Abstract
Along the southeastern coast of Costa Rica, a variety of pesticides are intensively applied to produce export-quality plantains and bananas. In this region, and in other agricultural areas, fish kills are often documented by local residents and/or in the national news. This study examines principal exposure pathways, measured environmental concentrations, and selected toxicity thresholds of the three most prevalent pesticides (chlorpyrifos, terbufos, and difenoconazole) to construct a deterministic risk assessment for fish mortality. Comparisons of observed pesticide concentrations, along with estimated biological effects and observations during actual fish kills, highlight gaps in knowledge in correlating pesticide environmental concentration and toxicity in tropical environments. Observations of fish kill events and measured pesticide concentrations in the field, along with other water quality indicators, suggest that a number of environmental conditions can interact to cause fish mortality and that current species toxicity datasets may not be applicable for estimating toxicological or other synergistic effects, especially in tropical environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Central America, pesticide use has essentially doubled over the past 20 years, with imports to the region estimated to be 57.9 million kilograms of active ingredients (a.i.) in 2010 (de la Cruz et al. 2014; Wesseling et al. 2005). In Costa Rica, approximately 12,000 kg of pesticide active ingredients were imported in 2011 for agricultural use, the majority being fungicides, followed by herbicides and insecticides (Castillo et al. 2012). Although 11–25 % of these pesticides are re-exported, transportation accidents, in addition to pesticide runoff or drift from agricultural areas, has had negative impacts on human and environmental health in Costa Rica (de la Cruz et al. 2014; Kumar et al. 2013; Castillo et al. 2012, 2000; Echeverría-Sáenz et al. 2012; Wesseling et al. 2001). Over the past two decades, massive fish kills around agricultural areas have been documented by local residents, scientists, and in the national news (de la Cruz et al. 2014; Castillo et al. 2012).
In the remote Sixaola watershed of southeastern Costa Rica, pesticide fate and transport is a concern, as many area residents directly work with pesticides to maintain agricultural production on household or commercial banana and plantain farms, as well as rely on river and groundwater resources for consumption, fishing activities, and household use. Several environmental and human poisonings have been documented in banana plantations in Central America (Barraza et al. 2011; Wesseling et al. 2005, 2001; Castillo et al. 2000, 1997). However, no comprehensive studies regarding environmental distribution, toxic effects on aquatic organisms, and/or general impact on ecosystems or human populations have been undertaken in the remote Sixaola region of Costa Rica (Barraza et al. 2011; Fieten et al. 2009; Polidoro et al. 2008), where the primary source of income for local residents is generated by banana and plantain cultivation. In this region of Costa Rica, relatively few biological, geophysical, social, or economic data exist to guide conservation planning decisions, sustainable agricultural activities, or to develop best management practices (Polidoro 2007; Borge and Villalobos 1994; Kapp 1989).
Our objective was to compare field measurements of pesticide concentration and observations of potential biological effects in the Sixaola watershed, Costa Rica with available toxicity thresholds to provide a screening level deterministic risk assessment (USEPA 1998, 1992). In light of regularly observed fish kills and detection of pesticide residues in the Sixaola region, the benefits and limitations of a deterministic assessment using a hazard quotient approach are discussed, and alternative measures for assessing risk are proposed for similar tropical floodplain and coastal landscapes where comprehensive biophysical data are sparse.
Methods
Study site description
Located in southeastern Costa Rica, the 2,700 km2 Sixaola watershed is a tropical floodplain-coastal landscape characterized by a network of highland and floodplain rivers that flow from the 3,800-m Talamanca mountains through a mosaic of forests, pasture, and agricultural lands to the sea, where near and offshore coral reefs are found. The floodplain soils of the Sixaola River and its tributaries are important areas for the production of export-quality plantain and banana (Polidoro et al. 2008). Farms located on the Sixaola River floodplains produce 52 % of the plantain, 90 % of the organic banana, and 6 % of the commercial banana in Costa Rica (Municipality of Talamanca 2003). It is estimated that 150 km2 or approximately 30 % of the entire Sixaola floodplain is cultivated with banana or plantain. Between 15 and 65 kg of a.i. per hectare (ha) of pesticides and an average of 375 kg a.i./ha of fertilizers (Castillo et al. 2012; Polidoro et al. 2008) are estimated to be applied to industrialized commercial banana and plantain farms in the Sixaola River Valley. Average rainfall in the Sixaola watershed is approximately 3,000 mm/year with 2,700 mm of runoff (Grant et al. 2004) Annual temperatures average 25–27 °C with evapotranspiration reported to be 1,565–1,710 mm/year (Kapp 1989). In this region of Costa Rica, unregulated or poorly managed pesticide applications, the clearing of riparian vegetation, combined with high rainfall, runoff, and regular flooding events create a high risk for pesticide contamination of aquatic resources (Polidoro et al. 2008).
Primary routes of exposure to humans and aquatic wildlife, along with pesticide application rates, in the Sixaola watershed have been previously identified (Polidoro et al. 2008). In summary, a variety of fungicides, nematicides, insecticides, and herbicides are applied year-round in plantain and banana production. Although pesticide products are primarily the same in plantain and banana production, the frequency and quantity of agrochemicals applied in the Sixaola watershed vary based on agricultural intensity, ranging from less than 2 kg a.i./ha/year in low-intensity plantain production, from 3 to 9 kg a.i./ha/year in moderate plantain, from 10 to 15 kg a.i./ha/year in intensive plantain, and between 45 and 65 kg a.i./ha/year in intensive banana production (Castillo et al. 2012; Polidoro et al. 2008). The fungicide, difenoconazole, the nematicide, terbufos, and the insecticide, chlorpyrifos, are the most widely used products in their pesticide class and combined represent approximately 75 % of pesticide loading in the Sixaola watershed (Polidoro et al. 2008, Polidoro 2007), although each is with a different application rate and method. Primary routes of pesticide exposure that pose a threat to both human and ecological health stem from a lack of regulatory infrastructure as well as the absence of appropriate application knowledge and safe-handling practices. Principal aquatic exposure routes include spray drift and direct spray from aerial application of fungicides, runoff from packing plants, runoff and leaching during storm events, and direct entry of pesticides or waste products due to accidents during transport or storage or the unavailability of waste disposal facilities (Polidoro et al. 2008). Many of these routes of exposure are similar to those reported in other parts of the world where farmers in poor agricultural areas are applying pesticides without sufficient knowledge or infrastructure (Dinham and Malik 2003; Mekonnen and Agonafir 2002; Aragon et al. 2001).
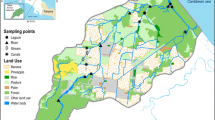
Seven sampling sites (Fig. 1) were chosen across the Sixaola watershed: five streams or rivers (Sixaola, Cana, Margarita, Sandbox, Shirolita), one coastal estuary (Gondoca), and a combination well and rain-fed drinking water source in the town of Sixaola. For comparison, data were also collected in an 8th site, representing intensive banana production in the adjacent Estrella watershed. The Estrella site is an oxbow lake (Aviarios Rescate) with little or no flow that is connected to the large Estrella River. The Sixaola and Margarita are upstream and downstream sections of the large Sixaola River (average annual flow 232 m3/s). The Cana is a medium-size river that drains into the Sixaola River and has been highly altered to serve as a principal drainage canal for a large banana plantation. The Shirolita and Sandbox are smaller streams located in upstream plantain growing areas. The Gondoca estuary is a coastal lagoon in the Gondoca-Manzanillo National Wildlife Refuge.
Water and passive sampling
Sampling consisted of water “grab” samples and deployment of passive samplers, known as semi-permeable membrane devices (SPMDs). Each site was sampled at least 8 times over an 18-month period (approximately every 2 months). All SPMD deployments and retrievals and water grab samples were accompanied by one to two field or trip blanks. Water “grab” sampling consisted of collecting 2 L of surface water in sterilized glass jars from a 10–30-cm depth, which were immediately put on ice and extracted within 24 h at the Institute for Studies of Toxic Substances (IRET) at the National University of Costa Rica in Heredia. Water grab sampling occurred during two “fish kill” events, where several species of fish and invertebrates were already dead or in the process of dying during sampling. Measurements of stream discharge, temperature, pH, dissolved oxygen, conductivity, and salinity were made during every sampling event. Stream discharge was calculated by multiplying average stream velocity by stream width and average depth. Stream velocity was measured by averaging flow meter (Ben Meadows, Janesville, WI, USA) readings at equal depths at three different points in the stream, while stream width was measured with a measuring tape when possible in smaller rivers or with a laser view finder in the case of the Sixaola River. Average stream depth measurements were made by first creating an integrated depth profile of the stream bottom, then choosing a daily reference point to ascertain regular changes in average depth. For larger rivers, such as the Sixaola River, a pre-installed depth chart on the side of the Panama-Costa Rica Bridge was used for average depth. A laboratory calibrated multimeter (YSI 556 Multiprobe, Yellow Springs, OH, USA) was used to record dissolved oxygen, pH, conductivity, salinity, and temperature during sampling.
Passive samplers, or SPMDs, have been used effectively to screen for the presence or absence of environmental contaminants, to monitor the spatial distribution and source of pollutants, as well as to provide a time-weighted average of aqueous concentrations and estimation of organism exposure (Vrana et al. 2005). Based on their construction, SPMDs effectively sequester compounds with octanol/water partition coefficients (K ow) > 1,000 (Huckins et al. 1993) and with molecular masses less than 600 (Petty et al. 2000). This solute size limitation samples only the dissolved and vapor phase of hydrophobic contaminants, and likely excludes large molecules as well as those that are adsorbed on colloids or humic acids (Vrana et al. 2005; Vrana and Schuurmann 2002; Petty et al. 2000) while mimicking the transfer of biomolecules through biomembranes (Vrana and Schuurmann 2002). In addition to providing an estimate of the potentially bioavailable contaminants in the region, the longer exposure time in SPMD field deployments can allow for the detection of lower concentrations of contaminants compared to routine grab samples.
Replicate SPMDs (two standard 91.4-cm SPMD membranes from Environmental Sampling Technologies) were placed in all six of the surface water sites in the Sixaola watershed for a period of 28 days. Two of the most highly pesticide impacted sites, Sixaola and Cana, were sampled more intensively (11 additional water samples and 3 additional months of SPMD deployments), as they became sites for SPMD experiments to estimate the effect of biofouling and time on SPMD uptake of pesticides (Polidoro et al. 2009). After field deployment, SPMDs were kept frozen until analysis at the Institute for Studies of Toxic Substances (IRET) at the National University of Costa Rica in Heredia.
Pesticide residue extraction and quantification
For each 2-L water sample, 1 L was extracted for HPLC analyses and the other liter for GC analyses. One liter water samples were extracted with SPE cartridges (Isolute ENV+ 200 mg/6 mL) conditioned with ethyl acetate, water, and methanol. For HPLC-PDA analysis, cartridges were eluted with methanol and concentrated to a final volume of 1 mL. For GC-ECD, FPD, and GC-MS analysis, cartridges were eluted with methylene chloride and acetone, the extract dried with sodium sulfate, and concentrated to approximately 0.1 mL with nitrogen gas. Acetone:cyclohexane (1:9) was added to achieve a final extract volume of 1 mL.
Before analysis, each SPMD was weighed before and after surface cleaning to calculate the weight of the biofilm as an estimate of biofouling and to calibrate uptake rates (Polidoro et al. 2009). SPMDs were dialyzed in hexane for 24 h, then again for 8 h. Dialyzed extracts were cleaned-up with gel permeation chromatography (GPC). SPMD extracts and blanks were analyzed with GC-ECD and FPD, with compound confirmation by GC-MS. All samples, including trip, lab, and storage blanks, were analyzed for 29 current-use pesticides (including three metabolites) in Costa Rica, with detection limits and log K ow values reported in Polidoro et al. (2009).
Pesticide residues sequestered in SPMD membranes were used to calculate time-weighted average concentrations in streams where they were deployed based on the methods described in Polidoro et al. (2009). Due to excellent precision among replicate membranes (±0.02 μg/mL extract), similar biofouling rates on membranes throughout the study period, and similar K ow values among the three pesticides (chlorpyrifos, difenoconazole, and terbufos) primarily sequestered in the SPMDs (log K ow of 4.9, 4.3, and 4.4, respectively), a generic uptake value of 5.0 L/day was chosen to estimate time-weighted average pesticide concentrations over the 28-day deployments (Polidoro et al. 2009).
Estimation of biological effect and hazard quotient
Deterministic risk assessments can calculate risk using a hazard quotient. In this approach, risk is determined by dividing the peak or average contaminant environmental concentration by a selected threshold of acute or chronic toxicity to represent the estimated biological effect (Van den Brink et al. 2003; Burns 2001; Versteeg et al. 1999). To estimate the toxicological effects of chlorpyrifos, difenoconazole, and terbufos, available literature (e.g., Giesy and Solomon 2014) and the US EPAs Ecotox database (EPA 2014) were searched for the lowest acute and chronic toxicity values for daphnia, crustaceans, and fish. For acute toxicity, only 96-h LC50 values were considered. For chronic toxicity, only maximum allowable toxic concentration (MATC) values were selected in order to standardize the various measures of chronic toxicity (i.e., growth, reproduction, enzyme activity, etc.), test length, and organism life stage. It is important to note that toxicity data available for chlorpyrifos far outnumber those available for most current-use pesticides. In the comprehensive online Pesticide Action Network Database (PAN 2014), over 3,962 aquatic acute and chronic toxicity records are listed for chlorpyrifos compared to 203 toxicity studies listed for terbufos and only 12 toxicity studies for difenoconazole. No toxicity data were found specifically for relevant tropical species. In calculation of the hazard quotient during a fish kill event, toxicity values were multiplied by a factor of 0.01 for acute toxicity and of 0.1 for chronic toxicity to account for uncertainties in extrapolation of data from one species to another and as an uncertainty factor (Kwok et al. 2007; Van den Brink et al. 2003). After application of the safety factors, a simple tier I hazard quotient (US EPA 1998) was calculated by dividing the maximum environmental concentration measured in grab samples and in SPMDs by the lowest acute toxicity values for acute risk quotient and the lowest chronic toxicity values for chronic risk quotient.
Results and discussion
Environmental exposure
Maximum pesticide concentrations detected in water grab samples and SPMDs are shown in Figs. 2 and 3. The most commonly found pesticides across all samples were difenoconazole and diuron in water grab samples and chlorpyrifos, difenoconazole, and terbufos in SPMD samples. In general, detection limits for analyses of water grab samples were well above the concentrations of chlorpyrifos and terbufos estimated by sequestration in SPMDs; but, unlike water grab samples, SPMDs are unable to capture “peak” concentrations as they can only provide an average concentration over time. All sites where SPMDs were deployed detected chlorpyrifos in concentrations calculated as 0.2 pg/L or greater. The majority of all pesticide detections were in the Cana, a stream that primarily drains a large banana plantation. At this site, the highest concentration pesticide observed was difenoconazole at 6 μg/L. The highest chlorpyrifos and terbufos concentrations calculated from SPMDs were 3.3 and 1.3 pg/L, respectively. Based on the study stream characteristics, study sites that had the most frequent detections and highest pesticide concentrations were smaller streams or canals adjacent to intensive banana plantations with little or no discharge or flow velocity (i.e., Cana in Sixaola watershed and the oxbow lake in Estrella).
Both chlorpyrifos and terbufos have been detected in streams, lagoons, and packing plant effluents adjacent to banana plantations in other areas of Central America in maximum concentrations of approximately 0.1 μg/L (Carvalho et al. 2002; Castillo et al. 2000). In other studies of current use pesticides in Central America, chlorpyrifos and terbufos have primarily been quantified in stream or marine sediments at concentrations as high as 320 and 154 μg/kg, respectively (Castillo et al. 2000; Albarca and Ruepert 1992; Readman et al. 1992). As difenoconazole is a relatively new fungicide that has primarily replaced propiconazole in banana production over the past 10 years, no studies to date have reported detection of difenoconazole in surface waters or sediments.
Difenoconazole is a systemic fungicide that is applied by airplane in intensive banana or motorized hand sprayer in plantain. In intensive banana and intensive plantain, it is often applied in combination with other fungicides, such as mancozeb, chlorothalonil, bitertanol, or tridemorph, to combat against black sigatoka disease (Mycosphaerella fijiensis; Marin et al. 2003; Matlock and de la Cruz 2002). Difenoconazole was detected in 75 % of water samples from the Cana with an average concentration of 0.5 μg/L and 20 % of water samples in the Shirolita with an average concentration of 0.3 μg/L. In this area, spray drift from aerial application of difenoconazole may be locally entering stream resources from either direct application or from movement with soil during flooding or high runoff events. In Costa Rican banana production regions, it has been estimated that in addition to direct spray, 15 % of aerially applied fungicides are estimated to be lost to wind drift, 40 % to falling on soil, and 35 % washed off by rain, resulting in a 90 % loss of formulated fungicides annually (Hernandez and Witter 1996). Mixed fungicide applications can occur as much as every 8 days all year in commercial banana plantations in the Sixaola region (Polidoro et al. 2008). Although few studies on the environmental behavior of difenoconazole exist (Huan et al. 2013; Wang et al. 2012), difenoconazole has a relatively short half-life in water (<1 day) and in air, with longer stability in soils (IRET 1999).
Terbufos is a granular nematicide with insecticidal activity that is applied to the soil around each banana or plantain plant both during and after seeding approximately three times a year in commercial banana production to protect against nematodes and the banana weevil (Cosmopolites sordidus Germar; Polidoro et al. 2008). Terbufos likely enters into aquatic resources with soil runoff during high rain and flooding events. In water resources, terbufos rapidly degrades through photolysis with half-lives reported as low as 1.2 day and through hydrolysis with half-lives reported of 2.2 weeks. The relatively short half-lives reported in temperate studies, combined with the fact that terbufos is only applied to the soil three times a year, may account for the very small amount of terbufos sequestered in SPMD stream samples.
Chlorpyrifos is the most widely used agrochemical in banana and plantain cultivation as it is impregnated into plastic bags that cover all maturing fruit in the region to prevent cosmetic damage caused by the thrips insect Chaetanaphothrips signipennis. Every banana or plantain plant is covered by the 40-g plastic bag impregnated with approximately 0.4 g chlorpyrifos at least once during the approximately 11- to 12-month crop cycle (Hernandez et al. 2000; IRET 2000). Volatilization and photolysis are likely the primary pathways of chlorpyrifos degradation due to its unique application method and exposure to high temperatures in tropical regions. A half-life of 2.6 days was reported in laboratory studies of chlorpyrifos photodegredation in moist air with 60 % relative humidity and a temperature of 25 °C (Fontaine and Teeter 1987 in Racke 1993). In the Sixaola watershed, chlorpyrifos likely enters aquatic resources from volatilization and redeposition during rain events, from chlorpyrifos sorbed to soils and sediments that are mobilized during erosional events, or from direct deposition when the impregnated plastic bags are blown or washed into streams during storms. Once in water resources, chlorpyrifos can be somewhat persistent if partitioned into soils or sediments. In freshwater systems at 25 °C and a pH 7.0, chlorpyrifos that is not partitioned into sediments or biota is primarily degraded through hydrolysis with reported half-lives ranging from 35 to 78 days (Howard 1991).
Biological Effect and Hazard Quotient
The lowest found acute and chronic toxicity values for chlorpyrifos, difenoconazole, and terbufos are shown in Table 1. All hazard-risk quotients calculated from maximum pesticide concentrations (both water grab samples and SPMDs) detected throughout the study period were less than 1 (e.g., environmental concentration < toxicity threshold), with the exception of chronic toxicity of chlorpyrifos in the Cana and Sixaola Drinking Water (0.025 μg/L in water grab samples > 0.004 μg/L lowest chronic value) and chronic toxicity of difenoconazole in the Cana (6 μg/L in water grab samples > 5.6 μg/L lowest chronic value).
Two fish kill events were observed during stream sampling in the Cana stream site during the study period. Primary fish species that had high mortality were Macrobrachium spp. (Palemonidae), Gobiomorus dormitor (Eleotridae), Centropomus spp. (Centropomidae), and Pomadasys crocro (Haemulidae). However, even though chlorpyrifos, terbufos, and difenoconazole were detected in SPMDs and water samples during the observed fish kill events, none of the measured concentrations provided a chronic or acute risk quotient above 1 (Table 2, Fig. 4), even with safety factors applied, indicating that measured water concentrations did not exceed the lowest published acute or chronic toxicity values for any species. In general, the fish kills were observed during periods of sun and high temperatures, 2 or 3 days after large rain events in drier months. It is theorized that pesticide residues “built up” on soils and plants during weeks of applications in dry periods and moved in to aquatic resources during a large rain event, which if followed by 1 or 2 days of high temperatures and no rain, would concentrate in water resources as stream flow rapidly decreased. As measured pesticide concentrations were below toxicity thresholds, it is difficult to provide evidence that fish mortality was directly caused by the pesticides found.
As several fill kills every year are regularly observed at this site, and many environmental and health organizations have recorded both human and environmental poisonings from pesticide applications in adjacent banana plantations (Polidoro et al. 2008), a combination of stressful environmental conditions and pesticide mixtures is suspected. During the observed fish kills, stream temperatures were relatively high, dissolved oxygen concentrations were low, and stream flow velocity was negligible (Table 2), all of which are conditions that can contribute to fish mortality (Alabaster and Lloyd 2013).
In tropical climates, pesticide degradation is more rapid, but pesticide solubility, rate of oxygen depletion, biological uptake, and impact of nutrients may be higher, which can result in lower toxicity thresholds or no effect concentrations (Henriques et al. 1997). For example, Mayer et al. (1994) showed that organophosphate toxicity thresholds are lowered by increases in temperature and suggested that the 96-h log LC50 value for organophosphate pesticides be lowered by a factor of 0.7 and all other chemicals by a factor of 0.5 for every 10 °C increase in temperature. These conditions in combination with excess nutrient loading from agricultural fertilizers can contribute to stressful or eutrophic conditions that can cause fish mortality, as well as can lower toxicity thresholds for aquatic organisms (Traas et al. 2004; Gunnarson et al. 1995). Although high concentrations of dissolved organic matter, humic acids, or suspended sediments can be linked to a decrease in pesticide bioavailability (Warren et al. 2003), increased pesticide bioavailability in the presence of increased organic matter or humic acids has also been reported under different environmental conditions such as increased salinity or for different life stages of aquatic organisms (Mezin and Hale 2004; Phillips et al. 2003). Finally, it is possible that other pesticides were present and were undetected. Very little is known about the combined effects of pesticide mixtures on toxicological thresholds or the synergistic effects between pesticides and other natural ecological stressors such as changes in predator–prey relationships or limited food resources (Raylea and Hoverman 2006; Preston 2002). In sum, better information on toxicity thresholds for tropical species under tropical conditions is needed to better understand these dynamics.
Conclusion
The fate and toxicity of pesticides in tropical ecosystems is not as well understood as in temperate regions (Damm and Van den Brink 2010), as the bulk of pesticide fate and toxicology research has been conducted in temperate zones. Thus, the applicability of temperate chemical behavior and surrogate single-species toxicity tests for use in tropical ecological risk assessments can be problematic. Aquatic resources in the Sixaola region do not appear to be under high risk of fish mortality based on current pesticide application rates and a simple hazard quotient approach. However, chronic toxicity of low, but frequently detected, concentrations of chlorpyrifos and difenoconazole in the Cana and in Sixaola drinking water may warrant further research. Single-species toxicity data derived from temperate toxicity datasets may not be representative of chronic or acute toxicological responses of tropical species. Especially in light of other factors that may be lowering toxicity thresholds, including “stressful” environmental conditions such as low dissolved oxygen or high temperatures, pesticide mixtures, excess nutrients, and synergistic effects related to intricate tropical food webs and complex species interactions.
However, given the vast complexity of environmental conditions and species toxicological responses, a single-species or even site-specific approach can be very costly, inefficient, and time-consuming to conduct experimentally. Where relatively few resources and data exist, alternate risk assessment methods that incorporate trait-based assessments for complete clades of species, and/or biodiversity impact indices at the site or landscape level may provide a more feasible and protective approach. Trait-based assessments that use phenotypic or ecological characters of a species to explain or predict variation in ecological systems are a new frontier in both ecological risk assessment and in bioassessment and biomonitoring of aquatic ecosystems (Van den Brink et al. 2011), and may provide a more viable alternative to single-species toxicity testing and taxonomically-based approaches to determine chemical risk to species and ecosystems.
References
Alabaster JS, Lloyd RS (2013) Water Quality Criteria for Freshwater Fish, No. 3117. Elsevier.
Albarca L, Ruepert C (1992) Plaguicidas encontrados en el valle de la estrella: estudio preliminar. Tecnología en Marcha 12:31–38
Aragon A, Aragon C, Thorn A (2001) Pests, peasants, and pesticides on the Northern Nicaraguan. Pacific Plain. Int J Occup Environ Health 7:295–302
Barraza D, Jansen K, van Wendel de Joode B (2011) Pesticide use in banana and plaintain production and risk perception among local actors in Talamanca, Costa Rica. Environ Res 111:708–717
Borge C, Villalobos V (1994) Talamanca en la Encrucijada. EUNED.
Burns LA (2001) Probabilistic aquatic exposure assessment for pesticides 1. Foundations. National Exposure Research Laboratory USEPA.
Carvalho FP, Villeneuve JP, Cattini C et al (2002) Ecological risk assessment of pesticide residues in coastal lagoons of Nicaragua. J Environ Monit 4:778–787
Castillo LE, de la Cruz E, Ruepert C (1997) Ecotoxicology and pesticides in tropical aquatic ecosystems of Central America. Environ Toxicol Chem 16:41–51
Castillo LE, Ruepert C, Solis E (2000) Pesticide residues in the aquatic environment of banana plantation areas in the north Atlantic zone of Costa Rica. Environ Toxicol Chem 19:1942–1950
Castillo L, Ruepert C, Ramírez F, VanWendel de Joode B, Bravo V, de la Cruz E (2012) Plaguicidas y otros contaminantes. Ponencia preparada para el Decimoctavo Informe Estado de la Nación. Programa Estado de la Nación, San José
Damm MA, Van den Brink PJ (2010) Implications of differences between temperate and tropical freshwater ecosystems for the ecological risk assessment of pesticides. Ecotoxicology 19:24–37
de la Cruz E, Bravo-Durán V, Ramírez F, Castillo LE (2014) Environmental hazards associated with pesticide import into Costa Rica, 1977-2009. J Environ Biol 35:43–55
Dinham B, Malik S (2003) Pesticides and human rights. Int J Occup Environ Health 9:40–52
Echeverría-Sáenz S, Mena F, Pinnock M et al (2012) Environmental hazards of pesticides from pineapple crop production in the Río Jiménez watershed (Caribbean Coast, Costa Rica). Science Total Environ 440:106–114
Fieten KB, Kromhout H, Heederik D, de Joode BVW (2009) Pesticide exposure and respiratory health of indigenous women in Costa Rica. Amer J Epidem 169:1500–1506
Giesy JP, Solomon KR (2014) Ecological risk assessment for chlorpyrifos in terrestrial and aquatic systems in the United States. Rev Environ Contam Toxicol 231: 219–265.
Grant A, Oreamuno R, Serrano A et al. (2004) Inundaciones en la Vertiente Altantica. Informe de Colegio Federado de Ingenieros y de Arquitectos de Costa Rica, San Jose, Costa Rica, May 2004.
Gunnarson J, Broman D, Jonsson P et al (1995) Interactions between eutrophication and contaminants: towards a new research concept for the European aquatic environment. Ambio 24:383–385
Henriques W, Jeffers RD, Lacher TE, Kendall RJ (1997) Agrochemical use on banana plantations in Latin America: perspectives on ecological risk. Environ Toxicol Chem 16:91–99
Hernandez CE, Witter SG (1996) Evaluating and managing the environmental impact of banana production in Costa Rica: a systems approach. Ambio 25:171–178
Hernandez C, Witter SG, Hall CAS et al. (2000) The Costa Rican banana industry: can it be sustainable? pp. 563-593 In Hall CAS [Ed.], Quantifying Sustainable Development. Academic Presss
Howard PH (1991) Ed. Handbook of environmental fate and exposure data for organic chemicals. Vol 3: pesticides. Lewis Publishers, Chelsea, MI, pp 5–13
Huan Z, Xu Z, Lv D, Xie D, Luo J (2013) Dissipation and residues of difenoconazole and azoxystrobin in bananas and soil in two agro-climatic zones of China. Bull Environ Contam Toxicol 91:734–738
Huckins JN, Manuweera GK, Petty JD et al (1993) Lipid-containing semipermeable membrane devices for monitoring organic contaminants in water. Environ Sci Tech 27:2489–2496
IRET (Regional Institute for Studies of Toxic Substances (1999) Manual de Plaguicidas: Guia para America Central, 2nd Ed. EUNA, Heredia, Costa Rica.
IRET (Regional Institute for Studies of Toxic Substances) (2000) Reducción del escurrimiento de plaguicidas al mar caribe: Informe Nacional: Costa Rica GEF/11000-99-04/PNUMA. Dec. 2000.
Kapp GB (1989) Perfil ambiental de la zona Baja de Talamanca, Costa Rica. Turrialba, Costa Rica CATIE. 97 p. (Informe Técnico No.155).
Kumar A, Colton MBM, Springer M, Trama FA (2013) Macroinvertebrate communities as bioindicators of water quality in conventional and organic irrigated rice fields in Guanacaste, Costa Rica. Ecol Indic 29:68–78
Kwok KWH, Lueng KMY, Lui GSG et al (2007) Comparison of tropical and temperate freshwater animal species’ acute sensitivities to chemicals: implications for deriving safe extrapolation factors. Integr Environ Assess Manage 3:49–67
Marin DH, Romero RA, Guzman M et al (2003) Black Sigatoka: an increasing threat to banana cultivation. Plant Dis 87:208–222
Matlock RB, de la Cruz R (2002) An inventory of parasitic Hymenoptera in banana plantations under two pesticide regimes. Agri Ecosyst Environ 93:147–164
Mayer FL, Marking LL, Bills TD et al (1994) Physiochemical factors affecting toxicity in freshwater: hardness, pH and temperature. In: Hamelink JL, Landrum PF, Bergman HL, Benson WH (eds) Bioavailability: physical, chemical and biological interactions. CRC Press, Boca Raton, pp 5–22
Mekonnen Y, Agonafir T (2002) Pesticide sprayers’ knowledge, attitude and practice of pesticide use on agricultural farms of Ethiopia. Occup Med 52:311–315
Mezin LC, Hale RC (2004) The effect of humic acids on toxicity of DDT and chlorpyrifos to freshwater and estuarine invertebrates. Environ Toxicol Chem 23:583–590
Municipality of Talamanca (2003) Plan local de desarrollo: 2003:2013. Municipality of Talamanca, Bribri, Costa Rica.
PAN (Pesticide Action Network Database) (2014) http://www.pesticideinfo.org. Accessed December 2014
Petty JD, Orazio CE, Huckins JN et al (2000) Considerations involved with the use of semipermeable membrane devices for monitoring of environmental contaminants. J Chrom A 879:83–95
Phillips TA, Summerfelt RC, Wu J et al (2003) Toxicity of chlorpyrifos adsorbed on humic colloids to larval walley (Stizostedion vitreum). Arch Environ Contam Toxicol 45:258–263
Polidoro BA (2007). Ecological risk assessment of current-use pesticides in the Sixaola Watershed, Costa Rica. Doctoral dissertation, University of Idaho.
Polidoro BA, Dahlquist R, Castillo LE et al (2008) Pesticide application practices, pest knowledge, and cost-benefits of plantain production in the Bribri-Cabecar Indigenous Territories, Costa Rica. Environ Res 108:98–106
Polidoro BA, Morra MJ, Ruepert CR et al (2009) Pesticide sequestration in passive samplers (SPMDs): considerations for deployment time, biofouling, and stream flow in a tropical watershed. J Environ Monit 11:1866–1874
Preston BL (2002) Indirect effects in aquatic ecotoxicology: implications for ecological risk assessment. Environ Manage 29:311–323
Racke KD (1993) Environmental fate of chlorpyrifos. Rev Environ Contamin Toxicol 131:1–154
Raylea R, Hoverman J (2006) Assessing the ecology in ecotoxicology: a review and synthesis in freshwater systems. Ecol Let 9:1157–1171
Readman JW, Kwong LLW, Mee LD et al (1992) Persistent organophosphorus pesticides in tropical marine environments. Mar Poll Bull 21:398–402
Traas TP, Janse JH, van den Brink PJ et al (2004) A freshwater food web model for the combined effects of nutrients and insecticide stress and subsequent recovery. Environ Toxicol Chem 23:521–529
US EPA (2014) Ecotox Database. http://cfpub.epa.gov/ecotox/, accessed December 2014.
USEPA (US Environmental Protection Agency) (1992) Framework for ecological risk assessment EPA/630/R-92/001. US Environmental Protection Agency, Washington, D.C, USA
USEPA (US Environmental Protection Agency) (1998) Guidelines for ecological risk assessment. US Environmental Protection Agency, Washington, D.C., USA
Van den Brink PJ, Sureshkumar SN, Daam MA et al. (2003) Environmental and human risks of pesticide use in Thailand and Sri Lanka. Alterra-rapport 789, MAMAS Report Series No. 3/2003. Alterra, Green World Research, Wageningen, Netherlands.
Van den Brink PJ, Alexander AC, Desrosiers M et al (2011) Traits-based approaches in bioassessment and ecological risk assessment: strengths, weaknesses, opportunities and threats. Integrated Environ Assess Manage 7(2):198–208
Versteeg D, Belanger S, Carr G (1999) Understanding single-species and model ecosystem sensitivity: data-based comparison. Environ Toxicol Chem 18:1329–1346
Vrana B, Schuurmann G (2002) Calibrating the uptake kinetics of semipermeable membrane devices in water: impact of hydrodynamics. Environ Sci Tech 36:290–295
Vrana B, Mills GA, Allan IJ et al (2005) Passive sampling techniques for monitoring pollutants in water. Trends Anal Chem 24:845–868
Wang K, Wu JX, Zhang HY (2012) Dissipation of difenoconazole in rice, paddy soil, and paddy water under field conditions. Ecotoxicol Environ Safety 86:111–115
Warren N, Allan IJ, Carter JE et al (2003) Pesticides and other micro-organic contaminants in freshwater sedimentary environments—a review. App Geochem 18:159–194
Wesseling C, van Wendel de Joode B, Monge P (2001) Pesticide related illness among banana workers in Costa Rica: a comparison between 1993 and 1996. Int J Occup Environ Health 7:90–97
Wesseling C, Corriols M, Bravo V (2005) Acute pesticide poisoning and pesticide registration in Central America. Toxicol Appl Pharmacol 207:S697–S705
Acknowledgments
This work was funded in part by NSF-IGERT Grant No. 0114304 and by NSF-EPSCoR. We thank the faculty and staff at the Regional Institute for Studies of Toxic Substances (IRET) at Universidad Nacional in Heredia, especially Clemens Ruepert and Luisa Castillo. We thank Thomas Cahill for his review and comments on the manuscript. We also thank the communities of Sixaola, Margarita, Bribri, and Shiroles for their community-based monitoring and dedication to the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Hutchinson
Rights and permissions
About this article
Cite this article
Polidoro, B.A., Morra, M.J. An ecological risk assessment of pesticides and fish kills in the Sixaola watershed, Costa Rica. Environ Sci Pollut Res 23, 5983–5991 (2016). https://doi.org/10.1007/s11356-016-6144-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6144-0