Xiao YH, Lei CY, Mo JM, Zhang W, Lu XK (2011) Nitrogen deposition and forest nitrogen cycling along an urban–rural transect in southern China. Glob Chang Biol 17:872–885
Flechard CR, Nemitz E, Smith RI, Fowler D, Vermeulen AT, Bleeker A, Erisman JW, Simpson D, Zhang L, Tang YS, Sutton MA (2011) Dry deposition of reactive nitrogen to European ecosystems: a comparison of inferential models across the NitroEurope network. Atmos Chem Phys 11:2703–2728
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland C, Green P, Holland E (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gromov SA, Ryaboshapko AG, Paramonov SG (2001) Dry deposition of atmospheric sulphur and nitrogen in Russia and the former USSR. Water Air Soil Pollut 130:589–594
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010
Hara H, Kitamura M, Mori A, Noguchi I, Ohizumi T, Seto S, Takeuchi T, Deguchi T (1995) Precipitation chemistry in Japan 1989–1993. Water Air Soil Pollut 85:2307–2312
Hong YM, Lee BK, Park KJ, Kang MH, Jung YR, Lee DS, Kim MG (2002) Atmospheric nitrogen and sulfur containing compounds for three sites of South Korea. Atmos Environ 36:3485–3494
Ju XT, Xing GX, Chen XP, Zhang SL, Zhang LJ, Liu XJ, Cui ZL, Yin B, Christie P, Zhu ZL, Zhang FS (2009) Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc Natl Acad Sci U S A 106:3041–3046
Khoder MI (2002) Atmospheric conversion of sulfur dioxide to particulate sulfate and nitrogen dioxide to particulate nitrate and gaseous nitric acid in an urban area. Chemosphere 49:675–684
Larssen T, Duan L, Mulder J (2011) Deposition and leaching of sulfur, nitrogen and calcium in four forested catchments in China: implications for acidification. Environ Sci Technol 45:1192–1198
Li SQ, Li SX (1999) Nitrogen added to ecosystems by wet deposition in Guanzhong area in Shaanxi. Agro-Environ Prot 18:97–101 (in Chinese)
Li SX, Cun DG, Gao YJ, He HX, Li SQ (1993) Mineral nitrogen introduced into soil by precipitation on Loess dry land. Agric Res Arid Areas 11:83–92 (in Chinese)
Li KH, Liu XJ, Song W, Chang YH, Hu YK, Tian CY (2013) Atmospheric nitrogen deposition at two sites in an arid environment of central Asia. Plos One 8:e67018
Liang T, Tong YA, Lin W, Qiao L, Liu XJ, Bai SC, Yang XL (2014a) Spaital–temporal variability of dry and wet deposition of atmospheric nitrogen in different ecological regions of Shaanxi. Acta Ecol Sin 34:738–745 (in Chinese)
Liang T, Tong YA, Liu XJ, Qiao L (2014b) Dynamics of atmospheric nitrogen wet deposition fluxes in Guanzhong Area, Shaanxi. J Agro-Environ Sci 33:2389–2394 (in Chinese)
Liu XJ, Ju XT, Zhang Y, He CE, Kopsch J, Zhang FS (2006) Nitrogen deposition in agroecosystems in the Beijing area. Agr Ecosyst Environ 113:370–377
Liu XJ, Duan L, Mo JM, Du EZ, Shen JL, Lu XK, Zhang Y, Zhou XB, He CE, Zhang FS (2011) Nitrogen deposition and its ecological impact in China: an overview. Environ Pollut 159:2251–2264
Liu XJ, Zhang Y, Han WX, Tang AH, Shen JL, Cui ZL, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, Zhang FS (2013) Enhanced nitrogen deposition over China. Nature 494:459–462
Loubet B, Asman WAH, Theobald MR, Hertel O, Tang SY, Robin P, Hassouna M, Dämmgen U, Genermont S, Cellier P, Sutton MA (2008) Ammonia deposition near hot spots: processes, models and monitoring methods. In: Sutton MA, Reis S, Baker SMH (eds) Atmospheric ammonia: detecting emission changes and environmental impacts. Springer, Netherlands, pp 205–251
Luo XS, Liu P, Tang AH, Liu JY, Zong XY, Zhang Q, Kou CL, Zhang LJ, Fowler D, Fangmeier A, Christie P, Zhang FS, Liu XJ (2013) An evaluation of atmospheric Nr pollution and deposition in North China after the Beijing Olympics. Atmos Environ 74:209–216
Luo XS, Tang AH, Shi K, Wu LH, Li WQ, Shi WQ, Shi XK, Erisman JW, Zhang FS, Liu XJ (2014) Chinese coastal seas are facing heavy atmospheric nitrogen deposition. Environ Res Lett 9:095007
Martins LD, da Silva Júnior CR, Solci MC, Pinto JP, Souza DZ, Vasconcellos P, Guarieiro ALN, Guarieiro LLN, Sousa ET, de Andrade JB (2012) Particle emission from heavy-duty engine fuelled with blended diesel and biodiesel. Environ Monit Assess 184:2663–2676
Ouyang Y, Wang TJ, Zhang Y, Hu ZY, Xu CK (2003) A method calculating dry deposition velocity of air pollutants and its application. J Nanjing Inst Meteorol 26:210–218 (in Chinese)
Pan YP, Wang YS, Tang GQ, Wu D (2012) Wet and dry deposition of atmospheric nitrogen at ten sites in Northern China. Atmos Chem Phys 12:6515–6535
Phoenix GK, Hicks WK, Cinderby S, Kuylenstiena JCI, Stock WD, Dentener FJ, Giller KE, Austin AT, Lefroy RDB, Gimeno BS (2006) Atmospheric nitrogen deposition in world biodiversity hotspots: the need for a greater global perspective in assessing N deposition impacts. Global Chang Biol 12:470–476
Qi JH, Shi JH, Gao HW, Sun Z (2013) Atmospheric dry and wet deposition of nitrogen species and its implication for primary productivity in coastal region of the Yellow Sea, China. Atmos Environ 81:600–608
Richter A, Burrows JP, Nüß H, Granier C, Niemeier U (2005) Increase in tropospheric nitrogen dioxide over China observed from space. Nature 437:129–132
Rockström J, Steffen W, Noone K, Persson A, Chapin FS, Lambin E, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ, Nykvist B, de Wit CA, Hughes T, van der Leeuw S, Rodhe H, Sörlin S, Snyder PK, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell RW, Fabrty VJ, Hansen J, Walker B, Liverman D, Richardson K, Crutzen FJ (2009) Planetary boundaries: exploring the safe operating space for humanity. Ecol Soc 14:32
Scheuner ET, Makeschin F (2005) Impact of atmospheric nitrogen deposition on carbon dynamics in two Scots pine forest soils of Northern Germany. Plant Soil 275:43–54
Schwede D, Zhang LM, Vet R, Lear G (2011) An intercomparison of the deposition models used in the CASTNET and CAPMoN networks. Atmos Environ 45:1337–1346
Shen JL, Tang AH, Liu XJ, Fangmeier A, Goulding K, Zhang FS (2009) High concentrations and dry deposition of reactive nitrogen species at two sites in the North China Plain. Environ Pollut 157:3106–3113
Shen JL, Liu XJ, Zhang Y, Fangmeier A, Goulding K, Zhang FS (2011) Atmospheric ammonia and particulate ammonium from agricultural sources in the North China Plain. Atmos Environ 45:5033–5041
Shen JL, Li Y, Liu XJ, Luo XS, Tang H, Zhang YZ, Wu JS (2013) Atmospheric dry and wet nitrogen deposition on three contrasting land use types of an agricultural catchment in subtropical central China. Atmos Environ 67:415–424
Song YZ, Qin BQ, Yang LY, Hu WP, Luo LC (2005) Primary estimation of atmospheric wet deposition od nitrogen to aquatic ecosystem of Lake Taihu. J Lake Sci 17:226–230 (in Chinese)
Song L, Bao XM, Liu XJ, Zhang F (2012) Impact of nitrogen deposition on plant community in a semi-arid temperate steppe in China. J Arid Land 4:3–10
Song W, Chang YH, Liu XJ, Li KH, Gong YM, He GX, Wang XL, Christie P, Zheng M, Dore AJ, Tian CY (2015) A multiyear assessment of air quality benefits from China’s emerging shale gas revolution: Urumqi as a case study. Environ Sci Technol Lett 49:2066–2072
Stelson AW, Seinfeld JH (1982) Relative humidity and temperature dependence of the ammonium nitrate dissociation constant. Atmos Environ 16:983–992
Stevens CJ, Dise NB, Mountford JO, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303:1876–1879
Tang YS, Simmons I, van Dijk N, Di Marco C, Nemitz E, Dammgen U, Gilke K, Djuricic V, Vidic S, Gliha Z (2009) European scale application of atmospheric reactive nitrogen measurements in a low-cost approach to infer dry deposition fluxes. Agric Ecosyst Environ 133:183–195
Tian Y, Haibara K, Chang SX, Toda H, Fang SZ (2013) Acid deposition strongly influenced element fluxes in a forested karst watershed in the upper Yangtze River region, China. For Ecol Manag 310:27–36
Turnipseed AA, Huey LG, Nemitz E, Stickel R, Higgs J, Tanner DJ, Slusher DL, Sparks JP, Flock F, Guenther A (2006) Eddy covariance fluxes of peroxyacetyl nitrates (PANs) and NOy to a coniferous forest. J Geophys Res 111:D09304
Wang XY (2014) Study on characteristics of the temporal and spatial variation of nutrient resource utilization in Shaanxi Province. Northwest A&F University, 2015
Wang WX, Wang T (1995) On the origin and the trend of acid precipitation in China. Water Air Soil Pollut 85:2295–2300
Wang XZ, Zhu JG, Gao R, Baochun JH (2004) Dynamics and ecological significance of nitrogen wet deposition in Taihu Lake region: taking Changshu agro-ecological experiment station as an example Chinese. J Appl Ecol 15:1616–1620 (in Chinese)
Wang TJ, Liu Q, Zhao H, Zhou J, Fan JL (2008a) Atmospheric nitrogen deposition in agroecosystem in red soil region of Jiangxi Province. Acta Pedol Sin 45:280–287 (in Chinese)
Wang W, Ren LH, Zhang YH, Chen JH, Liu HJ, Bao LF, Fan SJ, Tang DG (2008b) Aircraft measurements of gaseous pollutants and particulate matter over Pearl River Delta in China. Atmos Environ 42:6187–6202
Wang ZH, Zhang Y, Liu XJ, Tong YA, Qiao L, Lei XY (2008c) Dry and wet nitrogen deposition in agricultural soils in the Loess area. Acta Ecol Sin 28:3295–3301 (in Chinese)
Wei Y, Tong YA, Duan M, Qiao L, Tian HW, Lei XY, Ma WJ (2010a) Atmospheric dry and wet deposition in typical agricultural areas of North Shaanxi. Chin J Appl Ecol 21:255–259 (in Chinese)
Wei Y, Tong YA, Qiao L, Liu XJ, Duan M, Li J (2010b) Preliminary estimate of the atmospheric nitrogen deposition in different ecological regions of Shaanxi Province. J Agro-Environ Sci 29:795–800 (in Chinese)
Wesely ML (1989) Parameterization of surface resistances to gaseous dry deposition in regional-scale numerical-models. Atmos Enciron 23:1293–1304
Whitall DR, Paerl HW (2001) Spatiotemporal variability of wet atmospheric nitrogen deposition to the Neuse River Estuary, North Carolina. J Environ Qual 30:1508–1515
Xie HX (2012) Comprehensive evaluation of the quality of atmospheric environment in Shaanxi Province. J Anhui Agric Sci 40(9410–9411):9532 (in Chinese)
Xu XL, Ouyang H, Cao GM, Pei ZY, Zhou CP (2004) Nitrogen deposition and carbon sequestration in alpine meadows. Biogeochemistry 71:353–369
Xu W, Luo XS, Pan YP, Zhang L, Tang AH, Shen JL, Zhang Y, Li KH, Wu QH, Yang DW, Zhang YY, Xue J, Li WQ, Li QQ, Tang L, Lu SH, Liang T, Tong YA, Liu P, Zhang Q, Xiong ZQ, Shi XJ, Wu LH, Shi WQ, Tian K, Zhong XH, Shi K, Tang QY, Zhang LJ, Huang JL, He CE, Kuang FH, Zhu B, Liu H, Jin X, Xin YJ, Shi XK, Du EZ, Dore AJ, Tang S, Collett JL Jr, Goulding K, Zhang FS, Liu XJ (2015) Quantifying atmospheric nitrogen deposition through a nationwide monitoring network across China. Atmos Chem Phys 15:12345–12360
Zhang LM, Gong SL, Padro J, Barrie L (2001) A size-segregated particle dry deposition scheme for an atmospheric aerosol module. Atmos Enciron 35:549–560
Zhang Y, Wang TJ, Hu ZY, Xu CK (2004) Temporal variety and spatial distribution of dry deposition velocities of typical air pollutants over different landuse types. Clim Environ Res 9:591–604 (in Chinese)
Zhang Y, Liu XJ, Zhang FS, Ju XT, Zhou GY, Hu KL (2006) Spatial and temporal variation of atmospheric nitrogen deposition in North China Plain. Acta Ecol Sin 26:1633–1639 (in Chinese)
Zhang GS, Zhang J, Liu SM (2007) Characterization of nutrients in the atmospheric wet and dry deposition observed at the two monitoring sites over Yellow Sea and East China Sea. J Atmos Chem 57:41–57
Zhang FS, Wang JQ, Zhang WF, Cui ZL, Ma WQ, Chen XP, Jiang RF (2008a) Nutrient use efficiencies of major cereal crops in China and measures for improvement. Acta Pedol Sin 45:915–924 (in Chinese)
Zhang Y, Liu XJ, Fangmeier A, Goulding KTW, Zhang FS (2008b) Nitrogen inputs and isotopes in precipitation in the North China Plain. Atmos Environ 42:1436–1448
Zhang Y, Yu QL, Ma WC, Chen LM (2010) Atmospheric deposition of inorganic nitrogen to the eastern China seas and its implications to marine biogeochemistry. J Geophys Res: Atmos 115:D7
Zhang M, Tong YA, Guo JW, Zhang SL, Guo P (2011) Determination of reasonable nitrogen use and evaluation of application status in wheat/maize rotation system in Guanzhong area of Shaanxi Province. J Northwest A&F Univ (Nat Sci Ed) 152:158–164 (in Chinese)
Zhang L, Jacob DJ, Knipping EM, Kumar N, Munger JW, Carouge C, Van Donkelaar A, Wang YX, Chen D (2012a) Nitrogen deposition to the United States: distribution, sources, and processes. Atmos Chem Phys 12:4539–4554
Zhang Y, Song L, Liu XJ, Li WQ, Lü SH, Zheng LX, Bai ZC, Cai GY, Zhang FS (2012b) Atmospheric organic nitrogen deposition in China. Atmos Environ 46:195–204
Zheng LX, Liu XJ, Zhang FS (2007) Atmospheric deposition of organic nitrogen: a review. Acta Ecol Sin 27:3828–3834 (in Chinese)
Zhu ZL (2008) Research on soil nitrogen in China. Acta Pedol Sin 45:778–783 (in Chinese)
Acknowledgments
The research was financially supported by the National Natural Science Foundation of China (40675006, 40425007). We would like to thank Mr. Puhui Ji for his constructive comments and suggestions and Mr. Yimin Gao for helping us to draw the monitoring site distribution figure.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Gerhard Lammel
Rights and permissions
About this article
Cite this article
Liang, T., Tong, Y., Liu, X. et al. High nitrogen deposition in an agricultural ecosystem of Shaanxi, China. Environ Sci Pollut Res 23, 13210–13221 (2016). https://doi.org/10.1007/s11356-016-6374-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6374-1