Abstract
Removal of oil spillage from the environment is a global concern. Various methods, including the use of fibers as sorbents, have been developed for oil spill control. Oil palm empty fruit bunch (OPEFB) fiber is a plant biomass that may be acetylated by acetic anhydride using N-bromosuccinimide (NBS) as a catalyst; here, the extent of acetylation may be calculated in terms of weight percent gain (WPG). The modified fiber was used to remove Tapis and Arabian crude oils. The optimum time, temperature, and catalyst concentration were 4 h, 120 °C, and 3 %, respectively, and these parameters could achieve an 11.49 % increase in WPG. The optimized parameters improved the adsorption capacity of OPEFB fibers for crude oil removal. The acetylated OPEFB fibers were characterized by using Fourier transform infrared spectroscopy and field emission scanning electron microscopy to observe the functional groups available and morphology. Kinetic and isotherm studies were conducted using different contact times and oil/water ratios. The rate of oil sorption onto the OPEFB fibers can be adequately described by the pseudo-second-order equation. Adsorption studies revealed that adsorption of crude oil on treated OPEFB fiber could be best described by the Langmuir isotherm model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fossil fuels are important raw materials and energy sources used worldwide. However, improper handling of these fuels (e.g., during loading, transportation, and unloading) and human commercial activities can bring about hazardous impacts to marine life and the environment (Boopathy 2000). Oil leakage produces unpleasant odors and green algae blooms, which can cause drastic changes to the sea color and landscape (Annunciado et al. 2005). These problems have encouraged academicians, researchers, and industrial personnel to develop methods for oil removal. A robust and effective methodology must be developed to recover leaked oil and eradicate possible hazards caused by oil pollution. The existing management approach for oil spills includes the use of oil booms, skimmers, oil dispersants, oil gelling agents, in situ burning, bioremediation, and oil sorbents (Adebajo et al. 2003; Angelova et al. 2011; Asadpour et al. 2013; Zahed et al. 2010).
Sorbent materials have the potential to eradicate oil spill issues by cleaning and collecting the leaked oil. These materials can be categorized into three types, namely, inorganic materials, synthetic organic polymers, and organic natural materials. Synthetic sorbents, such as polypropylene and polyurethane, are utilized to retrieve oil in commercial applications (Adebajo et al. 2003). These sorbents generally have high oil sorption capacity, good buoyancy, and application simplicity but are non-biodegradable and can persist in the environment for long periods of time. Inorganic materials, such as vermiculite, organoclay, perlite, and exfoliated graphite, are also used as oil sorbents (Bastani et al. 2006; Carmody et al. 2007; Tryba et al. 2003). Unfortunately, most of these materials have low oil sorption capacity, low buoyancy, and poor reusability, except exfoliated graphite (Toyoda and Inagaki 2003), which is expensive. Organic sorbents, such as those from agricultural wastes, are candidate materials for oil sorption because they are relatively cheap and easily accessible. Kapok, kenaf, corn cob, cotton, peat moss, milkweed floss, sawdust, barks, bagasse, barley straw, and rice straw have been tested and proven as effective sorbents for oil spill cleanup (Annunciado et al. 2005; Asadpour et al. 2013). Natural organic sorbents are fully biodegradable and do not leave environmentally harmful residues.
In the situation where sorbents are used for cleaning oil spill incident, recovering from soaked materials is an important step to reduce their impacts on aquatic environment. However, it is possible that some sorbents may not be collected during the recovering process due to their sinking. This effect can be expressed in term of buoyancy. From “Standard Test Method for Sorbent Performance of Adsorbents” described in ASTM 726–06, if 10 % or more of the sorbent material has sunk, then the sorbent is considered to have failed as a sorbent.
Oil palm (Elaeis guineensis) is endemic to West Africa but now cultivated and considered the most important economic plantation crop in tropical countries. Oil palm fruits are a major source of edible oils and can produce around 38.5 × 106 t of oil annually (i.e., 25 % the total edible oil and fat production in the world) (Shuit et al. 2009). Oil palm empty fruit bunch (OPEFB) is an abundantly available natural fiber that is extracted from empty fruit bunch (EFB) for various uses (Isa et al. 2008). Of the 55.73 × 106 t of oil palm biomass annually produced in Malaysia, 17.0 × 106 t come from OPEFB. More than half the quantity of OPEFB products originate from this fiber (Shuit et al. 2009). The fiber can be obtained directly from the mill at low cost, making it an appropriate choice as a feedstock for various applications.
One of the main components of OPEFB is lignocellulose, which is composed of cellulose (43.0 %), hemicelluloses (35.8 %), and lignin (16.4 %) (Hamzah et al. 2011). Cellulose and hemicelluloses are hydrophilic, whereas the lignin is hydrophobic. These limitations are overcome by chemically modifying the fiber components. A method commonly used to do so involves changing the functional groups attached to cellulose, lignin, and hemicelluloses into hydrophobic groups through chemical modification (Asadpour et al. 2014b; Sidik et al. 2012; Sun et al. 2002). Alteration using acetic anhydride and fatty acids has been extensively applied by various researchers (Asadpour et al. 2014a, 2016; Sun et al. 2004), and results reveal that acetylation is an efficient way to enhance the properties of the fibers. Acetylation improves the quality of the fiber from its extremely hygroscopic nature, which causes swelling and shrinking via changes in humidity. Although it can improve fiber strength, dimensional stability, and decay resistance, the anhydride generally reacts with the hydroxyl groups of wood components and prepares the oleophilic surface by transforming some hydroxyl groups in lignocellulose into oleophilic groups (Asadpour et al. 2015; Sun et al. 2002; Teli and Valia 2013a, b).
To improve sorption capacity, several researchers have examined various methods for wood acetylation with or without catalysts. A variety of catalysts have been tested to speed up the reaction rate of acetic anhydride with wood. 4-Dimethylaminopyridine (DMAP), N-methylpyrrolidine (MPI), N-methylpyrrolidone (MPO), and N-bromosuccinimide (NBS) have been used as acetylation catalysts in chemical synthesis (Sun et al. 2002; Teli and Valia 2013a; Wang and Wang 2013). NBS, in particular, is commercially available, inexpensive, non-poisonous, and efficient (Karimi and Seradj 2001).
Hydroxyl groups in the cellulosic structure of lignocellulosic materials are converted into O–CO–CH3 (acetate groups) when subjected to acetylation (Eq. (1)). This reaction changes the hydrophilic properties of cellulosic material into hydrophobic by blocking cell wall fiber hydroxyl groups with acetyl groups (Sun et al. 2002).

The present research aims to explore the oil sorption capacity of raw and acetylated OPEFB fibers prepared in acetic anhydride by using NBS as a catalyst in an oil/water mixture. The impact of various factors, such as NBS content, reaction temperature, and time of acetylation, on oil sorption was examined for light (Tapis) and heavy (Arabian) crude oils. Fourier transform infrared (FTIR) spectroscopy and field emission scanning electron microscopy (FESEM) were utilized to investigate the reaction and morphology of the sorbent. The pseudo-first-order and pseudo-second-order models were used for kinetic studies, and the Langmuir and Freundlich isotherm models were tested to fit the experimental data.
Methods
Materials
OPEFB fibers were used in this study. The fiber was treated and tested to adsorb both Arabian and Tapis crude oils. Other chemicals used in this study included acetic anhydride, NBS, acetone, and ethyl alcohol.
Mechanical treatment of oil palm empty fruit bunch fiber
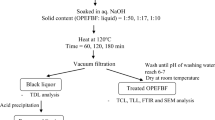
OPEFB fibers were mechanically treated by manual hammering. One- to two-centimeter-long fibers were packed in a plastic bag as single sheets and hammered for about 10 min. After hammering, the fibers were subjected to wash by immersion into a 500-mL beaker containing 0.1 N NaOH. The mixture was stirred for 2 h. The treated fibers were washed with distilled water several times to remove the alkaline solution and then dried in an oven at 80 °C for 8 h. Mechanical treatment was used to eliminate SiO2, which can increase fiber pore sizes and surface roughness, on the fiber surface.
Preparation of acetylated OPEFB fiber
Acetate groups feature higher molecular weights than hydroxyl groups and are thus bulkier than the latter. Thus, the acetylated sample is predicted to show larger weight increases, which are reported in terms of weight percent gain (WPG), compared with the raw sample (Teli and Valia 2013a). The acetic acid produced after reaction of the cellulosic material and acetic anhydride must be washed and removed from the sorbent material before the fiber is used. A calculated amount of OPEFB fiber was placed in a 500-mL two-necked round-bottom flask containing acetic anhydride (solid to liquid ratio, 1 g/30 mL) and NBS (1–3 %) as the reaction catalyst under atmospheric pressure. A reflux condenser, a thermometer, and a mechanical stirrer were attached to the flask. The flask then placed in an oil bath set to 90 or 120 °C for 2–6 h. The flask was removed from the oil bath after the required time, the mixture inside the flask was decanted, and the fibers were thoroughly washed with ethyl alcohol and acetone to remove unreacted acetic anhydride and acetic acid by-products. The modified fibers were placed in an oven at 80 °C overnight and weighed to evaluate the WPG. Each treatment was conducted three times under similar conditions, and average values of WPG, which was calculated according to Eq. (2), are reported.
To show increase in the hydrophobicity of sorbent, the method proposed by Sidik et al. (Sidik et al. 2012) was applied. Base on the method, 1 g of sorbent was mixed with 30 mL of distilled water and was stirred for 20 min. After that, 30 mL of hexane was added and further agitated for 3 min. After termination time, the beaker was put in the static condition for 5 min to separate two immiscible phases. The upper layer of the mixture which placed in the hexane was separated by decanting. The residual was filtered and dried an oven at 110 °C to reach constant weight and weighed. The hydrophobicity degree of hydrophobicity of the sorbent which shows the presence of adsorbent transferred into the organic phase was determined as the ratio of the mass of OPEFB fiber transferred to hexane (g) and the initial OPEFB fiber mass (g), by Eq. (3).
To investigate how many sorbents are floated on the aquatic system, buoyancy test was carried out. Both types of sorbents modified and unmodified were tested to compare their floating characteristics. The test was carried out in a 500-mL glass beaker, it was filled with 300 mL water and was slowly added 30 mL crude oil, and then, 1 g of sorbents was placed in the middle of the oil slick. The mixture was put on the automatic shaker and set its frequency to 100 rpm and shake for 15 min. At the end of the test, the floated sorbent and oil were gathered by filter and suction, respectively. The settled materials which remain in the beaker were collected of the water, dried, and weighed. The percentage of buoyancy was calculated with the following equation (Eq. (4)):
where w s and w 0 are the mass of settled part and initial of sorbent, respectively.
Characterization of OPEFB fiber and experimental oils
FTIR was performed from 400 to 4000 cm−1, and results were analyzed to compare the functional groups of the raw and acetylated fiber samples. The surface morphology and cross section of the sorbents were observed by FESEM to determine the surface roughness and pore diameter of the sorbent. The physical features of the OPEFB fiber, including Brunauer–Emmett–Teller (BET) surface area and pore volume, were measured via N2 (g) adsorption in an ASAP 2020 analyzer (Micromeritics, USA) in liquid N2 using the BET method. A density meter (Anton Paar, Austria) and EV1000 viscometer (Anton Paar) were used to measure the density and viscosity of the experimental oils, respectively.
Measurements of oil sorption capacity
In a 500-mL beaker, 40 mL of the crude oil samples (Tapis) was mixed with 400 mL of standard artificial seawater (3.5 % NaCl). About 1 g each of the raw and acetylated sorbents was then added to the oil/water system at room temperature. The system was kept for 10, 20, 30, 40, or 50 min, and the raw and acetylated OPEFB fibers were filtered using a mesh screen for 1 min. The OPEFB fibers were then re-weighed to calculate their oil sorption capacity (g oil/g sorbent) using Eq. (5).
where w s is the weight of the wetted sorbent, w c is the initial weight of the sorbent, and w w is the weight of water. The weight of the absorbed water in the sample was measured using the Karl Fischer technique as well described in ASTM D1533. The experiment was repeated using heavy crude oil (Arabian).
To find the effect of oil concentration to water ratio on oil sorption capacity of sorbent, different amounts of oil in water from 12,500 to 125,000 mg/L were tested. One gram of the acetylated sorbent was used in this experiment. The sorption capacity of the sorbent was measured after 35- and 40-min contact time for Tapis and Arabian crude oils, respectively.
Results and discussion
Characterization of OPEFB fiber as a sorbent
Morphology identification of sorbent
The main sorption mechanisms include adsorption and absorption. The oil sorption capacity of sorbents is closely associated with the molecular arrangement, chemical properties, and physical properties of the sorbent; these properties include surface roughness, hollow lumens, porosity, twists, and crimps. Oleophilicity and surface energy are significant characteristics associated with the molecular arrangement and chemical properties of the sorbents, while the adsorption mechanism and capillary action are related to their physical configuration (Choi and Moreau 1993).
The physical configuration of the sorbent can be examined using FESEM images. The surface of OPEFB fibers contains numerous cylindrical holes of about 10 μm in diameter, some of which are filled with SiO2 (Fig. 1a, b). If the SiO2 is detached from the fiber surface, the resulting voids can improve the surface roughness and pore volume of the fibers, which, in turn, allows the oil to adhere to the surface of the fibers and diffuse into their porous interior through capillary action. Most of the SiO2 can be removed by hammering (Ghazali et al. 2009) and washing by 0.1 N NaOH. The treatment results showed reduced attachment of SiO2 molecules and increased surface roughness of the OPEFB fibers (Fig. 1c). The EDEX result shows that the silica content (atomic) reduced from 11.29 to 0.28 (as shown in Table 1). However, hammering treatment also destroyed the hollow tubular structure of the fibers and made them flat (Fig. 1d). Therefore, because flat structures and less porosity adsorb less oil, we can conclude that hammering exerts no effect on the oil sorption capacity of OPEFB fibers.
The FESEM cross section of the OPEFB fibers is shown in Fig. 1e, f. OPEFB fibers have a hollow tubular structure with diameters of 10–14 μm and a highly porous interior. During adsorption, the oil penetrates into the porous interior and hollow structure of the fiber. We can conclude that the sorption mechanism in this study involves adsorption of crude oil by filling the porous interior of the sorbent through capillary action.
FTIR analysis of sorbent
Figure 2 shows the FTIR spectra of raw and acetylated OPEFB fibers at different reflux time.
The important bands recognized in the spectra are
- 3404 cm−1 :
-
Stretching vibrations of hydroxyl groups (O–H) present in cellulose, hemicellulose, and lignin of OPEFB fiber.
- 2930 cm−1 :
-
C–H asymmetric stretching of CH2 groups.
- 1740 cm−1 :
-
Stretching vibration of the carbonyl group C = O in ester.
- 1640 cm−1 :
-
Bending mode of the absorbed water.
- 1375 cm−1 :
-
C–H bending vibration in –O–(C = O)–CH3.
- 1240 cm−1 :
-
C–O stretching vibration in ester.
Comparison of the raw and acetylated fiber spectra indicates four major differences. Very weak adsorption at 1740 cm−1 in raw fiber FTIR spectrum was dramatically increased in the acetylated fiber which shows an increase in carbonyl group C = O in the ester. Although the intensity of two ester bands around 1375 and 1240 cm−1 were increased, the reduction of the hydroxyl groups at 3404 and 1640 cm−1 demonstrated the substitution of the hydroxyl groups with the acetyl group. The effect of reaction time on the FTIR spectrum was also investigated by the peak intensity of acetylated OPEFB fibers. As shown in Fig. 2, an increase in reflux time from 2 to 6 h resulted in an increase in the intensity of the three ester bands at 1240, 1375, and 1740 cm−1 but a decrease in hydroxyl groups (O–H), demonstrating a raise of acetylation, which agreed to the data of WPG as will be discussed in “Effect of different parameters on WPG” section. Although by comparing the FTIR spectra of the 4 and 6 h, there is no obvious change in the intensity of spectrum with increase in the reaction time, increase in carbonyl group in acetylated fiber create a non-polar layer on the surface of the fiber, which leads to a dramatic increase in degree of hydrophobicity (%DH) as approved by the experimental result before and after acetylation from 21 to 57 %.Thus, all evidence confirms that the OPEFB fiber was successfully modified.
Although, increasing the reaction time from 2 to 6 h increases the intensity of the peak at the relative bands. This shows the effect of reaction time on the acetylation degree; nevertheless, there are only minor differences between the bands at 4 and 6 h. The bands that appeared at 3600 and 3800 cm−1 are due to the O–H stretch of H2O which appeared at 6-h reflux time. Varying degree of hydrogen bonding interactions among hydroxyl groups in the cellulose, hemicellulose, and lignin will result in wider peak in the OH stretching vibration region (3000–3800 cm−1) (Barnette et al. 2012).
Surface area measurement
The physical properties of raw and treated OPEFB fiber were analyzed. Table 2 shows surface area and average pore diameter of sorbent. According to the criterion established by the International Union of Pure and Applied Chemistry (IUPAC), pore sizes are classified into the following three groups: micropores (diameter (d) < 20 Å), mesopores (20 Å < d < 500 Å), and macropores (d > 500 Å) (Rouquerol et al. 1994). Based on the BET analyzes, raw and acetylated OPEFB fibers have a surface area of 0.4077 and 0.3463 m2/g, respectively. The BET surface areas of all samples were analyzed in triplicate. Based on the morphology of fibers discussed in “Morphology identification of sorbent” section and shown in Fig. 1d–f, OPEFB fiber has a porous interior and hollow structure, the main factors which contribute to enhance oil sorption. This low value of surface area does not explain the apparent high oil sorption capacity of fibers compared to mangrove bark, published in our previous work (Asadpour et al. 2014a). It is reported elsewhere (Eric et al. 1998) (RAHMAH 2009) that some intermolecular structures of agricultural biomass collapsed during N2 gas adsorption in the BET test. This happened during dehydration process to dry water molecules by vacuum drying (conditions of sample preparation before the N2 adsorption). During this process, the intramolecular water molecules were removed during vacuum drying, which was followed by the collapsing cellulose chains. Simultaneously, all the pores in the intermolecular structure are clogged. This linkage is so strong to repair by N2 molecules. Therefore, only the external surface of fibers was measured (Eric et al. 1998). The similar result was archived for cotton and kapok fiber which have high sorption capacity, but BET method showed the low surface area that only measured the external surface area which does not represent the true values (Eric et al. 1998; RAHMAH 2009). It is suggested that the same phenomenon resulted in measurement of low BET surface area in this study as well. The average pore diameter of the sorbent was slightly increased after acetylation.
Effect of hammering on sorption capacity
No significant change in sorption capacity was observed in the raw and pre-treated samples. As mentioned in “Morphology identification of sorbent” section, some of the SiO2 are removed from the fiber surface during hammering treatment, and improvements in surface roughness and pore size may be expected. Although oil adheres to the surface and diffuses into the porous interior of the fibers, the flat interior structure of the sorbent cannot adsorb as much oil as the raw fibers. Thus, oil remained on the fiber surface and filled up its pores. This theory confirms the results achieved in this study. Figure 3 illustrates the sorption capacity of raw, hammered treated fibers. Pre-treatment (hammering) exerted no significant effect on oil sorption capacity in this study.
Effect of different parameters on WPG
Optimization of the experimental data was carried out by changing various parameters affecting WPG and oil sorption capacity, including reaction time, temperature, and catalyst percentage. Changes in these parameters were correlated with the acetylation conditions. The acetylated fibers showed more hydrophobicity and adsorbed more oil. The maximum oil sorption capacity was also associated with the maximum WPG. Table 3 describes the various parameters employed in each experiment. The experimental data showed that the maximum WPG (11.49 %) may be observed at 4 h of reaction time, refluxing at 120 °C, and 3 % NBS as the catalyst. Under these conditions, sorption of Tapis and Arabian crude oils reached 6.80 and 7 g/g, respectively.
One of the main factors affecting acetylation is reaction time. WPG increased with increasing reaction time up to 4 h and slightly decreased afterward. Diffusion of the acetylation agent into the OPEFB fiber molecules increased with increasing time. However, prolonged reaction times negatively affected WPG because the acetic acid released as a by-product reduced the acetic anhydride concentration and, consequently, the reaction rate. The optimum reaction time was found to be 4 h, after reflux.
Refluxing temperatures of 90 and 120 °C were tested. Table 1 shows that a higher WPG could be achieved at the higher temperature. During acetylation, hydrogen bond networks with hydroxyl groups are broken to allow the acylating agent to modify the intermolecular structure of the fibers. High temperature assists the breaking of hydrogen bond networks and swelling of the OPEFB fibers to create reactive chemical sites that are favorable for accepting the acylating agent; thus, the acetylation process is enhanced. Therefore, a refluxing temperature of 120 °C is more effective than 90 °C. Temperatures higher than 120 °C were also tested, and the results show no significant WPG change. Therefore, to obtain an energy-efficient and cost-effective procedure, 120 °C was selected as the optimum temperature.
Catalyst concentration is another factor that plays an important role in acetylation. Increases in NBS concentration considerably accelerated the acetylation process. With 3 % NBS and refluxing at 120 °C for 4 h, WPG reached a maximum value of 11.49 %. NBS (C4H4BrNO2) contains Br, which can activate the carbonyl group of acetic anhydride and yield-reactive acylating agents (Teli and Valia 2013b). However, since the WPG at 5 % NBS was similar to that at 3 % NBS, the optimum NBS concentration was considered to be 3 %. In summary, the highest WPG (11.49 %) and oil sorption capacity (6.80 g/g for Tapis and 7 g/g for Arabian crude oils) were obtained by refluxing at 120 °C for 4 h with 3 % NBS.
Figure 4 shows the relationship between the oil sorption capacities of OPEFB fibers and their WPG. The oil sorption capacity of OPEFB fibers was directly correlated with their WPG; oil sorption capacity increased with increasing WPG.
Effect of contact time on sorption capacity
Low- and high-viscosity crude oils were immediately adsorbed by the sorbent within the first 15 min of contact (Fig. 5). The sorption process continued slowly until equilibrium was achieved at around 35 and 30 min for the Arabian and Tapis crude oils, respectively. Sorption indicated three stages, namely, the rapid, slow, and steady stages. In the rapid stage, crude oil was taken up at a high rate, likely because of the bare surface and interiors of the sorbent fibers. In the slow stage, adsorption increased gradually as small oil droplets were created by breakage of the crude oil that diffused into the interior of the fibers (Stang et al. 1994). Finally, the steady stage occurred after 35 and 30 min of sorption for the Arabian and Tapis crude oils, respectively. In this stage, adsorption remained constant and reached equilibrium because the OPEFB fibers were saturated by the crude oil particles. The adsorption time differed between the two oils because of differences in their viscosity. Tapis oil is a low-viscosity oil that flows and penetrates faster than Arabian oil, which is a high-viscosity oil. Arabian crude oil reached the porous interior of the sorbent within 35 min.
Buoyancy test
The result of the test shows that buoyancy of OPEFB fiber was varied from 95.3 to 93.7 after 5- and 15-min buoyancy time, although the raw sample shows the lowest value of 91.2 which is acceptable by ASTM. The good buoyancy of acetylated OPEFB fiber goes them into an effective adsorbent in real conditions for the removal of crude oil spills.
Kinetic study
To understand the sorption process, a kinetic adsorption study was conducted. Two well-known models, namely, the pseudo-first-order and pseudo-second-order models, were used to understand the kinetics of adsorption of crude oil onto the OPEFB fiber. The linear forms (Eqs. (6) and (7)) of these two models are as follows:
where q e and q t (mg/g) are the amounts of oil adsorbed onto the sorbent at equilibrium and time t, respectively, and K 1 and K 2 (1/min) are the rate constants of first-order and second-order models, respectively. Figure 6a, b shows the linear plots of the two models. K 1 and q e were determined from the plot of log (q e − q t ) against t for the first-order model, and K 2 and q e were calculated from the plot of t/q t versus t for the second-order model. The calculated parameters, namely, R 2, K 1, K 2, and theoretical q e , for both models, are listed in Table 4. The pseudo-second-order model showed a better fit with the experimental data because this model yielded a higher R 2 value than the first-order model as well as fairly matching experimental and theoretical q e values.
Effect of oil concentration to water ratio and isotherm study
Figure 7 shows the effect of oil concentration on sorption capacity. The highest adsorption rate was achieved at 87,500 mg/L oil concentration. At equilibrium, no further adsorption was observed. This equilibrium can be attributed to the saturation of active sites on the OPEFB fiber at high oil concentrations.
In this study, two isotherms models, namely, the Langmuir and Freundlich models, were used to describe the mechanism of oil sorption. Equations (8) and (9), respectively, represent the linear forms of the Langmuir and Freundlich isotherm models.
where q e (mg/g) is the amount of oil adsorbed at equilibrium time or monolayer adsorption, C e (mg/L) is the equilibrium concentration of oil, q 0 is the theoretical maximum adsorption capacity, and b is the Langmuir constant related to the rate of adsorption. The critical features of the Langmuir isotherm can be explained by the constant separation factor R l , which is dimensionless. Here, the isotherm model is favorable if 0 < R l < 1, unfavorable if R l > 1, linear if R l = 1, or irreversible if R l = 0. R l is calculated using Eq. (10).
where C 0 is the initial oil concentration.
In the Freundlich equation, q e (mg/g) is the maximum adsorption capacity, C e is the equilibrium concentration of oil (mg/L), K f (mg/g) (L/mg)1/n is the adsorption capacity in multilayer adsorption, and n is the dimensionless Freundlich constant. When 1/n is between 0 and 1, the adsorption process is favorable. The values of n and K f are obtained from the slope and intercept of the plot of log q e versus log C e . All of the calculated values are shown in Table 5. Figure 8a, b illustrates the Langmuir and Freundlich plots of the sorption data for Arabian and Tapis crude oil sorption onto the acetylated OPEFB fibers. The Freundlich model describes the multilayer adsorption of the crude oil onto a heterogeneous sorbent surface, whereas the Langmuir model assumes that adsorption of oil onto the adsorbent surface is monolayer in nature and homogeneous (Salarirad et al. 2011).
The results show that fitting via the Langmuir isotherm model produced a higher correlation coefficient (R 2) than fitting via the Freundlich isotherm model. The R l obtained (0.073 and 0.080 for Arabian and Tapis crude oils, respectively) reveals that adsorption favored the Langmuir model. Although the 1/n Freundlich constant is between 0 to 1 (indicating favorable fitting), this model produces an R 2 lower than that obtained from the Langmuir model. These findings indicate that sorption of the crude oils onto OPEFB fibers occurs via a monolayer approach through homogeneous sites with uniform energy-level distributions.
Conclusion
Acetylated OPEFB fibers are more hydrophobic than raw fibers. The optimum conditions for acetylation of the fibers included acetic anhydride with 3 % NBS as a catalyst at 120 °C and 4 h of refluxing time. Significant differences in the FTIR spectra of the raw and acetylated fibers were observed with increasing acetyl and ester groups and decreasing hydroxyl groups. The optimum contact time for oil adsorption was influenced by the oil viscosity. Arabian crude oil (heavy crude) required 35 min to reach equilibrium, whereas Tapis (light crude) required only 30 min. The sorption rate and adsorption mechanism of crude oil onto the acetylated sorbent can be described by the pseudo-second-order model. The oil concentration equilibrium fitted the Langmuir isotherm model. The results of this study reveal the potential use of waste materials such as OPEFB fibers as an environmentally acceptable and economical sorbent for oil spill cleanup.
References
Adebajo MO, Frost RL, Kloprogge JT, Carmody O, Kokot S (2003) Porous materials for oil spill cleanup: a review of synthesis and absorbing properties. J Porous Mater 10:159–170
Angelova D, Uzunov I, Uzunova S, Gigova A, Minchev L (2011) Kinetics of oil and oil products adsorption by carbonized rice husks. Chem Eng J 172:306–311
Annunciado T, Sydenstricker T, Amico S, Annunciado T, Sydenstricker T, Amico S (2005) Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar Pollut Bull 50:1340–1346
Asadpour R, Sapari NB, Tuan ZZ, Jusoh H, Riahi A, Orji KU (2013) Application of sorbent materials in oil spill management: a review Caspian. J Appl Sci Res 2:46–57
Asadpour R, Sapari NB, Isa MH, Orji KU (2014a) Enhancing hydrophobicity of mangrove bark by esterification for oil adsorption. Water Sci Technol 70:1220–1228. doi:10.2166/wst.2014.355
Asadpour R, Sapari NB, Isa MH, Orji KU (2014b) Investigation of modified mangrove bark on the sorption of oil in water. Appl Mech Mater 567:74–79
Asadpour R, Sapari NB, Isa MH, Kakooei S, Orji KU (2015) Acetylation of corn silk and its application for oil sorption. Fibers Polym 16:1830–1835. doi:10.1007/s12221-015-4745-8
Asadpour R, Sapari NB, Isa MH, Kakooei S, Orji KU, Daneshfozoun S (2016) Esterification of corn silk fiber to improve oil absorbency. Adv Mater Res 1133:552–556
Barnette AL et al (2012) Quantification of crystalline cellulose in lignocellulosic biomass using sum frequency generation (SFG) vibration spectroscopy and comparison with other analytical methods. Carbohydr Polym 89:802–809
Bastani D, Safekordi A, Alihosseini A, Taghikhani V (2006) Study of oil sorption by expanded perlite at 298.15 K. Sep Purif Technol 52:295–300
Boopathy R (2000) Factors limiting bioremediation technologies. Bioresour Technol 74:63–67
Carmody O, Frost R, Xi Y, Kokot S (2007) Adsorption of hydrocarbons on organo-clays—implications for oil spill remediation. J Colloid Interface Sci 305:17–24
Choi HM, Moreau JP (1993) Oil sorption behavior of various sorbents studied by sorption capacity measurement and environmental scanning electron microscopy. Microsc Res Tech 25:447–455
Eric H, Chongrak K, Noureddine A, Jean P (1998) Application of methylene blue adsorption to cotton fiber specific surface area measurement. J Cotton Sci 2:164–173
Ghazali A, Wan Rosli W, Law K (2009) Pre-treatment of oil palm biomass for alkaline peroxide pulping. Cellul Chem Technol 43:331–338
Hamzah F, Idris A, Shuan TK (2011) Preliminary study on enzymatic hydrolysis of treated oil palm (Elaeis) empty fruit bunches fibre (EFB) by using combination of cellulase and β 1–4 glucosidase. Biomass Bioenergy 35:1055–1059
Isa MH, Ibrahim N, Aziz HA, Adlan MN, Sabiani NHM, Zinatizadeh AAL, Kutty SRM (2008) Removal of chromium (VI) from aqueous solution using treated oil palm fibre. J Hazard Mater 152:662–668
Karimi B, Seradj H (2001) N-bromosuccinimide (NBS), a novel and highly effective catalyst for acetylation of alcohols under mild reaction conditions. Synlett 4:519–520
RAHMAH AU (2009) Characterization of physicochemical properties and process design for oil removal using Ceiba pentandra (L.) Gaertn. as a natural sorbent. Dissertation, Universiti Teknologi Petronas
Rouquerol J et al (1994) Recommendations for the characterization of porous solids (Technical report). Pure Appl Chem 66:1739–1758
Salarirad MM, Behnamfard A Modeling of equilibrium data for free cyanide adsorption onto activated carbon by linear and non-linear regression methods. In: International Conference on Environment and Industrial Innovation, Singapour, 2011. IACSIT Press.
Shuit SH, Tan KT, Lee KT, Kamaruddin A (2009) Oil palm biomass as a sustainable energy source: a Malaysian case study. Energy 34:1225–1235
Sidik S, Jalil A, Triwahyono S, Adam S, Satar M, Hameed B (2012) Modified oil palm leaves adsorbent with enhanced hydrophobicity for crude oil removal. Chem Eng J 203:9–18
Stang M, Karbstein H, Schubert H (1994) Adsorption kinetics of emulsifiers at oil–water interfaces and their effect on mechanical emulsification. Chem Eng Process Process Intensif 33:307–311
Sun X-F, Sun R, Sun J-X (2002) Acetylation of rice straw with or without catalysts and its characterization as a natural sorbent in oil spill cleanup. J Agric Food Chem 50:6428–6433
Sun X, Sun R, Sun J (2004) Acetylation of sugarcane bagasse using NBS as a catalyst under mild reaction conditions for the production of oil sorption-active materials. Bioresour Technol 95:343–350
Teli M, Valia SP (2013a) Acetylation of banana fibre to improve oil absorbency. Carbohydr Polym 92:328–333
Teli M, Valia SP (2013b) Acetylation of jute fiber to improve oil absorbency. Fibers Polym 14:915–919
Toyoda M, Inagaki M (2003) Sorption and recovery of heavy oils by using exfoliated graphite. Spill Sci Technol Bull 8:467–474
Tryba B, Morawski AW, Kalenczuk RJ, Inagaki M (2003) Exfoliated graphite as a new sorbent for removal of engine oils from wastewater. Spill Sci Technol Bull 8:569–571
Wang J, Wang A (2013) Acetylated modification of kapok fiber and application for oil absorption. Fibers Polym 14:1834–1840
Zahed MA, Aziz HA, Isa MH, Mohajeri L, Mohajeri S (2010) Optimal conditions for bioremediation of oily seawater. Bioresour Technol 101:9455–9460
Acknowledgments
The authors would like to thank Universiti Teknologi PETRONAS for providing the facilities and funding through the University Internal Research Funding (URIF Grant No 0153AA-B18) and Ministry of Higher Education (FRGS Grant No 0153AB-I09) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Santiago V. Luis
Rights and permissions
About this article
Cite this article
Asadpour, R., Sapari, N.B., Isa, M.H. et al. Acetylation of oil palm empty fruit bunch fiber as an adsorbent for removal of crude oil. Environ Sci Pollut Res 23, 11740–11750 (2016). https://doi.org/10.1007/s11356-016-6349-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6349-2