Abstract
China’s natural waters are experiencing an increasingly anthropogenic perturbation widely including acidification and hypoxia, and toxic metals including copper (Cu) are subject to a series of reactions including chemical speciation and transformation. However, there is still little information available regarding such alterations of metal behaviors in China’s natural waters. By using solid phase extraction technique, this study for the first time measured total dissolved Cu, and different Cu species: toxic labile Cu (referred to those free cupric ions and some weakly organic compounds adsorbed onto Chelex-100 resins), the organic refractory Cu (referred to those adsorbed onto C18 resins after passing through Chelex-100 resins), and residual Cu (obtained by subtracting labile and organic refractory fractions from the total) in a freshwater lake (the Lover) and a saltwater lagoon (the Yundang) in Xiamen, China. Our results demonstrated that both waters were characterized with relatively low levels of total dissolved Cu (5–10 nM), as a result of a net removal process dominated by particle adsorption and precipitation. Relatively high proportion of organic refractory Cu (as high as 50 %) was observed in the saltwater Yundang lagoon as a result of organic matter production and/or discharges followed by complexation nearby. On the other hand, the toxic labile Cu accounted for >40 % of the total dissolved Cu pool in these waters, and particularly the increased proportion of toxic labile Cu (as high as 70 %) occurred in the bottom sulfidic Lover Lake. Our study provides clear evidence that toxic labile Cu could be transformed under reducing environments such as deep sulfidic waters of the Lover Lake (Xiamen, China), and the releases of toxic labile metals are increasingly threatening nearby aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper (Cu) is commonly used as a pure metal or alloy in industrial activities and daily life around the world, and Cu compounds are also widely applied in agriculture or aquaculture as nutritional supplements or fungicides. Therefore, land runoff or direct sewage and industrial effluents could easily carry a large amount of Cu into nearby waters. Over the past decades, metal contaminants including Cu have been increasingly released into natural waters around the world, e.g., in the Long Island Sound (Beck and Sañudo-Wilhelmy 2007), and in the Pearl River estuary (Wang et al., 2012), in the South San Francisco Bay (Buck and Bruland 2005), in the Seine Estuary (Chiffoleau et al. 1994), and in the Scheldt Estuary (Paucot and Wollast 1997). Although a small amount of Cu is essential in algal metabolisms including photosynthesis (Morel and Price 2003; Peers and Price 2006) and high affinity iron uptake (Maldonado et al. 2006; Kustka et al. 2007), dissolved Cu beyond the tolerance levels could exert toxic effects to algal growth as in photosynthetic reaction and cellular division (Cid et al. 1995). In particular, those free cupric ions of as low as 10−11 mol/L might be toxic to certain phytoplankton species, such as cyanobacteria (Kozelka and Bruland 1998; Muller, 1996; Bruland et al. 1991).
In natural waters, dissolved metals are subject to a series of geochemical reactions, including organic complexation, particle adsorption, and precipitation (e.g., Tubbing et al. 1994), which further alter the metals’ availability and toxicity to aquatic systems (Kozelka and Bruland 1998; Brooks et al. 2008). On the other hand, these geochemical reactions are also subject to human perturbation such as subtle the temperature rise, and redox decreases in coastal waters, leading to more releases of toxic labile Cu from sediment porewaters into coastal waters (Beck and Sañudo-Wilhelmy 2007). Over the past decades, China has experienced a rapid economic development (Wang et al. 2014). Particularly, anthropogenic metal contaminants are increasingly emitted into nearby soils, aerosols, and sediments (Cheng 2003; Pan and Wang 2012). Eventually, a large amount of these metals reached nearby waters via land runoff, direct sewage, and industrial effluents. For example, the total discharges of metals (Cu, Pb, Zn, Cd, Hg, and As) into China’s coastal waters reached the level of as high as 27,000 tons in 2012, among which Cu was 3700 tons (from China Oceanic Information Network, http://www.coi.gov.cn/). However, previous studies have mostly focused on those highly human impacted metal-enriched sediments (e.g., Duan et al. 2014; Xu et al. 2014; Zhang et al. 2012; Feng et al. 2011). There are still limited data available regarding dissolved metals in China’s waters so far, e.g., in the rivers of Hainan (Fu et al. 2013), in the East China Sea (Li et al. 2014), in the Pearl River estuary (Wang et al. 2012), and in the Changjiang river (Yang et al., 2014; Shulkin and Zhang 2014), and even little published data are available regarding the chemical speciation and transformation of these metals such as Cu. As far as the authors know, there are only few articles examining the dissolved metal speciation in China (e.g., South China Sea, Wen et al. 2006; Danshuei River, Jiann et al. 2005).
This study selected two typical China’s urban waters: the Lover freshwater lake and the Yundang seawater lagoon in the Xiamen Island, South China, and examined the total dissolved Cu and different Cu species (toxic labile, organic refractory, and residual fractions) along with hydrological and chemical parameters. The objectives of the study were to determine the speciation and transformation of dissolved Cu in these typical human impacted waters, and to explore the geochemical responses of toxic metals to the recently increasing human perturbation in China.
Materials and methods
Study area
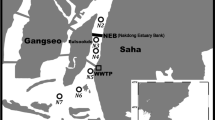
The Lover Lake is located in a mountainous valley in the southern part of the Xiamen Island, with an area of ~38,000 m2, and an average water depth of ~7.5 m and a 10-m deepest site (Fig. 1). The lake is surrounded with forests and receives freshwater from mountain streams, and influenced by human activities such as a floriculture greenhouse and a golf field nearby, and high land runoffs commonly occurred during heavy rain precipitation, characteristic of DOC concentrations of ~400 μmol/L. The surface water here appears brownish all the year around with high primary productivity (as indicated by high levels of Chl-a: 33–67 μg/L), and phytoplankton is dominated with the following species: Scenedesmus quadricanda, Crucigenia quadrata, Oscillatoria tenuissima, and Melosira granulate (Duan et al. 2011).
The Yundang Lake is located at the west side of the Xiamen Island (Fig. 1), with a surface area of 1.5 km2, and an average depth of 2–3 m. The lake is actually a saltwater lagoon, connecting with seawater in the Xiamen Bay via a gateway. The Yundang Lake is subject to a substantial amount of municipal sewage discharges from the city of Xiamen. Particularly, the city of Xiamen has experienced a rapid industrialization and economic development for more than two decades with a population of ~3.67 million in 2012 (Xiamen Municipal Bureau of Statistics 2013). Chl-a was ~4.5 μg/L in the lake during our sampling period (Chen 2012), with the following algal species dominated: Skeletonema costatum, and Euglena sp. (Xu 2005).
Sampling
Field investigations were conducted in the lakes of the Lover and the Yundang on June 23, 2011 and March 22, 2011, respectively (Fig. 1). Water samples in the Lover Lake were taken from a water column of 10 m deep via using a peristaltic pump and plastic tubing marked at 1.0 m intervals. Similarly, the water samples in the Yundang Lake were obtained from a water column of 1.2 m deep at 0.2 m intervals. Filtered samples were collected first via capsule filters (0.22 μm). All the samples were collected in the order of increasing depth, and the water sample from the preceding depth interval was purged at each new depth by flushing the lines with ambient water.
Hydro-chemical parameters such as temperature, salinity, pH, and DO were determined on site by using a WTW analyzer. A portion of filtered samples were fixed in 0.5 M Zn-acetate on site and analyzed for H2S in the laboratory using the methylene blue method (Cline 1969). Water samples for Chl-a were filtered under a pressure of less than 50 kPa, and extracted for 24 h in 90 % acetone in 0 °C, and the fluorescent values were finally measured in a Hitachi 850 Fluorospectrometer (Parsons et al. 1984).
Separation and measurements of different Cu species (toxic labile, organic refractory, and residual fractions)
Samples for dissolved Cu species were directly filtered on site into acid-washed PE bottles through acid-cleaned polypropylene capsule filter (0.22 μm). Once collected, the water samples were immediately returned to the laboratory. All the separation protocols were processed in a Class-1000 clean room laboratory at the State Key Laboratory of Marine and Environmental Science, Xiamen, China. The samples were processed within 6–8 h by using a two-column solid-phase extraction technique (resin Chelex-100 and C-18) under nitrogen condition adapted from Beck and Sañudo-Wilhelmy (2007).
Dissolved Cu in natural waters is generally composed of labile and refractory organically complexed and residual forms (e.g., Kozelka and Bruland 1998). The former (Chelex-adsorbed) refers to the “bioavailable” fraction including the free metal ions, inorganic complexes, and some small active organic molecules; the organic refractory (C18 adsorbed) mainly includes refractory organically bound complexes (Velasqueza et al. 2002; Waeles et al. 2004); and the last one refers to neutral organic molecules with little affinity with either Chelex-100 or C18 resins as defined as the residual fraction throughout the context. After extracted onto Chelex-100 and C-18 resins, Chelex-adsorbed metals (toxic labile) were eluted out with 10 mL 0.3 N HNO3, directly into 30 mL wide-mouth LDPE bottles. C-18 columns were eluted with 10 mL methanol (Optima grade) into acid-washed 30 mL vials (organic refractory). All the eluates of Chelex-100 and C-18 columns were placed under a Class-100 clean hood and evaporated to dryness at the temperature of 60 °C. The dry sample residues (residual) were then reconstituted in 2 mL 0.3 N HNO3 before analysis. All the samples were then analyzed on an Agillent ICPMS (State Key Laboratory of Marine Environmental Science, Xiamen University) by using standard addition techniques. The total dissolved Cu was obtained by summing all three fractions up. The detection limits for all the dissolved Cu species were estimated by three times of the standard deviation of triplicate procedural blanks with <0.1 nmol/L. The accuracy of the total dissolved metal determination was checked with the certified reference materials (river water SLRS-4 and coastal water CASS-4) with <5 % deviation.
Results and discussion
Hydro-chemical settings in the lakes
All the hydro-chemical parameters and the dissolved Cu speciation data in the two lakes are summarized in the Appendix Table 1. Our results showed that the freshwater Lover Lake was generally stratified throughout the water column, with a thermocline layer at 2.0–4.0 m during the sampling period (June 2011) (Fig. 2). The water temperature was 30 °C in the upper lake waters, and 23 °C in the deep lake waters. In addition, the upper water body was characterized with high oxygen and pH (<2.0 m O2 > 8.0 mg/L, pH >8.2) as exposed to atmospheric oxygen and with a high primary productivity (indicated by relatively high Chl-a: 33–67 μg/L), while the deep water body was characterized with extremely low oxygen and pH (4.0–9.0 m <0.3 mg/L, pH = 6.1–6.9). Particularly, high levels of sulfides (42–65 μmol/L) occurred in the deep waters (4.0 m until bottom) (Fig. 2) suggesting of a sulfidic setting there.
In contrast, the saltwater Yundang Lake was fully aerobic with high oxygen levels (5.2–07.1 mg/L) throughout the water column during our sampling period (March 2011) (Fig. 2), probably as influenced from the strong seawater intrusion from the Xiamen Bay. In addition, the Yundang Lake was characterized with high salinity (24–26), relatively low levels of Chl-a (4.5 μg/L, Xu 2005), slightly high pH (7.3–7.6), low temperature (15–16 °C), and low levels of H2S (undetectable to 0.4 μmol/L) throughout the whole water column.
Speciation of dissolved Cu
Our study showed that the concentrations of dissolved Cu were generally low in these urban lakes (4–10 nmol/L), and varied slightly in our study waters: 8.5 ± 2.2 nmol/L and 4.1 ± 0.5 nmol/L in the upper and deeper waters of the Lover Lake, respectively, and 9.1 ± 2.2 nmol/L in the Yundang Lake (Fig. 3). In general, dissolved Cu concentrations were lower than those in moderately human perturbed waters (Fig. 4), e.g., in the Mississippi delta (23 nmol/L, Shiller and Boyle 1991); in the Delaware River (37 nM, Church and Scudlark 1997); the Long Island Sound (10–40 nmol/L, Buck et al. 2005); the Danshuei River (25–60 nmol/L, Jiann et al. 2005); the Seine Estuary (10–60 nmol/L, Chiffoleau et al. 1994); the Scheldt Estuary (5–60 nmol/L, Paucot and Wollast 1997), the South San Francisco Bay (47 nM, Buck and Bruland 2005), and the Great South Bay, NY (30 nM, Beck et al. 2010).
Relative low concentrations of dissolved Cu in both Lover and Yundang waters could not simply be attributed to low inputs of anthropogenic inputs, particularly due to the fact that sewage discharges from the city of Xiamen were severely loaded into the Yundang Lake and nearby waters (e.g., Chen 2012, 2009; Zhou et al. 2010), and the sediments in the lake and nearby waters were heavily enriched with heavy metals such as Cu (Lin et al. 2009). In addition, high levels of pollutants also commonly occurred in the Lover Lake as a result of land runoff during heavy rains (Zhou et al. 2007), and both lakes were characterized with high levels of suspended sediments and organic materials (Wang et al. 2006; Lin et al. 2011). Indeed, riverine fluxes of particles were high in nearby waters, e.g., the sediment fluxes from the Jiulong River contributed a substantial amount of materials to coastal waters including the Xiamen Bay (244 × 104 tons/year, Cai et al. 1999). The Yundang Lake was characterized with high levels of total suspended matter (a few to 50 mg/L, Lin et al. 2011), and total suspended matter could also be as high as 14–16 mg/L in the Lover Lake (as observed in our study). High discharges of organic materials due to terrigenous runoff, sewage, and anthropogenic effluents also occurred in the nearby waters (e.g., ~21,000 tons CODMn/year, Wang et al. 2006). Therefore, metal contaminants were subject to particle adsorption and/or organic complexation in these water bodies, as suggested elsewhere previously (e.g., Davis 1984). Consistently, the coupling of comparatively low dissolved Cu levels with high sulfide in the deep sulfidic Lover Lake waters (sulfide 42–65 μmol/L; Cu 4.1 ± 0.5 nmol/L) suggested that Cu was mainly removed and precipitated as sulfides (e.g., CuS or Cu2S), and finally accumulated into sediments, as consistent with previous studies in the lake sediments (Lin et al. 2011).
Similar to the total dissolved Cu, different dissolved Cu species also behaved dynamically in these waters. Generally, organic refractory Cu ranged from 1.0 to 6.0 nmol/L, accounting for 30–50 % of the total dissolved Cu. Organic refractory Cu was also different in these waters: 2.9 ± 0.7 nmol/L, and 1.1 ± 0.2 nmol/L in the upper and deeper waters of the Lover Lake, and 4.1 ± 1.1 nmol/L in the Yundang Lake, respectively. These organic refractory Cu species was probably produced during organic discharges nearby, e.g., sewage effluents in the Yundang Lake, and land runoff in the Lover Lake, as consistent with previous reports in the Danshuei River estuary (e.g., Jiann et al. 2005). Residual Cu was mostly undetectable (<0.1 nmol/L) in the Lover Lake, and elevated concentrations of residual Cu occurred at the depth of 2.0 m (4.5 nmol/L) along with high TSM (12–16 mg/L). Residual Cu mainly occurred (as high as 2.1 nmol/L) near surface in the Yundang Lake and decreased downwards until undetectable. These residual Cu species might also be related to direct sewage discharges or land runoffs locally, and alternatively residual Cu could also be produced from organic materials via bacterial decomposition.
Toxic labile Cu was higher in the upper waters (4.0 ± 0.5 nmol/L) than in the deep waters of the Lover Lake (2.9 ± 0.6 nmol/L) and was 3.8 ± 1.2 nmol/L in the Yundang Lake (Fig. 4). As the dominant species in the Lover Lake, toxic labile Cu accounted for ~49 ± 13 and ~70 ± 7 % in the upper oxic and bottom sulfidic zones, respectively (Fig. 4) and ~40 % of total dissolved Cu in the Yundang Lake. Our results showed that the proportions of labile Cu were higher than those in the USA (<20 %), e.g., in the Great South Bay (Beck et al., 2010) and in the Long Island Sound (Beck and Sañudo-Wilhelmy 2007, Fig. 4).
Toxic labile Cu might contribute from direct sewage effluents, and upward diffusion from sediment porewaters. Alternatively, the higher proportion of toxic labile Cu in these China’s natural waters could also be partly attributable to the production from organic refractory Cu. For example, toxic labile Cu in the bottom waters might result from the reduction of organic materials in those sulfidic environments, while the photoreduction of organic refractory to labile Cu also occurred in the top surface waters as consistent with previous reports, e.g., in the low-latitude waters (Voelker et al., 2000). In addition, toxic labile Cu in bottom waters could diffuse upward from sediment porewaters into the overlying waters (e.g., Beck and Sañudo-Wilhelmy 2007).
Transformation of organic refractory to labile Cu and its possible mechanism
Dissolved metals, however, generally exist in natural waters as in several chemical species including labile, organic refractory, and residual fractions. These different chemical species behaved quite differently in terms of their particle affinity and reactivity (e.g., Turner et al. 1998; Charriau et al. 2011). For example, Charriau et al. (2011) reported that organic complexation might stabilize the metals in dissolved phases, while free metal ions could be readily precipitated with sulfides. Turner et al. (1998) also suggested that dissolved metals have low particle affinity once complexed with non-labile organic materials.
In addition to the direct sewage source, dissolved Cu undergoes a series of reactions including chemical speciation and transformation. These reactions could be largely dictated by environmental factors like temperature, pH and Eh, and even biogeochemical processes such as autochthonous organic matter production and remineralization (e.g., Zuo et al. 2012; Zhao et al. 2014; Tonietto et al. 2015). For example, highly reducing conditions favored the production of labile metals (e.g., Beck and Sañudo-Wilhelmy 2007), and high temperature conditions also trigger upward releases of labile metals from porewaters (e.g., Beck and Sañudo-Wilhelmy 2007). The high proportion of labile Cu in our study waters along with in other China’s natural waters (Fig. 3) suggested of its production from organic refractory Cu, leading to a net accumulation of labile Cu in these sulfidic waters. On the other hand, toxic labile Cu is also subject to precipitate out of water column under the conditions of high sulfides (e.g., Charriau et al. 2011), and via high affinity with particles (e.g., Turner et al. 1998). Under those strongly sulfidic conditions, the insoluble sulfide precipitates (e.g., CuS and Cu2S) could be readily formed (Dyrssen and Kremling 1990; Brugmann et al. 1997), due to the extremely low solubility product of CuS 6 × 10−16. Therefore, high levels of H2S in the deep sulfidic Lover Lake waters ultimately led to a net removal of dissolved Cu as precipitating free cupric ions out of the water column (Fig. 2).
In natural waters, dissolved Cu could precipitate out of the water columns following two reactions: deassociation of organic refractory Cu (as represented as Cu = Corg) to toxic labile Cu (as represented as Cu2+) under high levels of hydrogen ions, and then adsorption of toxic labile Cu onto particles, or precipitation as solid sulfides:
In particular, toxic labile Cu could be largely precipitated out of the water column under sulfidic conditions, while in those acidific waters, more proportion of labile Cu could also be dissoluted from organic refractory Cu via ion exchanges. As a result, more toxic labile Cu accumulated. China’s natural waters experienced a series of humane perturbation over the past years including intensified hypoxia in coastal waters (e.g., Dai et al. 2006), acidification of freshwater in land (e.g., Zhang and Chen 2002), and even hypoxia in freshwater and coastal waters (e.g., Zhang et al. 1999). Our study provides clear evidence that such alteration in terms of hydrogen ions and redox conditions could potentially impact the water chemistry including the cycling of the metal Cu, and particularly lead to a net accumulation of toxic labile Cu, threatening aquatic ecosystems.
Conclusions
By using the solid phase extraction technique, we for the first time separated labile and organic Cu species from the total dissolved Cu pool in two typical lakes in China. The results show that total dissolved Cu was relatively lower in our study waters (4–11 nmol/L) than those moderately human impacted waters, as influenced from a higher land runoff with suspended particles and organic materials in these waters. In addition, our results also show that dissolved Cu is subject to precipitate out of the water column as sulfides in those strongly reducing waters such as the bottom sulfidic Lover Lake. Organic refractory Cu could directly originate from sewage discharges and land runoffs, and organic refractory Cu could also be formed after organic complexation of free cupric ions.
Particularly, we observed with elevated levels of toxic labile Cu in these natural waters. These toxic labile Cu could be produced from the di-association of organic refractory Cu under high levels of free hydrogen ions via ion exchanges. In addition, high proportion of toxic labile Cu could also be produced during the precipitation of Cu sulfides. Therefore, along with the further acidification and redox changes in China’s natural waters, toxic labile fraction of dissolved Cu is expected to increase with the potential of impacting aquatic ecosystems in a larger extent in the near future.
References
Beck AJ, Sañudo-Wilhelmy SA (2007) Impact of water temperature and dissolved oxygen on copper cycling in an urban estuary. Environ Sci Technol 41:6103–6108
Beck AJ, Cochran JK, Sañudo-Wilhelmy SA (2010) The distribution and speciation of dissolved trace metals in a shallow subterranean estuary. Mar Chem 121:145–156
Brooks SJ, Bolam T, Tolhurst L, Bassett J, Roche JL, Waldock M, Barry J, Thomas KV (2008) Dissolved organic carbon reduces the toxicity of copper to germlings of the macroalgae. Fucus vesiculosus Ecotox Environ Safe 70(1):88–98
Brugmann L, Hallberg R, Larsson C, Loffler A (1997) Changing redox conditions in the Baltic Sea deep basins: impacts on the concentration and speciation of trace metals. Ambio 26:107–112
Bruland KW, Donat JR, Hutchins DA (1991) Interactive influences of bioactive trace metals on biological production in oceanic waters. Limnol Oceanogr 36(8):1555–1577
Buck KN, Bruland KW (2005) Copper speciation in San Francisco Bay: a novel approach using multiple analytical windows. Mar Chem 96:185–198
Buck NJ, Gobler CJ, Sañudo-Wilhelmy SA (2005) Dissolved trace element concentrations in the East River-Long Island Sound system: relative importance of autochthonous versus allochthonous sources. Environ Sci Technol 39:3528–3537
Cai F, Huang M, Su X, Zhang H (1999) Characteristics of silt movement and sedimentary dynamic mechanism in Jiulongjiang Estuary. J Oceanography Taiwan Strait 18(4):418–424 (in Chinese)
Charriau A, Lesven L, Leermakers M, Baeyens W, Ouddane B, Billon G (2011) Trace metal behavior in riverine sediments: role of organic matter and sulfides. Appl Geochem 26:80–90
Chen T (2012) Study on interrelationship between phytoplankton growth and phosphate, nitrate of Yundang Lake. Xiandai Kexue Yiqi 4:134–136 (in Chinese)
Chen, C. Study on the pollution status of metals and organic contaminants and microbiological remediation in the Yandang Lagoon, Xiamen. Master’s Thesis, Xiamen University (2009).
Cheng S (2003) Heavy metal pollution in China: origin, pattern and control. Environ Sci Pollut Res 10(3):192–198
Chiffoleau JF, Cossa D, Auger D, Truquet I (1994) Trace metal distribution, partition and fluxes in the Seine estuary (France) in low discharge regime. Mar Chem 47:145–158
Church TM, Scudlark JR (1997) Trace metals in estuaries: a Delaware Bay synthesis. In: Allen HE, Garrison WA, Luther GW III (eds) Metals in surface waters. Ann Arbor Press, Chelsea, Michigan, pp 1–21
Cid A, Herrero C, Torres E (1995) Copper toxicity on the marine microalga Phaeodactylum tricornutum: effects on photosynthesis and related parameters. Aquat Toxicol 31:165–174
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458
Dai M, Guo X, Zhai W, Yuan L, Wang B, Wang L, Cai P, Tang T, Cai WJ (2006) Oxygen depletion in the upper reach of the Pearl River estuary during a winter drought. Mar Chem 102:159–169
Davis JA (1984) Complexation of trace metals by adsorbed natural organic matter. Geochim Cosmochim Acta 48(4):579–691
Duan Y, Tian YQ, Mo Y, Huang BQ (2011) A preliminary study of alkaline phosphatase activity of phytoplankton in freshwater lakes around Xiamen. Huanjing Kexue yu Keji 34(12H):20–24 (in Chinese)
Duan D, Ran Y, Cheng H, Chen J, Wan G (2014) Contamination trends of trace metals and coupling with algal productivity in sediment cores in Pearl River Delta, South China. Chemosphere 103:35–43
Dyrssen D, Kremling K (1990) Increasing hydrogen sulfide concentration and trace metal behavior in the anoxic Baltic waters. Mar Chem 30:193–204
Feng H, Jiang H, Gao W, Weinstein MP, Zhang Q, Zhang W, Yu L, Yuan D, Tao J (2011) Metal contamination in sediments of the western Bohai Bay and adjacent estuaries, China. Mar Pollut Bull 64:712–720
Fu J, Tang XL, Zhang J, Balzer W (2013) Estuarine modification of dissolved and particulate trace metals in major rivers of East-Hainan, China. Cont Shelf Res 57:59–72
Jiann KT, Wen LW, Santschi PH (2005) Trace metal (Cd, Cu, Ni and Pb) partitioning, affinities and removal in the Danshuei River estuary, a macro-tidal, temporally anoxic estuary in Taiwan. Mar Chem 96:293–313
Kozelka PB, Bruland K (1998) Chemical speciation of dissolved Cu, Zn, Cd, Pb in Narragansett Bay, Rhode Island. Mar Chem 60:267–282
Kustka AB, Allen A, Morel FMM (2007) Sequence analysis and regulation of iron acquisition genes in two marine diatoms. J Phycol 43:715–729
Li Y, Yang R, Zhang A, Wang S (2014) The distribution of dissolved lead in the coastal waters of the East China Sea. Mar Pollut Bull 85:700–709
Lin J, Cai M, Qi A, Hu H et al (2009) Contamination level and speciation of heavy metals in sediments from Yundang Lake, Xiamen. Environ Comput Sci 65:48–52
Lin J, Zhu X, Huang L (2011) Spatial and temporal distributions of total suspended matter and particulate organic carbon, nitrogen, phosphorus in Yundang Lagoon. J Xiamen Univ (Nat Sci) 50(3):579–585
Maldonado MT, Allen AE, Chong JS, Lin K, Leus D, Karpenko N, Harris SL (2006) Copper-dependent iron transport in coastal and oceanic diatoms. Limnol Oceanogr 51(4):1729–1743
Morel FMM, Price NM (2003) The biogeochemical cycles of trace metals in the oceans. Science 300:944–947
Muller FLL (1996) Interactions of copper, lead and cadmium with the dissolved, colloidal and particulate components of estuarine and coastal waters. Mar Chem 52:245–268
Pan K, Wang WX (2012) Trace metal contamination in estuarine and coastal environments in China. Sci Total Environ 421/422:3–16
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, New York, p 173
Paucot H, Wollast R (1997) Transport and transformation of trace metals in the Scheldt estuary. Mar Chem 58:229–244
Peers G, Price NM (2006) Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature 441:341–344
Shiller AM, Boyle EA (1991) Trace elements in the Mississippi River delta outflow region: behavior at high discharge. Geochim Cosmochim Acta 55:3241–3251
Shulkin V, Zhang J (2014) Trace metals in estuaries in the Russian Far East and China: case studies from the Amur River and the Changjiang. Sci Total Environ 499:196–211
Tonietto AE, Lombardi AT, Choeri RB, Vieira AA (2015) Chemical behavior of Cu, Zn, Cd, and Pb in a eutrophic reservoir: speciation and complexation capacity. Environ Sci Pollut Res. doi:10.1007/s11356-015-4773-3.
Tubbing DMJ, Admiraal W, Cleven RFMJ, Iqbal M, Van de Meent D, Verweij W (1994) The contribution of complexed copper to the metabolic inhibition of algae and bacteria in synthetic media and river water. Water Res 28(1):37–44
Turner A, Nimmo M, Thuresson KA (1998) Speciation and sorptive behavior of nickel in an organic-rich estuary (Beaulieu, UK). Mar Chem 63:105–118
Velasqueza IB, Jacintoa GS, Valerab FS (2002) The speciation of dissolved copper, cadmium and zinc in Manila Bay, Philippines. Mar Pollut Bullet 45:210–217
Waeles M, Riso RD, Maguer JF, Le Corre P (2004) Distribution and chemical speciation of dissolved cadmium and copper in the Loire estuary and North Biscay continental shelf, France. Estuar Coast Shelf Sci 59(1):49–57
Wang W, Hong H, Zhang Y, Cao W (2006) Preliminary estimate for the contaminations fluxes from Jiulong River to the sea. Mar Environ Sci 25(2):45–48 (In Chinese)
Wang D, Lin W, Yang X, Zhai W, Dai M, Chen CTA (2012) Occurrences of dissolved trace metals (Cu, Cd, and Mn) in the Pearl River Estuary (China), a large river-groundwater-estuary system. Cont Shelf Res 50/51:54–63
Wang D, Zhao Z, Lin W, Dai M (2014) Tracing the recently increasing anthropogenic inputs into the East China Sea shelf sediments using Pb isotopic analysis. Mar Pollut Bull 79:333–337
Wen LS, Jiann KT, Santschi PH (2006) Physicochemical speciation of bioactive trace metals (Cd, Cu, Fe, Ni) in the oligotrophic South China Sea. Mar Chem 101:104–109
Xiamen Municipal Bureau of Statistics (2013) Statistical communiqué of the city of Xiamen, China on the 2012 National Economic and Social Development; also see http://www.stats-xm.gov.cn/.
Xu C (2005) Distribution of phytoplankton in Yundang Lagoon and ecological assessment. Fujian Shuichan 25(4):16–21 (in Chinese)
Xu G, Liu J, Pei S, Kong X, Hu G (2014) Distribution and source of heavy metals in the surface sediments from the near-shore area, north Jiangsu Province, China. Mar Pollut Bull 103:35–43
Zhang LT, Chen YQ (2002) Study on water quality changes in the Xijiang River. Acta Scientiarum Naturalium Universitatis Sunyatseni 41:97–100 (in Chinese)
Zhang J, Zhang ZF, Liu SM, Wu Y, Xiong H (1999) Human impacts on the large world rivers: would the Changjiang (Yangtze River) be an illustration? Global Biogeochem Cycles 13:1099–1105
Zhang W, Liu X, Cheng H, Zeng EY, Hu Y (2012) Heavy metal pollution in sediments of a typical mariculture zone in South China. Mar Pollut Bull 64:712–720
Zhao K, Fu W, Liu X, Huang D, Zhang C, Ye Z, Xu J (2014) Spatial variations of concentrations of copper and its speciation in the soil-rice system in Wenling of southeastern China. Environ Sci Pollut Res 21(11):7165–7176
Zhou, W. Temporal variations of chromophoric dissolved organic matter in different waters. Master’s degree thesis, Xiamen University (2007). (In Chinese)
Zhou J, Guo W, Deng X, Zhang Z, Xu J, Huang L (2010) Fluorescence excitation-emission matrix spectroscopy of cDOM from Yundang Lagoon and its indication for organic pollution. Spectrosc Spectr Anal 30(6):1539–1544
Zuo XJ, Fu DF, Li H (2012) Speciation distribution and mass balance of copper and zinc in urban rain, sediments, and road runoff. Environ Sci Pollut Res 19(9):4042–4048
Acknowledgments
The authors would like to thank Ms Violeta Léon Fernández for partly analyzing H2S, and Mr. Yi Xu for measuring pH and DO. The State Key Laboratory of Marine Environmental Science is thanked for providing all necessary facilities and equipment. This research was partly supported by the National Science Foundation of China (no. 41176060; no. 41476060).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Céline Guéguen
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Wang, D., Gao, Y., Larsson, K. et al. Speciation of dissolved copper in human impacted freshwater and saltwater lakes. Environ Sci Pollut Res 23, 10832–10840 (2016). https://doi.org/10.1007/s11356-016-6140-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6140-4