Abstract
Hydrogeochemical analysis, statistical analysis, and geochemical modeling were employed to evaluate the impacts of coal mining activities on karst water chemistry in Niangziguan spring catchment, one of the largest karst springs in Northern China. Significant water quality deterioration was observed along the flow path, evidenced from the increasing sulfate, nitrate, and TDS content in karst water. Karst water samples are Ca-Mg-HCO3 type in the recharge areas, Ca-Mg-HCO3-SO4 type in the coal mining areas, and Ca-Mg-SO4-HCO3/HCO3-SO4 type in the rural areas and discharge areas. A four-factor principal component analysis (PCA) model is conducted which explains over 82.9 % of the total variation. Factor 1, which explained the largest portion (45.33 %) of the total variance, reveals that coal mining activities and natural water-rock interaction as the primary factors controlling karst water quality. Anthropogenic effects were recognized as the secondary factor with high positive loadings for NO3 − and Cl− in the model. The other two factors are co-precipitation removal of trace elements and silicate mineral dissolution, which explained 20.96 % of the total variance. A two-end mixing modeling was proposed to estimate the percentage of coal wastewater giving on karst water chemistry, based on the groundwater sulfate chemistry constrains rather than sulfur isotopes. Uncertainty of sulfur isotope sources led to an overestimation of coal mining water contribution. According to the results of the modeling, the contribution of coal mining waste on karst water chemistry was quantified to be from 27.05 to 1.11 % which is ca. three times lower than the values suggested using a sulfur isotope method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Karst water quality degradation has posed a great restriction in satisfying local water supply and economic development in many countries (Bakalowicz 2005; Jiang et al. 2009; Lang et al. 2006; López-Chicano et al. 2001). China has carbonate rocks covering a surface of approximately 3.25 million km2, including bare karst of 1.25 million km2 and covered or buried karst of 2 million km2 (Yu 1994; Li et al. 2010). The Niangziguan spring group is one of the largest karst springs in Northern China, located in Yangquan City, Shanxi, China. Karst aquifers are a risk of inputting of various pollutants, due to the native low self-protection ability. Coal mining activities, widely distributed in the Niangziguan spring catchment, have been considered as the biggest threat to karst water quality since the economic boom in the 1980s in China. Most water quality parameters showed a higher value than the Chinese standard value, indicating the serious pollution of karst water in the area (Li et al. 1998). Coal mining waste pollution, uncontrolled urban sewage effluents, industrial discharges, and intensive use of fertilizers have been recognized as factors causing karst water quality deterioration (Hao et al. 2012). Karst water pollution reduces the available water supply and is a threat to the further development of local economy in this semiarid area in Northern China (Shahbaz et al. 2015). To address the potential impact of coal mining activity on karst water chemistry, a sulfur isotope method was introduced to calculate the percentage of coal mining wastewater input. It is supposed that the pyrite source sulfate accounts for ca. 60–70 % (Li et al. 1998) or 99.7 % (Duan and Liang 2006) of the total sulfate in karst water. However, these values are not always right and a little higher than the real contribution of coal mining wastewater, due to the multiple sources of 34S in karst water. Therefore, evaluation with diverse methods is needed to depict the impact of coal mining activities on karst water quality.
Traditionally, multivariate statistical techniques, like factor analysis (FA), principal component analysis (PCA), cluster analysis (CA), and discriminant analysis (DA), are successful employed to assess groundwater quality (Masoud 2014; Singh et al. 2013; Yidana et al. 2008; Zhou et al. 2010). They are also often used to evaluate the hydrochemical characteristic of groundwater (Cloutier et al. 2008; Farnham et al. 2003; Galazoulas and Petalas 2014; Lambrakis et al. 2004; Suk et al. 1999) and to identify the hydrogeochemical processes and factors controlling the compositions in groundwater (Belkhiri et al. 2010; Cloutier et al. 2008; Dassi 2011; Lu et al. 2008; Wang et al. 2001). Among all those statistical techniques mentioned above, PCA is one of the most powerful and common methods for reducing the large data matrix to smaller dimensions that consist of principal component scores and loadings. However, choosing only the multivariate statistical techniques is not judicious sometime. Putting statistical methods and hydrochemical analysis together could obtain relatively reasonable interpretations for hydrogeochemical mechanisms in controlling water quality variation. Combination of multivariate statistical and hydrochemical analysis is successfully used in extracting main factors contributing to groundwater quality: seawater intrusion, microbial activity, and chemical fertilizers (Kim et al. 2005), and geochemical modeling becomes another tool in elucidating the chemical reactions affecting water chemistry (Güler and Thyne 2004). In conclusion, the combined use of statistical methods, hydrochemical analysis, and geochemical modeling may offer greater benefits for groundwater pollution identification.
In the present study, statistical models, hydrogeochemical analysis, and geochemical (mixing) modeling were employed to evaluate and estimate the influence of coal mining activity on karst groundwater quality in the Niangziguan karst spring catchments, Northern China.
Topography and hydrology
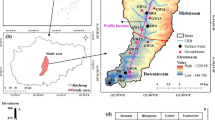
Niangziguan spring catchment is one of the largest karst springs in Northern China, located in the Mianhe Valley with latitude ranging from 36° 55′ to 37° 50′ and longitude ranging from 112° 20′ to 113° 55′ (Fig. 1). This area consists of districts of Pingding, Yuxian, Heshun, Zuoquan, Xiyang, and Shouyang counties and Yangquan City, with an area of 7394 ha (Zhang 2004).
The geomorphology of this area is low in the central with higher altitude in the north, west, and south mountain areas of the catchments. The lowest part is located in the Mianhe Valley (Niangziguan Town) with an altitude of 342 m, and the highest part is located in the north mountain area, Yuxian County (1847 m). The climate here is temperate continental monsoon with a mean annual temperature of 10.9 °C. The mean annual precipitation here is 505.23 mm, and the rainfall peaks occur from July to September. Larger tributaries running through the area are the Wenhe River and the Taohe River. These two rivers are converging as the Mianhe River in Niangziguan Town (Fig. 1).
Geology and hydrogeology
Tectonically, the Niangziguan spring catchment is situated in the northeast of the Qinshui Synform, sloping down from northwest to east. The basement rocks of which are mainly Archaean metamorphic rocks. It is covered by Paleozoic carbonates with some sandstone, shale, and Cenozoic alluvium including silt clay, sand, and gravel. In the western part of the area, the covered strata are primarily Permian and Triassic sandstones and shales with a thickness of 90–1200 m. The underlying strata from shallow to deep include Carboniferous limestone (thickness of 80–220 m), middle Ordovician limestone (thickness of 467–628 m), and lower Ordovician carbonate (thickness of 120–200 m). Carbonate complex, including middle Ordovician limestones, lower Ordovician carbonates, and Cambrian dolomites, is air exposed in the east part of the area. Karst water here is capable to be recharged by the infiltration of precipitation.
Groundwater occurs mainly in the Carboniferous and Permian fracture aquifers and deep karst aquifers in the study area. The formation of karst aquifers is composed of Carboniferous limestone, Ordovician limestone, and occasionally, Cambrian dolomite. As an enclosed karst water system, precipitation is a dominated source of water supply in the Niangziguan spring catchment. Karst water aquifers are recharged by precipitation, surface water, Quaternary water, as well as fissure water from the Upper Carboniferous and Permian strata. The karst water table is ca. 500–750 m in the western mountain area and 230–380 m in the eastern river valley area. Well extraction and natural springs are the main discharge patterns of karst water which occurred in the study area.
Sampling and methods
A total of 54 water samples, including rainwater, coal mine water, surface water, karst well water, seepage water, and spring water, were collected from the major coal mining areas and the following downstream areas at the Niangziguan karst water catchment during September 2013 (Fig. 2, Table 1).
When sampling, the water samples were collected only after the in situ physicochemical parameters, including temperature and pH, were stable and all the parameters were measured within 5 min using a Hanna portable pH meter (HI8424, pH ±0.01, T ±0.4 °C) that had been calibrated beforehand. Each sample was collected in three bottles, one for anion analysis, one with extra acid for cation analysis, and the rest kept in a refrigerator for experimental usage in the future. The total alkalinity was measured on the sampling day using the Gran titration method with the triple repetition analyze error <±2 %. The concentrations of Cl−, SO4 2−, and NO3 − were determined by ion chromatography (IC) (Dionex 120; Dionex, Sunnyvale, CA, USA). For cation analysis, reagent-quality HNO3 was added to one of these polyethylene bottles until the pH value of the samples was less than 2. Major cations (K+, Na+, Ca2+, and Mg2+) and trace elements (Sr and Si) were measured by inductively coupled plasma-atomic emission spectrometry (ICP-AES) (IRIS Intrepid XSP; Thermo Elemental, Madison, WI, USA). The analytical precision for the measurements of cations and anions is indicated by the ionic balance error, which is observed to be within the standard limit of ±5 %. The software PHREEQC V2.8 was used for mixing the modeling and calculating the saturation indices (SIs) and the charge balance of each sample. The average charge balance is 0.44 with a largest value of 8.96 (sample SF3) and a smallest value of −7.55 (sample SP6), all of which are in acceptable limit.
Factor analyses of the water hydrochemistry data were performed using the statistical software SPSS version 9.0. Factor extraction was carried out by principal component analysis to reduce immense datasets of high complexity. It can determine a small number of variables which represent the greatest variance in all of the original variables of the groundwater quality. Principal component analysis was used to define the major geochemical processes that control the chemical compositions in groundwater. In this present study, a dataset was made up, including 54 samples and 13 chemical parameters (NO3 −, Cl−, HCO3 −, SO4 2−, Ca2+, K+, Mg2+, Na+, Ba, Fe, Mn, Si, and Sr).
Results and discussion
Hydrochemistry
Surface water quality is good in the upstream. Sample SF9, from the upstream, has a low K+, Na+, Mg2+, Cl−, and NO3 − concentration and a medium SO4 2−, Ca2+, and HCO3 − concentration (Table 1). A significant increase of sulfate, nitrate, and chloride concentration was observed in surface water when it flows through the mining areas and inhabitant areas. High content of sulfate in surface water may come from coal mining wastewater pollution, especially in the coal mining areas (Fig. 3). The high nitrate content and medium chloride content in surface water may result from the contamination of agriculture activity and municipal sewage. This contaminated surface water may threat the karst water quality directly, due to the close hydraulic connection between the surface water and groundwater in the area.
A simple concept model of hydraulic circulation in the study area. Mine wastewater may either recharge the lower carbonate aquifers or discharge into the surface water (1 karst aquifer in Middle Ordovician; 2 coal mining goaf; 3 relative aquitard in Lower Ordovician dolomite; 4 Quaternary aquifer; 5 spring; 6 water table; 7 runoff; 8 Permo-Carboniferous system)
Karst well water samples showed large variations in all major ions except for K+ which makes little impact on water quality (Fig. 5a). Ca2+ and Mg2+ concentrations fluctuate in a narrow range at this karst area. The sufficient source of Ca and Mg from carbonate maintains the stable Ca2+ and Mg2+ concentrations in karst water via water-carbonate rock interaction. The value of HCO3 − ranges from 92.45 to 582.41 mg/L (except for W26), and SO4 2− ranges from 9.52 to 750.3 mg/L. Karst water samples, collected in non-coal mining areas in Yuxian County, are all fresh water. Karst water samples with a high value of SO4 2− were mainly collected from coal mine areas. Mining activities should respond for the elevated sulfate in karst water. The karst water quality becomes worse in Pingding County and the suburb of Yangquan City.
Mine wastewater is normally characterized as having low pH, low bicarbonate content, and high content of sulfate, sodium, calcium, and magnesium. However, a simple pretreatment, called neutralization by calcite or CaO powder, would cause an elevation of pH value and bicarbonate content and a reduction of sulfate content in mining wastewater. In this case study, most mining wastewater samples showed an alkaline pH value (7.40–8.14) (Fig. 4a), medium to high bicarbonate content, and medium to high sulfate content due to the pretreatment with calcite or CaO powder. There are only two samples (M5 and M7) that are acidic (pH 3.8–4.5) with low bicarbonate content and sulfate content over 2000 mg/L (Table 1). They are recognized as the original raw coal mining wastewater. These two samples also display low content of nitrate and chloride and medium to high content of sodium, calcium, and magnesium. Briefly, the discharged coal mining wastewater is one of the major sources of sulfate pollution in the area based on our field investigation.
Spring waters are with better quality than karst well water and surface water (Fig. 5c). Two spring samples (SP1 and SP7), collected from the recharge mountain areas in Yangquan City, are with the low ion concentration due to a shorter circulation time and distance and an insufficient water-rock interaction with surrounding strata. An increase of major ion contents was found in all the karst spring waters in the discharge areas.
The water samples collected are classified into five hydrochemical facies: Ca-Mg-HCO3 type (G1), Ca-Mg-HCO3-SO4 (G2), Ca-Mg-SO4-HCO3 (G3), Ca-Mg-SO4/Cl (G4), and Na-SO4-HCO3 (G5) on the basis of hydrochemical compositions through AquaChem software. Figure 6 presents the major ions of water types and groups using a piper diagram. Samples with hydrochemical types of Ca-Mg-HCO3 are mostly found in the recharge area in Yuxian County where the predominant rocks are limestone and dolomite. The karst groundwater chemistry type converts to Ca-Mg-HCO3-SO4 type occurring in the coal mining areas of Yuxian County, Pingding County, and Yangquan City. This phenomenon could be explained by the factors of coal mining activity: (1) coal layers existing in Carboniferous-Permian stratum are rich in organic and inorganic sulfur, and this sulfur could be released into the water by coal mining activity, and (2) acidic coal mining wastewater would promote the dissolution of minerals, e.g., gypsum, in the surrounding strata and enable the increase of SO4 2− concentration in karst water. Karst water samples is a Ca-Mg-SO4-HCO3 or Ca-Mg-HCO3-SO4 type in the rural areas and flow-through areas with medium to high content of nitrate, indicating the potential impact of other human activities except for coal mining.
Identification of coal mining activity impact
In general, principle component analysis (PCA) of extracts correlates and reduces the number of data into components that explain a portion of the total variance between the chemical parameters. This explained that variance is mainly related to the chemical parameters showing the highest loading factor (>0.7) obtained using the varimax rotation (Davis 1986). These highly loaded components are further regarded as reference for identifying the major geochemical processes involved. PCA was employed to identify the major geochemical processes involved in the water quality degradation in the Niangziguan karst system, coupled with water chemistry study. In this case study, four factors were defined and a total cumulative variance of 82.95 % was obtained based on the Kaizer criterion (Table 2).
Factor 1
Factor 1 explained by mining activities and natural water-rock interactions has the largest portion (45.33 %) with high positive loadings (>0.748) for SO4 2−, Mg2+, Na+, Fe, Mn, and Si (Table 3). Basically, the source of major ions in groundwater or surface water is derived from lixiviation, also regarded as water-rock interaction. Because of the presence of calcite (CaCO3), dolomite (CaMg(CO3)2), and gypsum (CaSO4) in the Ordovician limestone aquifers, the dissolution of these minerals could bring a high content of HCO3 −, SO4 2−, Ca2+, and Mg2+ into karst water. With these dissolution processes, the milliequivalent ratio between HCO3 −+O4 2− and Ca2++Mg2+ would be near 1:1. Figure 7 shows the milliequivalent ratio scatter map indicating that the source of HCO3 −, SO4 2−, Ca2+, and Mg2+ in groundwater mostly attributes to the dissolution of calcite, dolomite, and gypsum—fall into the areas close to the 1:1 line. Therefore, high loadings of SO4 2− and Mg2+ reflect the contribution from gypsum and dolomite dissolution.
A scatter plot of (SO4+HCO3) vs. (Ca+Mg). The milliequivalent ratio between HCO3 −+SO4 2− and Ca2++Mg2+ would be near 1:1 with the dissolution of calcite (CaCO3), dolomite (CaMg(CO3)2), and gypsum (CaSO4) present in the Ordovician limestone aquifers. Most of the groundwater falls into the areas close to the 1:1 line, indicating that the source of HCO3 −, SO4 2−, Ca2+, and Mg2+ in them mostly attributes to the dissolution of calcite, dolomite, and gypsum
However, natural water-rock interaction could not well illustrate the extremely high SO4 2− content and high trace element loadings in factor 1. Coal mining activities induced by sulfide oxidation/organic degradation may respond to their additional source (Gao et al. 2011; Fig. 7). Coal mines are widely spread in the areas of Yuxian, Yangquan, and Pingding counties where serious environmental contamination by mining activities was reported (Wang and Gao 2009). Mining water is discharged by the way as a supply of underground karst water by seepage or pumped into surface water with little/simple pretreatment, like lime neutralization. From the hydrochemical dataset given by surface water samples, all eight samples are contaminated by sulfate with the highest concentration of 976.5 mg/L (sample SF1). Considering the rich sulfur content in coal-bearing strata, natural inorganic sulfides and organic sulfur could contact with karst water to generate the sulfate-rich groundwater during mining. The processes involved can be described using the following reactions:
These three reactions could increase the concentrations of SO4 2− and Fe and reduce the pH in an aqueous environment. Mining activities could also bring high concentrations of trace elements like Mn, Ni, Si, and Sr by the way of acidic weathering dissolution of sulfides, silicate, and carbonate minerals in the strata (Qiao et al. 2011). The high loadings of sulfate, Fe, Mn, Ni, and Si and the medium loading of Sr demonstrate the significant impact of coal mining activity on the karst water chemistry.
Based on the statement above, the medium loading of Ca2+ (0.58) in this case study is feasible. In the karst areas, dissolution of carbonates, such as calcite and dolomite, brings high content of calcium into karst water. Calculation of the SI of calcite and dolomite indicates that most of karst water samples are at equilibrium or oversaturated with them (Fig. 8). The SIdolomite (from 0.0 to over 2.5) shows a more rapid increased trend than SIcalcite (from 0.2 to 1.5) (Fig. 8), which means that more Mg2+ remains in the karst water even the solubility of calcite is higher than that of dolomite in this low-temperature situation. The reason for this phenomenon can be well described as those increased SO4 2− and Ca2+ concentrations (Fig. 9) tend to remove more Ca2+ out of the solution as calcite precipitation, due to the common ion effect and solubility difference between calcite and gypsum (gypsum is more soluble than calcite at around 20 °C). In the study area, karst water is suffering from gypsum dissolution and coal mining wastewater recharge, both of which could bring block sulfate and calcium into karst water. This increased sulfate and calcium concentration in karst water will further enhance the secondary precipitation of calcite and dissolution of dolomite, which named as dedolomitization or calcitization. To sum up, factor 1 is a comprehensive factor to which lixiviation and coal mining water input make most contributions. It could be regarded as natural and mining factor.
Factor 2
Factor 2 explained by effects of agricultural activity and sewage water discharge has a portion of 16.66 % with high positive loadings for NO3 − and Cl−. This component is closely associated with NO3 − (0.889) and Cl− (0.852) and weakly correlated with Ca2+ (0.561) and K+ (0.538). High positive loadings of Cl− and NO3 − indicate an influence by human activities, like agricultural activity and sewage water discharge. Generally, NO3 − concentration is low in atmospheric precipitation. However, rainwater with NO3 − concentration of 14.3 mg/L was observed in this karst area with a pH value of lower than 6.5 (Wang and Gao 2009; Gao et al. 2011; and this study). As one of the major coal mining and coal-using areas in Shanxi Province, Northern China, the air in the atmosphere is seriously polluted in Yangquan City where the rainwater was collected. So, the remarkable nitrate concentration in rainwater may be ascribed to nitrogenous fertilizer volatilization and fossil fuel combustion, such as coal and gasoline. However, the appearance of some high-nitrate-concentration (>100 mg/L) karst water suggests that there should be other sources of nitrate entering the karst water aquifers. A lithological source could not make contributions to such a high content of NO3 − in this karst area where the strata are mainly composed of lime, dolomite, some shales and sandstones, and little Quaternary sediments, and there are no other significant sources for nitrate importing in the area except for human being sources. So, it is inferred that domestic wastewater and agricultural fertilizers are the major sources of NO3 − content (Heaton 1986). Individual agricultural production which is the major food supply for local inhabitants has caused heavy overuse of fertilizer and pesticides in China. These fertilizers and pesticides enter the karst water via leakage through naked carbonate rocks or thin-covered soil via precipitation or polluted surface water leaching. Discharge of domestic wastewater with little pretreatment also increased the risk of groundwater and surface water pollution. As a consequence, karst water and surface water with high content of nitrate concentration are all found in the areas with heavy human activities in this case study.
A positive loading of Cl− in this factor demonstrates other contributors except for dissolution of halite during natural water-rock interaction. To identify the potential source of Cl−, a scatter map (Fig. 10) of Na+ vs. Cl− was employed. Some karst well samples and surface water samples are located below the halite dissolution line where Cl− is excessive over Na+. On the basis of the lithological context, there are no other bulk chloride-bearing minerals in the karst area. So, the significant increase of chloride content in the karst water is not possibly coming from natural water-rock interaction. According to our field investigation, there is no other industrial giver for Cl− in the karst area. Therefore, leakage of municipal wastewater and recharge of polluted surface water and shallow groundwater should be the factors resounding for the extra chloride in karst water and the high positive loading of Cl− in this PCA model.
Based on the analyses above, a conclusion could be drawn that the anthropogenic factors like overuse of nitrogenous fertilizer, municipal wastewater discharge, and fossil fuel (coal and gasoline) combustion seriously affect the chemistry of karst water in the Niangziguan karst area.
Factor 3
Factor 3 explained by the co-precipitation removal of trace elements, such as Ba, has a small portion of 10.78 % with a high negative loading for Ba2+ (−0.956). The high negative loading value of Ba2+ (−0.956) in factor 3 reminds that the occurrence of trace elements in karst water is pivotal for water chemistry. Generally, as a trace element, groundwater has low barium concentration which originates primarily from natural sources of water-bearing igneous and sedimentary rocks through the dissolution of barium-bearing minerals (Mokrik et al. 2009). Dissolution of barium co-occurrence carbonate rocks is one of the main natural sources of Ba2+ in the karst aquifers. Coal mining activity could be the most important anthropogenic source for high content of trace elements, such as Ba, Fe, Mn, Sr, and so on in this karst area. As the most important water supply source, excessive amounts of trace elements in karst water would cause a body health problem. Good news is that the negative loading of barium in the PCA model which declares a removal of it from the karst groundwater. This can be interpreted as follows: (1) in the recharge area, barium content in karst water is low, controlled by the natural water-rock interaction; (2) along the flow path, karst water obtains other barium supplies, such as coal mining wastewater, polluted surface water, or polluted sediment groundwater; and (3) meanwhile, the sulfate content in karst water increases rapidly due to short-time coal mining acid water input and longtime dissolution of gypsum in the carbonate strata. Increased sulfate content in karst water promoted the re-precipitation of barite which is indicated by the positive SIBarite value (Fig. 11).
Actually, mineral precipitation happens frequently during the longtime water-rock interaction in this open karst system. Calcium and magnesium may precipitate as calcite and dolomite along the karst water flow path (Fig. 8). Precipitation of calcite and dolomite could remove Ba and other trace elements via isomorphism or co-precipitation. Fe is tending to separate from karst water as goethite and Fe(OH)3 in the catchment area, which benefits the removl of trace elements from aqueous phases (Fig. 12) via adsorption. This partly explained the decreased content of trace elements in the karst water located in the downstream of the coal mine areas.
Factor 4
The last factor accounts for the lowest portion of 10.19 % with a high loading of HCO3 − (0.801) and a moderate loading of K+ (0.51) and Na+ (0.502). This factor represents the contribution of silicate weathering dissolution on water chemistry. In the study area, bicarbonate is generally one of the dominant anions in groundwater. The natural sources of bicarbonate in groundwater may come from natural organic matter degradation and carbonate and silicate mineral dissolution. As a typical karst area, the absence of organic matter in the major strata limits the amount of produced organic bicarbonate. Therefore, bicarbonate mainly comes from inorganic mineral dissolution. However, the low loadings of Ca2+ and Mg2+ in factor 4 suggest that carbonate mineral dissolution is not going to respond for the high positive loading of bicarbonate here. The exclusive possibility is silicate mineral dissolution which is consistent with the modest positive loading of K+ and Na+. During the infiltration of rainwater, surface water, and irrigated water, the silicate minerals available along the path will be dissolved depending on the availability of dissolved CO2 and carbonic acid. During the leakage or cross-recharge processes, HCO3 −, K+, and Na+ will be brought into groundwater. The low loading value of K+ and Na+ may attribute to the multi-sources and/or cation exchange in Quaternary aquifers which may modify the water chemistry.
Estimation of coal mining water contribution
A two-end mixing modeling was employed to estimate the contribution of coal mining wastewater on karst water chemistry, with the software PHREEQC V2.8 (see Supplementary Material 1). In the modeling, karst water samples W3, W4, and W5, collected from the upstream of the coal mining areas, were used to work out as end member 1 (fresh upstream karst water). The chemistry data of end member 1 was obtained by calculating the mean value of W3, W4, and W5. It is characterized as HCO3-Ca-type water with a temperature of 18.0 °C, pH 7.58, K 0.1 mg/L, Na 4.9 mg/L, Ca 68.2 mg/L, Mg 22.6 mg/L, Cl 10.6 mg/L, HCO3 269.0 mg/L, and SO4 22.7 mg/L. Coal mining wastewater was taken as the second mixing end member in this case study. The ions’ mean value of raw coal mining wastewater samples was used as the chemistry data of end member 2. End member 2 belongs to SO4-Na-type water with pH 2.4, K 5.7 mg/L, Na 635.7 mg/L, Ca 318.3 mg/L, Mg 177.4 mg/L, Cl 44.1 mg/L, HCO3 0.0 mg/L, and SO4 2701.7 mg/L. A pH value of 2.4 was used to represent the strong acidic condition in coal mining water. An equation was obtained by mixing different percentages of end members 1 (fresh upstream karst water) and 2 (mining water) to describe the coal mining water addition on sulfate concentration variation in karst water (see Supplementary Material 2, data not show). Two assumptions were made in the modeling: (1) sulfate ions were conservative and not or little affected by the mixing reaction. This assumption is tenable that major sulfate minerals were undersaturated throughout the modeling experiments, except for minor barite. Barite is going to be oversaturated in the karst water and showing a trend of precipitation. However, the precipitation of barite would remove little sulfate from karst water, due to the lower Ba concentration in groundwater (Table 1). (2) Another assumption is that the contribution of natural aquifer sulfate mineral dissolution on karst water is insignificant during the mixing process. During the mining and following mixing processes, the acid mining water could promote the further dissolution of reservoir rock minerals, including calcite, dolomite, gypsum, feldspar, and even quartz in the area. In this case study, we are willing to attribute this sulfate input to coal mining activity contribution rather than natural mineral dissolution. The sulfate that comes from the natural mineral dissolution would be negligible in this stage due to the inefficient contact time. Based on the above assumptions, the karst water samples collected from the coal mining areas were supposed to be the result of the mixing reaction between the recharge water from the upstream karst aquifers and the coal mining wastewater.
The contribution percentage of coal mining water on karst water chemistry was calculated using the equation (see Supplementary Material 3) obtained from the mixing percentage vs. sulfate concentration curve (Supplementary Material 2). The highest contribution was found in karst water sample W2 with a coal mining water mixing percentage of 27.05 %, while the lowest contribution was 1.11 %. Compared with a previous study from others (Duan and Liang 2006; Li et al. 1998), our results indicate a smaller sulfate proportion from coal mining activity. In the previous work of Duan and Liang (2006) and Li et al (1998), both of them used δ34S as the indicator. Their assumption was that 34S in groundwater was derived from the mixing of karst water existing in the Ordovician reservoir and mine wastewater. However, the sources of δ34S are not limited within these two sources in the area. The widely distributed coal stratum was another biggest reservoir of δ34S. The δ34S composition of water from the coal strata was similar to the mining wastewater, because the sulfate in them was coming from pyrite oxidation. So, the δ34S sourcing from the coal strata was mistaken and calculated as mining wastewater contribution. Therefore, they were going to overestimate the contribution of coal mining water on groundwater chemistry, based on the δ34S method. In this case study, the percentage of coal mining wastewater was calculated using the sulfate balance as the tracer and the result here is more acceptable.
Conclusions
-
1.
Significant karst water quality deterioration was observed in the Niangziguan spring catchment. Karst water samples collected are water. Coal mining activity caused the karst water type to change from Ca-Mg-HCO3 type (in the upstream recharge areas) to Ca-Mg-HCO3-SO4 type in the coal mining areas and Ca-Mg-SO4-HCO3/HCO3-SO4 type with high nitrate content in the rural areas and discharge areas.
-
2.
Statistical methods and hydrochemical analysis were successfully used together to interpret the main hydrogeochemical mechanisms controlling karst water quality. A four-factor principal component analysis (PCA) model is conducted which explains over 82.9 % of the total variation. Coal mining activities and natural water-rock interaction were extracted as the first primary factors controlling karst water quality. Anthropogenic effects were recognized as the secondary factor with high positive loadings for NO3 − and Cl− in the model. The other two factors are co-precipitation removal of trace elements and silicate mineral dissolution, which explained 20.97 % of the total variance.
-
3.
A two-end geochemical mixing model was proposed with upstream karst water and coal mining wastewater as the end members. An equation, used for the calculation of coal mining water mixing percentage, was set up based on the result of mixing modeling. The contribution of coal mining waste on karst water chemistry was quantified to be from 27.05 to 1.11 % which is ca. three times lower than the values suggested using a sulfur isotope method. Using the sulfate balance as the tracer, the result here is acceptable.
References
Bakalowicz M (2005) Karst groundwater: a challenge for new resources. Hydrogeol J 13:148–160
Belkhiri L et al (2010) Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater—a case study: Ain Azel plain (Algeria). Geoderma 159:390–398
Cloutier V et al (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353:294–313
Dassi L (2011) Investigation by multivariate analysis of groundwater composition in a multilayer aquifer system from North Africa: a multi-tracer approach. Appl Geochem 26:1386–1398
Davis JC (1986) Statistics and data analysis in geology, 2nd edn. Wiley, New York, p 646
Duan GW, Liang YP (2006) Study on sulfate pollution in Yangquan City using 34S isotope. West-China Explor Eng 1:35–38
Farnham IM et al (2003) Factor analytical approaches for evaluating groundwater trace element chemistry data. Anal Chim Acta 490:123–138
Galazoulas EC, Petalas CP (2014) Application of multivariate statistical procedures on major ions and trace elements in a multilayered coastal aquifer: the case of the south Rhodope coastal aquifer. Environ Earth Sci 72:4191–4205
Gao XB et al (2011) Anthropogenic effects on hydrochemistry of Niangziguan karst water. Proc Inst Civ Eng Water Manage 164(10):495–510
Güler C, Thyne GD (2004) Hydrologic and geologic factors controlling surface and groundwater chemistry in Indian Wells-Owens Valley area, southeastern California, USA. J Hydrol 285:177–198
Hao YH et al (2012) Investigation of karstic hydrological processes of Niangziguan Springs (North China) using wavelet analysis. Hydrol Process 26:3062–3069
Heaton TH (1986) Nitrate pollution of groundwater. S Afr J Sci 82:279–287
Jiang Y et al (2009) Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. J Contam Hydrol 109:49–61
Kim JH et al (2005) Multivariate statistical analysis to identify the major factors governing groundwater quality in the coastal area of Kimje, South Korea. Hydrol Process 19:1261–1276
Lambrakis N et al (2004) The use of multicomponent statistical analysis in hydrogeological environmental research. Water Res 38:1862–1872
Lang Y et al (2006) Geochemistry of surface and ground water in Guiyang, China: water/rock interaction and pollution in a karst hydrological system. Appl Geochem 21:887–903
Li YL et al (1998) Pollution analysis of SO4 2−, Ca2+, Mg2+ in karst water in Niangiguan spring area. Geol Sci Technol Inf 17(2):111–114
Li X et al (2010) The use of environmental isotopic (C, Sr, S) and hydrochemical tracers to characterize anthropogenic effects on karst groundwater quality: a case study of the Shuicheng Basin, SW China. Appl Geochem 25:1924–1936
López-Chicano M et al (2001) Factors which determine the hydrogeochemical behaviour of karstic springs. A case study from the Betic Cordilleras, Spain. Appl Geochem 16:1179–1192
Lu HY et al (2008) Identification of the origin of salinization in groundwater using multivariate statistical analysis and geochemical modeling: a case study of Kaohsiung, Southwest Taiwan. Environ Geol 55:339–352
Masoud AA (2014) Groundwater quality assessment of the shallow aquifers west of the Nile Delta (Egypt) using multivariate statistical and geostatistical techniques. J Afr Earth Sci 95:123–137
Mokrik R et al (2009) The origin of barium in the Cambrian-Vendian aquifer system, North Estonia. Est J Earth Sci 58:193–208
Qiao XJ et al (2011) Influence of coal mining on regional karst groundwater system: a case study in West Mountain area of Taiyuan City, Northern China. Environ Earth Sci 64:1525–1535
Shahbaz M et al (2015) Do coal consumption and industrial development increase environmental degradation in China and India? Environ Sci Pollut Res 22(5):3895–3907
Singh E et al (2013) Groundwater quality in Imphal West district, Manipur, India, with multivariate statistical analysis of data. Environ Sci Pollut Res 20:2421–2434
Suk H et al (1999) Characterization of a ground water hydrochemical system through multivariate analysis: clustering into ground water zones. Ground Water 37:358–366
Wang YX, Gao XB (2009) Geochemical evolution of the Niangziguan karst water system under the impact of human activities. Corsologica Sin 28:10–19
Wang Y et al (2001) Geostatistical and geochemical analysis of surface water leakage into groundwater on a regional scale: a case study in the Liulin karst system, northwestern China. J Hydrol 246:223–234
Yidana SM et al (2008) A multivariate statistical analysis of surface water chemistry data—the Ankobra Basin, Ghana. J Environ Manag 86:80–87
Yu P (1994) Surface collapse in the karst mining areas in China. Mine Water Environ 13(2):21–26
Zhang DJ (2004) Carbonate water contamination and genetic analysis of Niangziguan spring area. Saf Environ Eng 11:57–59
Zhou XH et al (2010) Application of factor analysis in the assessment of groundwater quality. Water Res Low Carbon Energy 1251:33–36, 2nd international symposium on aqua science
Acknowledgments
This research was financially supported by the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (41521001), National Natural Science Foundation of China (41372251), the Specialized Research Fund for the Doctoral Program of Higher Education (20130145120014), and the International Postdoctoral Exchange Fellowship Program by the Office of China Postdoctoral Council and the Natural Science Foundation of Hubei Province of China (2013CFB410).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 118 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Li, X. & Gao, X. Hydrochemistry and coal mining activity induced karst water quality degradation in the Niangziguan karst water system, China. Environ Sci Pollut Res 23, 6286–6299 (2016). https://doi.org/10.1007/s11356-015-5838-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5838-z