Abstract
The aim of this study was to assess the potential of newly isolated yeast strains Schwanniomyces etchellsii M2 and Candida pararugosa BM24 to produce yeast biomass on olive mill wastewater (OMW). Maximum biomass yield was obtained at 75 % (v/v) OMW, after 96 h of incubation at 30 °C and 5 % (v/v) inoculum size. The optimal carbon/nitrogen (C/N) ratio was in the range of 8:1 to10:1, and ammonium chloride was selected as the most suitable nitrogen source. Under these conditions, a maximum biomass production of 15.11 and 21.68 g L−1 was achieved for Schwanniomyces etchellsii M2 and Candida pararugosa BM24, respectively. Proteins were the major constituents of yeast cells (35.9–39.4 % dry weight), lipids were 2.8–5 % dry weight, and ash ranged from 4.8 to 9.5 % dry weight. Besides biomass production, yeast strains were also able to reduce toxicity and polluting parameter levels of the spent OMW-based medium. The practical results presented show that pH rose from initial value of 5.5 to 7.24–7.45 after fermentation. Approximately 23.1–41.4 % of the chemical oxygen demand (COD) and 15.4–19.2 % of the phenolic compounds were removed. The removal of phenolic compounds was associated with their biodegradation and their partial adsorption on yeast cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tunisia is the world’s fourth largest olive oil producer behind Mediterranean giants Spain, Italy, and Greece. Olive oil production is unfortunately associated with the generation of large quantities of by-products, such as olive mill wastewater (OMW), which are produced at a rate above 7 × 105 m3 year−1 (Hachicha et al. 2006). The difficulty of disposing OMW includes its high biochemical oxygen demand (BOD), chemical oxygen demand (COD), and phenolic compounds that may exhibit antimicrobial, ecotoxic, and phytotoxic properties (Lanciotti et al. 2005). However, OMW also contains valuable resources such as a high organic matter concentration and a variety of assimilable carbon sources that could be recycled and used as a substrate for the culture of microorganisms. The organic fraction includes sugars, tannins, polyphenols, polyalcohols, pectins, and lipids (Papanikolaou et al. 2008). The value of these by-products for food supply fortification or for consumption as single-cell protein (SCP) can be an alternative way to both reduce the pollutant load and solve the problem of worldwide protein shortage. Currently, SCP is produced from many species of microorganisms, including algae, fungi, and bacteria. The production of these high protein content cells can be continuous and not dependent on environmental conditions (Lee and Lee 1996). Yeast was the first microorganism whose importance as animal food supplement was recognized almost a century ago. It is suitable for SCP because of its high nutritional quality (Nasseri et al. 2011). Yeasts such as Candida utilis (Torula yeast), Candida lipolytica, Candida tropicalis, Candida novellas, Candida intermedia, and Saccharomyces spp. (Gharsallah 1993) are used as SCP producers to convert effluents from olive mill into microbial biomass protein.
The present investigation aimed to extend the possibilities of using OMW as a substrate for SCP production. Thus, the main purposes of this work were (a) to evaluate the biomass production from OMW using yeast isolates following enrichment, isolation, and identification, (b) to improve cell growth yield, and (c) to decrease the OMW acute toxicity.
Material and methods
Isolation and identification of yeasts
Yeast strains were isolated from different biotopes such as soil irrigated with OMW, sludge from OMW evaporating ponds, and fresh OMW collected from traditional mills located in Sfax-Tunisia. For yeast isolation, 1 g of each sample was suspended in 9 mL sterilized physiological water. The suspension was incubated at 30 °C, 150 rpm for 2 h, and 1 mL was serially (1:10) diluted with sterilized physiological water. Of each dilution, 0.1 mL was spread on Sabouraud Glucose Agar (peptone 5 g L−1, glucose 20 g L−1, agar 20 g L−1, pH 5.5) containing 50 mg L−1 chloramphenicol to inhibit bacterial growth. The plates were incubated for 48 h at 30 °C. Colonies with distinct morphological differences such as color, shape, and size were picked and purified by streaking at least three times on yeast peptone glucose agar (YPGA) (yeast extract 5 g L−1, peptone 5 g L−1, glucose 10 g L−1, and agar 20 g L−1) medium. The purified isolates were stored on YPGA plates at 4 °C. The selected strains were identified based on their internal transcribed spacer (ITS) sequences. The ITS region was amplified by PCR using ITS1 and ITS4 primers (forward ITS 1-5′ TCC GTA GGT GAA CCT GCG G 3′ and Reverse ITS 4-5′ TCC TCC GCT TAT TGA TAT GC 3′) and was directly sequenced (White et al. 1990). The obtained sequences were compared to ITS sequences available in GenBank.
Optimization of biomass production by yeast species
Yeast isolates were individually inoculated in YPG medium at 30 °C on a rotary shaker at 150 rpm for 24 h. The precultures were used to inoculate 50 mL of water-diluted OMW (25 %, v/v) with 1 mL of a mid-exponential growth phase containing 4 × 108 cells mL−1 (v/v), and then, the cultures were incubated at 30 °C in a shaker at 150 rpm for 48 h. OMW media were supplemented with ammonium chloride at a concentration of 2 g L−1. All media were sterilized by autoclaving at 121 °C for 20 min.
Cell growth was monitored by dry weight of yeast biomass in 10 mL culture following centrifugation at 8000 rpm for 15 min. Dry cell matter was determined after evaporating its water content at 105 °C until a constant mass.
Yeast strains showing the highest biomass growth yield on OMW were further studied. To investigate the effect of initial OMW concentrations on biomass production, several experiments were conducted with different proportions of OMW ranging between 25 and 100 %. OMW at the desired concentration was prepared by mixing raw OMW with distilled water and was supplemented with ammonium chloride at a concentration of 2 g L−1 . In order to study the nitrogen requirements of the yeast strains, various organic nitrogen sources (yeast extract, soy peptone, and a mixture of yeast extract/soy peptone (1:1)) and inorganic nitrogen sources (ammonium sulfate, sodium nitrate, and ammonium chloride) were added to OMW-based media (at the desired concentration) to produce carbon/nitrogen (C/N) ratios of 10. The effect of C/N on the biomass production was tested using ammonium chloride as nitrogen source at different C/N ratios (4:1, 6:1, 8:1, 10:1, 12:1, 15:1, 20:1). Inoculum sizes ranging from 2 to 10 % were added to OMW basal medium. Different pH levels (4, 4.5, 5, 5.5, 6, and 7) were set for the experiment. The effect of temperature on the growth of yeast strains was also tested using different temperatures (25, 30, and 37 °C).
Chemical characterization of OMW
COD, solids (total, volatile, and dissolved), and nitrogen (Kjeldahl) were determined according to Standard Methods (APHA et al. 1989). Phosphorous content was determined by vanadomolybdophosphoric acid method (American Public Health Association 1992). pH was measured using a combination electrode and pH meter. Total phenols were assessed by the Folin-Ciocalteu method (Wolfe et al. 2003) using gallic acid as a standard. Reducing sugars were measured by DNS method (Miller 1959). The concentration of the following minerals (Na, K, Mg, Ca, Cl, and Fe) has been determined by atomic absorption spectrometry.
To avoid spontaneous fermentation, OMW was stored at −20 °C. Before use, OMW was centrifuged at 8000 rpm for 15 min at 4 °C to remove solid particles and the supernatant was used for further fermentation studies.
Characterization of yeast biomass and spent OMW-based medium
Yeast cells were separated from the spent OMW-based medium by centrifugation (8000 rpm for 15 min). The yeast biomass was washed, then freeze-dried, and analyzed for crude protein content by the Kjeldahl method (N × 6.25) and for crude lipid content according to the method of Folch et al. (1957). The ash content was determined after combusting samples at 550 °C for 2 h.
Spent OMW-based medium was characterized with regard to its pH, COD, phenolic compounds, and reducing sugars as mentioned above.
Phytotoxicity tests
Phytotoxicity was assessed using the germination index of Lycopersicon esculentum defined by Zucconi et al. standard method (1981).
Statistical analysis
All experiments were conducted in duplicate. Data were expressed as means ± standard error and were statistically analyzed by one-way analysis of variance (ANOVA) followed by the Tukey post-test. Values of p < 0.05 were considered significant.
Results and discussion
OMW characterization
Table 1 lists the characteristics of OMW sample. It can be seen that OMW contains high levels of organic matter expressed in term of COD content (214.3 g L−1) and phenolic compounds (11.8 g L−1), which are toxic. It is an acidic effluent with a high nutrient content which offers a selective environment for yeast growth. In contrast, the total nitrogen concentration was extremely low, at 0.1 g L−1. Thus, addition of a nitrogen source is needed, generally in the form of ammonium salts, aqueous ammonia, soluble proteins, or urea (Gutiérrez et al. 2012).
Isolation, screening, and identification of yeast strains for biomass production
A total of 11 yeast strains named M1, M2, M3, M31, M32, M33, BM1, BM22, BM24, SM10, and SM14 were isolated from different OMW-contaminated biotopes such as soil irrigated with OMW, sludge from OMW evaporating ponds, and fresh OMW. Yeast strains were identified relying on their ITS region, and the sequences obtained were compared with those available in the GenBank. The results are presented in Table 2.
Preliminary experiments were conducted to test the potential of diluted OMW (at the rate of 25 % (v/v)) in supporting yeast growth. All tested strains were significantly able to grow in OMW-based medium (Table 2). Among all the isolated strains, Schwanniomyces etchellsii M2, Candida pararugosa BM24, Candida pararugosa BM22, Candida diddensiae SM10, Candida diddensiae SM14, and Debaryomyces etchellsii, BM1 showed the highest biomass production (p < 0.05). However, there was no significant difference on cell mass within the abovementioned strains (p > 0.05). To the best of our knowledge, Schwanniomyces etchellsii M2 and Candida pararugosa BM24 have not yet been reported for SCP production. Therefore, they were tested throughout this study for SCP production.
Schwanniomyces etchellsii also known as D. etchellsii, Pichia etchellsii, Torulaspora etchellsii, Kloeckera faecalis, and Pichia faecalis can be found in many habitats such as soil, vegetables, and foods and rarely in clinical samples (Kurtzman et al. 2011). This strain exhibits high levels of cell wall-bound β-glucosidases, an important property responsible for the hydrolysis of natural glucosides and influencing food aroma (Wallecha and Mishra 2003).
Candida pararugosa, formerly known as Cryptococcus aggregates and Torulopsis aggregata, not only was first isolated from human sources (Nakagawa et al. 2004 and Giammanco et al. 2004), but also was recovered in other food ecosystems such as dairy products (Spanamberg et al. 2004), a low-alcohol grain-based beverage (Botes et al. 2007), and commercial red wines (Jensen et al. 2009) and recently from dried meat (Matsheka et al. 2014). According to Pennacchia et al. (2008), this strain exhibits a satisfactory survival percentage in intestinal fluid (>70 %) after preexposure to gastric juice demonstrating a high tolerance to bile salts and pancreatic exposure. On the other hand, it showed considerable lipolytic and proteolytic activities, two important properties in the event of using this strain in food fermentation processes.
In the recent literature, there are no reports of human diseases related to these species. Therefore, they could be used as new supplements in foods or pharmaceutical preparations.
Effect of initial OMW concentrations
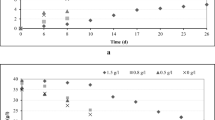
In order to test the effect of initial OMW concentration on the growth kinetics of these two yeast species, experiments were performed in the presence of increasing concentrations of OMW: 25, 50, 75, and 100 % (v/v) in distilled water to which 2 g L−1 NH4Cl was added (Fig. 1). It can be seen that both strains were able to grow in all tested OMW concentrations. However, in the case of strain M2, an inhibition effect was observed for undiluted OMW since the quantity of biomass produced was only 1.49 g L−1. In spite of the high organic load of OMW, higher dilutions were disadvantageous for BM24 and the lower biomass production (3.27 g L−1) was shown at 25 % of OMW. Both strains grew better at 75 % of OMW, and the maximum cell mass production was 6.69 and 7.63 g L−1 for M2 and BM24, respectively, after 96-h incubation period. Despite the high content of phenolic compounds in 75 % OMW, no inhibition growth was observed during the yeast strain cultivation. Similar results were reported by Gharsallah (1993) for Saccharomyces rouxii who showed that the majority of OMW phenolic compounds did not affect the growth of Saccharomyces rouxii except cinnamic acid and vanillic acid. Compared to other microorganisms, yeasts are more adapted to high concentrations of phenolic compounds and low pH values of mill wastes allowing them to grow in this stressful environment (Ben Sassi et al. 2006).
Optimization of medium composition and culture conditions
Effect of nitrogen source on biomass production
Nitrogen is the basic nutrient for yeast growth and should be adjusted to provide a C/N ratio in the range of 7:1 to 10:1 to favor high protein content (Halasz and Lasztity 1990). Thus, to improve yeast cell production in 75 % of OMW, several organic nitrogen sources (yeast extract, soy peptone, and yeast extract + soy peptone) and inorganic nitrogen sources (ammonium sulfate, sodium nitrate, or ammonium chloride) were added to get a C/N ratio of 10:1. Results showed that biomass production varied considerably depending on the types of nitrogen source used (Fig. 2). The amount of yeast biomass produced in the fermentation medium, containing only OMW as sole carbon and nitrogen sources, was lower than those obtained under nitrogen supplementation. Biomass production was significantly enhanced (p < 0.05) by both ammonium salts reaching 16.61 and 15.56 g L−1 with NH4Cl and 15.29 and 13.57 g L−1 with (NH4)2SO4 for BM24 and M2, respectively. According to Halasz and Lasztity (1990), ammonium salts are suitable nitrogen sources for yeasts of interest in SCP production. The results of the present study also agree with that of Choi and Park (2002) who showed that when cultured in juice extracted from cabbage waste, the addition of ammonium sulfate increased the cell mass of Pichia stipitis. It is water soluble and does not produce any toxic effect toward the majority of microbial enzymes. Bamforth (2005) reported that nitrogen in ammonium salt is readily assimilated by yeast; its replacement with organic nitrogen sources resulted in poor biomass production.
Effect of organic (yeast extract (YE), soy peptone (SP), and yeast extract + soy peptone (YE + SP)) and inorganic (KNO3, NH4Cl, and (NH4)2SO4) nitrogen source addition on biomass production of strains BM24 and M2. Experimental conditions are as follows: 75 % OMW (v/v), 2 % inoculum size, 30 °C, 150 rpm, 4-day incubation, and 50-mL working volume. a–e and A–F Means without a common letter differ (p < 0.05)
Effect of C/N ratio on biomass production
To determine the effect of C/N ratio on the growth of yeast strains M2 and BM24 on OMW-based medium, ammonium chloride as nitrogen source was added to give media with the following C/N ratios (4:1, 6:1, 8:1, 10:1, 12:1, 15:1, 20:1) (Fig. 3). It can be seen that for both strains, the optimal range for C/N ratio is 8–10 with a maximum biomass production of 15.48 and 16.6 g L−1 for strains M2 and BM24, respectively. The above differences in cell mass values are indeed statistically significant at p < 0.05 when different C/N ratios were applied. An optimal C/N ratio is the key factor to enhance microbial biomass protein (Zheng et al. 2005a). A C/N ratio of 10:1 or less favors high protein contents, while higher C/N ratios favor intracellular lipid or carbohydrate accumulation, which are undesirable in the final product (Harder and Brooke 1990).
Effect of inoculum size on biomass production
Inoculum size is one of the key factors in starting the fermentation process. The effect of different inoculums sizes on biomass production is shown in Fig. 4. In the case of the strain BM24, the addition of 2 to 5 % inoculum from a mid-exponential growth phase preculture containing 4 × 108 cells mL−1 led to maximum biomass production (p < 0.05) after 4 days of incubation. Besides, 7 % inoculum caused a drop in yeast biomass to approximately 28 % as compared to biomass obtained with 5 % inoculum size. Similarly, a slight decrease (10 %) of the biomass production of M2 was observed when the inoculum size exceeds 7 % (v/v). However, there was no significant difference in biomass production of this strain (p > 0.05), when different inoculum sizes were tested. These findings indicated that with large inoculum sizes (>7 % (v/v)), the nutrients in the growth medium might be rapidly consumed which results in low rate of yeast growth and cell survival. According to Reade and Gregory (1975), the increase of initial inoculum size may possibly favor autolysis. Therefore, additional nutrient is needed to maintain the cell survival. However, lower concentrations of inoculum size may not be enough for initiating of the cell growth.
pH and temperature effect on biomass production
As pH is important for yeast biomass production, different pH levels (4.0, 4.5, 5.0, 5.5, 6.0, and 7.0) were set for the experiments. The initial pH of the medium seemed to have a greater effect on biomass production. Indeed, both strains M2 and BM24 reached a maximum biomass at pH 5.5. Further increase in pH values resulted in a significant decrease of the biomass of BM24 (p < 0.05). However, no significant drop of the cell mass of M2 was observed (p > 0.05) (Fig. 5). Similar results were reported by Reiser and Gasperik (1995) and Vega et al. (2007) for Saccharomycopsis fibuligera and Kluyveromyces cicerisporus, respectively. The pH of the fermentation medium has an effect not only on the solubility of some nutrients but also on the permeability of cell membrane and, thus, the cell growth (Felipe et al. 1997).
Temperature also influenced the biomass production. Yeast biomass increased with temperature to reach its maximum at 30 °C for both strains (22.03 and 14.64 g L−1 for BM24 and M2, respectively) (Fig. 6). However, at higher and lesser temperatures, biomass production decreased significantly (p < 0.05). Low temperature can inhibit nutrients to cross cell membrane, while high temperature may inactivate enzymes of metabolic pathway. This result was in good accordance with that of Torija et al. (2003) who reported that the growth of different strains of Saccharomyces cerevisiae was optimal at 30–35 °C.
Composition and nutritive value of single-cell biomass
The optimum OMW medium for single-cell biomass production of yeast strains M2 and BM24 was obtained with 75 % of OMW. Ammonium chloride was added as a nitrogen source to get a C/N ratio in the range of 8:1–10:1. Inoculum size was of 5 %. The pH was adjusted to 5.5, and the temperature was maintained at 30 °C. The chemical composition analyses of both strains M2 and BM24 used for biomass production are shown in Table 3.
Crude protein contents
Almost 35.9 and 39.4 % of crude protein contents were estimated in dried cell biomass of strains BM24 and M2, respectively. For both strains, the protein content was quite similar to that produced by P. stipitis CBS 5776 grown on cabbage juice medium (Choi and Park 2002). On the other hand, the values obtained were higher than those reported for Candida utilis which was only 26 % when cultivated in the presence of salad oil manufacturing wastewater (Zheng et al. 2005a) and 16 % when cultivated in silage effluent (Arnold et al. 2000). Nevertheless, crude proteins in strains M2 and BM24 were lower than those reported in previous studies for other yeast species and substrates. Nigam (1998) and Choi et al. (2002) produced cell biomass of Candida utilis with protein contents of 55.3 and 43 %, respectively. The presented results agree with those of Lee et al. (1993) and Petkov et al. (2002) who demonstrated that production of yeast biomass using fatty substrates resulted in 34–45 % protein concentration. Protein contents in dried cell biomass of strains M2 and BM24 indicate its potential use as a substitute of other traditional protein sources.
Crude lipid contents and fatty acid composition
As shown in Table 3, strains M2 and BM24 contained low amounts of lipids, 5 and 2.8 %, respectively. These results are in good accordance with those of Miller and Litsky (1976) who reported that yeast contains 2 to 6 % of lipids. However, the values obtained were lower than the 9 % value reported for Candida utilis grown in salad oil manufacturing wastewater effluent (Zheng et al. 2005a) and higher than 0.4 % reported on binary mixed yeast biomass (Candida halophila and Rhodotorula glutinis) from glutamate fermentation wastewater (Zheng et al. 2005b). Generally, the total lipid and protein contents in dry biomass depend on the composition of growth medium. Fatty acid analysis of lipids accumulated by the strains M2 and BM24 showed that the major cellular fatty acid produced is oleic acid (Δ9 C18:1) followed by linoleic (Δ9,12C18:2), palmitic (C16:0), and palmitoleic (Δ9C16:1) acids (Table 4). The high content of monounsaturated and polyunsaturated fatty acids (PUFAs) found in lipids extracted from strains M2 and BM24 is suitable when yeast biomass is used for nutritional purposes.
Ash contents
Ash or the mineral content constituted about 9.5 and 4.8 % of the yeast biomass for strains M2 and BM24, respectively. Brown et al. (1996) reported that ash content ranged from 4.7 to 13 % dry weight in yeast strains. Ash content of Candida utilis was 7 % of dry matter (Han et al. 1976), while ash content of Yarrowia lipolytica cultured in pure glycerol varied only from 3.47 to 4.7 % (Juszczyk et al. 2013).
Adsorption of phenolic compounds to yeast cells
The uptake capacity of phenolic compounds by M2 and BM24 was found to be 38.4 and 43.8 mg g−1, respectively. Both strains displayed a good adsorption performance when compared to Saccharomyces cerevisiae (26.95 mg g−1) (Moyo et al. 2012). The adsorbed quantity of phenolic compounds may confer to the produced biomass several biological activities. In general, all phenolic compounds exhibit potential antioxidant activities which act through elimination of free radicals in cells and provide protection against oxidative stress in biomolecules (e.g., proteins, lipids, and DNA) (Boskou 2006). They also represent natural antibacterial, antiviral, and antifungal agents (Aziz et al. 1998; Koutsoumanis et al. 1998; Tassou and Nychas 1995; Vagelas et al. 2009). Schaffer et al. (2007) tested the effect of hydroxytyrosol-rich OMW extract (an o-diphenol) in vitro and ex vivo. No toxic effects have been shown when a dose of 100 mg of hydroxytyrosol per kilogram body weight was applied for 12 days. Interestingly, this treatment improved the resistance of dissociated brain cells to oxidative stress.
Characterization of the spent OMW-based medium
Organic pollution parameters
Spent OMW-based medium was characterized after the fermentation for a range of parameters such as pH, COD, and phenolics, which were chosen as indicators of pollution parameters of the effluent. OMW had an adjusted initial pH of 5.5 which increased after cultivation of both strains M2 and BM24 to pH 7.45 and 7.24, respectively. The substantial pH increases observed can be presumably due to ammonium from protein degradation or to the degradation of COO− and OH− groups of phenolic compounds and organic acids such as lactic and acetic acids as suggested by several authors (Ercoli and Ertola 1983; El Hajjouji et al. 2008). It was also reported that at neutral or alkaline pH, the treated effluent becomes more economically friendly and less problematic (Arnold et al. 2000). The results given in Table 5 show a noticeable capacity for reducing the organic matter present in OMW. Reducing sugars decreased markedly after cultivation. This means that both yeast species are able to use sugars present in OMW as carbon sources.
In all cases, the COD removal after cultivation was approximately about 41.4 and 23.1 %, respectively, for M2 and BM24. Total phenol removals in 75 % of OMW were found to be only 15.4 and 19.2 % for M2 and BM24, respectively. Strain M2 seemed to be more efficient than strain BM24 for degrading the COD in OMW, but strain BM24 degraded better the phenolic compounds. Hainal et al. (2011) reported the ability of yeasts to degrade and to use polyphenolic compounds as a carbon source. More dilution before subjecting OMW to biodegradation or employing a stepwise cultivation technique for adaptation seemed to be necessary for high degree of dephenolization and COD removal. These results are in agreement with those of Jarboui et al. (2011) who reported that the reduction of phenols in OMW by Rhodotorula mucilaginosa decreased with increasing the initial phenol concentration. In the same line, Sayadi and Ellouz (1995) and Ahmadi et al. (2006) showed that high OMW dilutions should be used to reduce the initial organic and phenolic content. Fungal remediation of OMW has been tested using three types of organisms: white rot fungi, Aspergillus sp., and different yeasts. Several strains of yeasts have revealed interesting capacities for the removal of problematic OMW compounds. Many authors have examined the ability of Y. lipolytica to degrade OMW (Scioli and Vollaro 1997; Lanciotti et al. 2005; Lopes et al. 2009; Wu et al. 2009). Y. lipolytica appears quite effective, achieving removal rates as high as 80 % for COD and 68 % for phenolics (Lopes et al. 2009). Reduction of COD and phenol concentrations for more than 80 % was achieved using Trichosporon cutaneum (Chtourou et al. 2004). Various other yeast strains have already been used to reduce COD from OMW and to remove phenolic compounds, namely Candida tropicalis (Martinez-Garcia et al. 2007), Trichosporon cutaneum (Chtourou et al. 2004), and Saccharomyces sp. (Gharsallah 1993; Gonçalves et al. 2009).
Phytotoxicity of the spent OMW-based medium
The effect of spent OMW-based medium (75 % OMW) and crude OMW 75 % on seed germination of L. esculentum was evaluated using the germination index (GI) as defined by Zucconi et al. (1981). The test was conducted on different OMW 75 % dilutions (1/1, 1/2, 1/4, 1/10, 1/25, and 1/50). The results showed that no germination was registered when untreated OMW dilution was lower than 1/10. On the other hand, data presented in Fig. 7 show that the treated OMW with both strains improved significantly GI even when diluted 1/10 was used. The GI increased up to 51.89 and 35.34 % for M2 and BM24, respectively. Treated OMW diluted to 1/10, 1/25, and 1/50 showed positive effects on germination ratio which was significantly higher than in untreated OMW at the same dilutions and control (distilled water). Therefore, many authors suggested that the phytotoxicity of OMW is principally due to the content of phenolic and organic acids present in untreated OMW at high concentrations (Komilis et al. 2005; Amaral et al. 2012).
Conclusions
The presented study suggests that the large amount of OMW generated from olive oil industries could be readily converted by selected yeast isolates into SCP without costly pretreatment. Furthermore, these strains were able to slightly reduce the pollution load of OMW. However, the degree of purification was insufficient to allow direct discharge to a water course without supplementary treatment.
Further research, including the scale-up and the use of the produced yeast biomass as a major protein source in animal diets, is required in order to ascertain whether the results achieved by the current study can be turned into viable process.
References
Ahmadi M, Vahabzadeh F, Bonakdarpour B, Mehranian M (2006) Empirical modeling of olive oil mill wastewater treatment using loofa-immobilized Phanerochaete chrysosporium. Process Biochem 41:1148–1154
Amaral C, Lucas MS, Sampaio A, Peres JA, Dias AA, Peixoto F, Anjos MDR, Pais C (2012) Biodegradation of olive mill wastewaters by a wild isolate of Candida oleophila. Int Biodeter Biodegr 68:45–50
American Public Health Association (1992) Standard methods for the examination of water and waste water, 18th edn. American Public Health Association, Washington
APHA, AWWA, WPCF (1989) Standard methods for the examination of water and wastewater, 17th edn. APHA, AWWA, WPCF, Washington
Arnold JL, Knapp JS, Johnson CL (2000) The use of yeasts to reduce polluting potential of silage effluent. Water Res 34:3699–3708
Aziz NH, Farag SE, Mousa LAA, Abo-Zaid MA (1998) Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios 374:43–54
Bamforth CW (2005) Food, fermentation and microorganisms. Blackwell Science, Oxford
Ben Sassi A, Boularbah A, Jaouad A, Walker G, Boussaid A (2006) A Comparison of olive oil mill wastewaters (OMW) from three different processes in Morocco. Process Biochem 41:74–78
Boskou D (2006) Sources of natural phenolic antioxidants. Trends Food Sci Technol 17:505–512
Botes A, Todorov S, von Mollendorff JW, Botha A, Dicks LMT (2007) Identification of lactic acid bacteria and yeast from boza. Process Biochem 42:267–270
Brown MR, Barrett SM, Volkma JK, Nearhos SP, Nell JA, Allan GL (1996) Biochemical composition of new yeasts and bacteria as food for bivalve aquaculture. Aquaculture 143:341–360
Choi MH, Park YH (2002) Production of yeast biomass using waste Chinese cabbage. Biomass Bioenergy 25:221–226
Choi MH, Ji GE, Koh KH, Ryu YW, Jo DH, Park HY (2002) Use of waste Chinese cabbage as a substrate for yeast biomass production. Bioresour Technol 83:251–253
Chtourou M, Ammar E, Nasri M, Medhioub K (2004) Isolation of a yeast, Trichosporon cutaneum, able to use low molecular weight phenolic compounds: application to olive mill waste water treatment. J Chem Technol Biotechnol 79:869–878
El Hajjouji H, Bailly JR, Winterton P, Merlina G, Revel JC, Hafidi M (2008) Chemical and spectrophotometric analysis of olive mill wastewater during a biological treatment. Bioresour Technol 99:4958–4965
Ercoli E, Ertola R (1983) SCP production from olive black water. Biotechnol Lett 7:457–462
Felipe MGA, Vitolo M, Mancilha IM, Silva SS (1997) Fermentation of sugar cane bagasse hemicellulosic hydrolysate for xylitol production: effect of pH. Biomass Bioenergy 13:11–14
Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 199:833–841
Gharsallah N (1993) Production of single cell protein from olive mill wastewater by yeasts. Environ Technol 14:391–395
Giammanco GM, Melilli D, Piuseppe G (2004) Candida pararugosa isolation from the oral cavity of an Italian denture wearer. Res Microbiol 155:571–574
Gonçalves C, Lopes M, Ferreira JP, Belo I (2009) Biological treatment of olive mill wastewater by non-conventional yeasts. Bioresour Technol 100:3759–3763
Gutiérrez A, Chiva R, Sancho M, Beltran G, Arroyo-López FN, Guillamon JM (2012) Nitrogen requirements of commercial wine yeast strains during fermentation of a synthetic grape must. Food Microbiol 31:25–32
Hachicha S, Chtourou M, Medhioub K, Ammar E (2006) Compost of poultry manure and olive mill wastes as an alternative fertilizer. Agron Sustain Dev 26:135–142
Hainal AR, Ignat I, Volf I, Popa VI (2011) Transformation of polyphenols from biomass by some yeast species. Cellul Chem Technol 45:211–219
Halasz A, Lasztity R (1990) Use of yeast biomass in food production. CRC Press, USA
Han IK, Hochstetler HW, Scott ML (1976) Metabolizable energy values of some poultry feeds determined by various methods and their estimation using metabolizability of the dry matter. Poult Sci 55:1335–1342
Harder W, Brooke AC (1990) Methylotrophic yeasts. In: Verachtert H, De Mot R (eds) Yeast biotechnology and biocatalysis. Marcel Dekker, New York, pp 395–428
Jarboui R, Baati H, Fetoui F, Gargouri A, Gharsallah N, Ammar E (2011) Yeast performance in wastewater treatment: case study of Rhodotorula mucilaginosa. Environ Technol 33:951–960
Jensen SL, Umiker NL, Arneborg N, Edwards CG (2009) Identification and characterization of Dekkera bruxellensis, Candida pararugosa, and Pichia guilliermondii isolated from commercial red wines. Food Microbiol 26:915–921
Juszczyk P, Tomaszewska L, Kita A, Rymowicz W (2013) Biomass production by novel strains of Yarrowia lipolytica using raw glycerol, derived from biodiesel production. Bioresour Technol 137:124–131
Komilis DP, Karatzas E, Halvadakis CP (2005) The effect of olive mill wastewater on seed germination after various pretreatment techniques. J Environ Manage 74:339–348
Koutsoumanis K, Tassou CC, Taoukis PS, Nychas GJ (1998) Modelling the effectiveness of a natural antimicrobial on Salmonella enteritidis as a function of concentration, temperature and pH, using conductance measurements. J Appl Microbiol 84:981–987
Kurtzman CP, Fell JW, Boekhout T (2011) The yeasts: a taxonomic study. Elsevier Science BV, Amsterdam
Lanciotti R, Gianotti A, Baldi D, Angrisani R, Suzzi G, Mastrocola D, Guerzoni ME (2005) Use of Yarrowia lipolytica strains for the treatment of olive mill wastewater. Bioresour Technol 96:317–322
Lee K, Lee S (1996) Continuous process for yeast biomass production from sugar beet stillage by a novel strain of Candida rugosa and protein profile of the yeast. J Chem Technol Biotechnol 66:349–354
Lee C, Yamakwa T, Komada T (1993) Rapid growth of thermotolerant yeast on palm oil. World J Microbiol Biotechnol 9:187–190
Lopes M, Araújo C, Aguedo M, Gomes N, Gonçalves C, Teixeira JA, Belo I (2009) The use of olive mill wastewater by wild type Yarrowia lipolytica strains: medium supplementation and surfactant presence effect. J Chem Technol Biotechnol 84:533–537
Martinez-Garcia G, Johnson AC, Bachmann RT , Williams CJ, Burgoyne A, Edyvean RGJ (2007) Two-stage biological treatment of olive mill wastewater with whey as co-substrate. Inter Biodeter Biodeg 59:273–282
Matsheka MI, Mpuchane S, Gashe BA, Allotey J, Khonga EB, Coetzee SB, Murindamombe G (2014) Microbial quality assessment and predominant microorganism of biltong produced in butcheries in Gaborone, Botswana. Food Nutr Sci 5:1668–1678
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Miller BM, Litsky W (1976) Single cell protein in microbiology. McGrow-Hill Book Co, New York
Moyo M, Mutare E, Chigondo F, Nyamunda BC (2012) Removal of phenol from aqueous solution by adsorption on yeast, Saccharomyces cerevisiae. IJRRAS 11:486–494
Nakagawa Y, Robert V, Kawarazakai J, Epping W, Smith MT, Poot GA, Mizuguchi I, Kanbe T, Doi M (2004) Recurrent isolation of an uncommon yeast, Candida pararugosa, from a sarcoma patient. Med Mycol 42:267–271
Nasseri AT, Rasoul-Amini S, Morowvat MH, Ghasemi Y (2011) Single cell protein: production and process. Am J Food Technol 6:103–116
Nigam JN (1998) Single cell protein from pineapple cannery effluent. World J Microbiol Biotechnol 14:693–696
Papanikolaou S, Galiotou-Panayotou M, Fakas S, Komaitis M, Aggelis G (2008) Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Bioresour Technol 99:2419–2428
Pennacchia C, Blaiotta G, Pepe O, Villani F (2008) Isolation of Saccharomyces cerevisiae strains from different food matrices and their preliminary selection for a potential use as probiotics. J Appl Microbiol 105:1919–1928
Petkov K, Rymowicz W, Musiał I, Kinal S, Biel W (2002) Nutritive value of protein Yarrowia lipolytica, yeast obtained on various lipid substrates. Folia Univ Agri Stetin, Zootechnica 227:95–100
Reade AE, Gregory KF (1975) High temperature production of protein enriched feed from cassava by fungi. Appl Microbiol 30:897–903
Reiser V, Gasperik J (1995) Purification and characterization of the cell-wall associated and extracellular α-glucosidases from Saccharomycopsis fibuligera. Biochem J 308:753–760
Sayadi S, Ellouz R (1995) Roles of lignin peroxidase and manganese peroxidase from Phanerochaete chrysosporium in the decolorization of olive mill wastewaters. Appl Environ Microbiol 61:1098–1103
Schaffer S, Podstawa M, Visioli F, Bogani P, Muller WE, Eckert GP (2007) Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J Agric Food Chem 55:5043–5049
Scioli C, Vollaro L (1997) The use of Yarrowia lipolytica to reduce pollution in olive mill wastewaters. Water Res 31:2520–2524
Spanamberg A, Hartfelder C, Fuentefria AM, Valente P (2004) Diversity and enzyme production by yeasts isolated from raw milk in Southern Brazil. Acta Sci Vet 32:195–199
Tassou CC, Nychas GJE (1995) Inhibition of Salmonella enteritidis by oleuropein in broth and in a model food system. Lett Appl Microbiol 20:120–124
Torija MJ, Rozès N, Poblet M, Guillamón JM, Mas A (2003) Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae. Int J Food Microbiol 80:47–53
Vagelas I, Kalorizou H, Papachatzis A, Botu M (2009) Bioactivity of olive oil mill wasterwater against plant pathogens and post harvest diseases. Biotechnol Biotechnol Equip 23:1217–1219
Vega MP, Da Silva Leite R, de Oliveira MA CL (2007) Mathematical modeling of single cell protein and ethanol production by Kluyveromyces cicerisporus fermentation on whey. In: Plesu V, Agachi PS (eds) 17th European symposium on computer aided process engineering-ESCAPE 17
Wallecha A, Mishra S (2003) Purification and characterization of two β-glucosidases from a thermo-tolerant yeast Pichia etchellsii. Biochim Biophys Acta 1649:74–84
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfland DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wolfe K, Wu X, Lu RH (2003) Antioxidant activity of apple peel. J Agric Food Chem 51:609–614
Wu L, Ge G, Wan J (2009) Biodegradation of oil wastewater by free and immobilized Yarrowia lipolytica W29. J Environ Sci 21:237–242
Zheng S, Yang M, Yang Z (2005a) Biomass production of isolate from salad oil manufacturing wastewater. Bioresour Technol 96:1183–1187
Zheng S, Yang M, Yang Z, Yang Q (2005b) Biomass production from glutamate fermentation wastewater by the co-culture of Candida halophila and Rhodotorula glutinis. Bioresour Technol 96:1522–1524
Zucconi FA, Pera MF, De Bertoldi M (1981) Evaluating toxicity of immature compost. Biocycle 22:54–57
Acknowledgments
The authors would like to thank Mr. Abdel Majid Dammak for revising the language of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Arous, F., Azabou, S., Jaouani, A. et al. Biosynthesis of single-cell biomass from olive mill wastewater by newly isolated yeasts. Environ Sci Pollut Res 23, 6783–6792 (2016). https://doi.org/10.1007/s11356-015-5924-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5924-2