Abstract
This study investigated the effects of various soil conditions, including drying-rewetting, nitrogen deposition, and temperature rise, on the quantities and the composition of dissolved organic matter leached from forest and wetland soils. A set of forest and wetland soils with and without the nitrogen deposition were incubated in the growth chambers under three different temperatures. The moisture contents were kept constant, except for two-week drying intervals. Comparisons between the original and the treated samples revealed that drying-rewetting was a crucial environmental factor driving changes in the amount of dissolved organic carbon (DOC). The DOC was also notably increased by the nitrogen deposition to the dry forest soil and was affected by the temperature of the dry wetland soil. A parallel factor (PARAFAC) analysis identified three sub-fractions of the fluorescent dissolved organic matter (FDOM) from the fluorescence excitation–emission matrices (EEMs), and their compositions depended on drying-rewetting. The data as a whole, including the DOC and PARAFAC components and other optical indices, were possibly explained by the two main variables, which were closely related with the PARAFAC components and DOC based on principal component analysis (PCA). Our results suggested that the DOC and PARAFAC component information could provide a comprehensive interpretation of the changes in the soil-leached DOM in response to the different environmental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil organic matter is a ubiquitous mixture of plant litter and microbial biomass (Kögel-Knabner 2002), which provides a labile substrate for microbial metabolism and undergoes humification processes. Various biotic and abiotic processes, such as degradation, mineralization, partitioning, dissolution, and aggregation, are associated with the dynamics of dissolved organic matter (DOM) leached from soils that result in a wide variety of molecular weights and chemical structures. It is imperative to understand the contributions of environmental changes to the quantity and the quality of DOM because some of these biotic and abiotic processes are sensitive to many environmental factors, including temperature, moisture content, and nutrient levels of soils.
A dramatic increase in agricultural activity has employed a great amount of fertilizer application in Asia since the early 1900s. More dramatic increase in nitrogen deposition is projected to continue until 2050 (Galloway et al. 2004). Fog (1988) and Guggenberger (1994) reported a positive correlation between nitrogen deposition and the amount of DOC that originated from refractory soil organic matter. In contrast, Aber (1992) claimed that adding nitrogen could stimulate DOM consumption and consequently reduce the DOC concentration. Although the correlation between the nitrogen deposition and DOC value seems contradictory, the underlying biogeochemical processes share a common rule that nitrogen is an important feed for microbial growth and activity. However, there is lack of understanding of the relative importance of co-existing environmental factors, such as temperature variation, site variation, and soil drying-rewetting, on DOM quantities or quality. In addition, soil in terrestrial ecosystems experiences frequent drying followed by rewetting, which can cause a continuous increase in the DOC (Clark et al. 2006). Although the overall impact of a drought and subsequent rewetting on soil organic carbon has been reported with respect to traditional parameters, such as dissolved or total organic carbon and the amounts of inorganic nitrogen or sulfur (Birch 1960; Palomo et al. 2013; Clark et al. 2006), the corresponding biogeochemical processes have still not been well defined.
It is generally believed that enhanced microbial growth and activity results in an increase in DOC. Because microbial degradation and respiration alter the composition of natural organic carbon by consuming the labile and bioavailable carbon, while leaving behind the relatively refractory compounds (Ogawa et al. 2001), probing DOM compositions may unveil the extent of microbial activities. However, Lundquist et al. (1999) presented the complex nature of the relationship between DOC and microbial activity in the field. The results of previous studies suggest that DOM quantities and quality are dependent on many factors such as temperature variation, site variation, nitrogen deposition, and soil drying-rewetting. The relative importance of co-existing environmental factors is not fully understood.
With advances in spectroscopic methods, the identification of the specific chemical constituents of DOM has become more versatile. The specific UV–visible absorbance (SUVA) and fluorescence excitation–emission matrices (EEMs) have been widely used as rapid and non-destructive analytical methods for monitoring DOM (Leenheer and Croue 2003). Kalbitz et al. (2007) reported that long-term increases in DOM production on the forest floor in response to climate change could lead to an increase in the DOC of surface water based on SUVA and humification index data. Recently, parallel factor analysis (PARAFAC) has become a very popular method for extracting a set of representative fluorescence components from EEMs (Stedmon et al. 2003). For example, Hall et al. (2005), Stedmon and Markager (2005), and Hur and Cho (2012) have classified estuarial DOM into several groups with different compositions based on the PARAFAC component scores.
The objective of this study was to gain information on the contributions of selected environmental factors, such as temperature, nitrogen deposition, and drying-rewetting, on the quantity and quality of forest and wetland soil DOM. Multivariate statistical tools were adopted to evaluate the significance of the selected environmental factors in both qualitative and quantitative ways. Special focus was given to the extent of the changes in the fluorescent DOM because the fluorescence components can be used as surrogates for important biogeochemical processes. The results of this study are expected to elucidate the response of the size and characteristics of the DOM pool to various scenarios of climate change, cultivating practices, and water resource management.
Materials and methods
Study sites
The Kyeongan watershed covers approximately 567 km2 along the primary stream with a length of 48.27 km. The overall land-use consists of 66.3 % forest and wetland, 21.4 % agriculture, and 3.6 % urban (Nguyen et al. 2010). The annual average temperature in this region in 2013 was 11.4 °C, and it ranged from −22.5 to 34.3 °C. The total annual precipitation in 2013 was 1367 mm from 118 days of rain or snow at a rate of over 0.1 mm/day. The remaining 247 days were relatively dry, with less than 0.1 mm/day of precipitation.
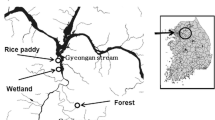
The soil samples were collected from a wetland (37°21′56.83″ N, 127°18′08.65″ E) and a forest (37°21′16.14″ N, 127°27′45.22″ E) that are located within the Kyeongan watershed (Fig. 1) on the 15–16th of May 2013. The dominant types of reeds in the wetland were Phragmites australis and Zizania latifolia (Cho and Kim 1994). The forest was dominated by Quercus (white oak) species and Robinia pseudoacacia Linnaeus (black locust). The predominant species among the weeds was Persicaria thunbergii (Polygonaceae), which commonly grows near rivers in Korea (Hur 2011).
Experimental design
The soil samples were taken from the top 10 cm of the surface horizon at each site. Each type of soil represents a composite of three individual soil samples taken within an area of 0.1 ha. After collection, the soil samples were kept in an icebox and shipped to the laboratory, where the soil samples were homogenized, air-dried for two weeks, and passed through a 2-mm sieve (Cai et al. 2013; Chen and Shrestha 2012). The water contents of the homogenized wetland and forest soils were 54.8 and 20.7 %, respectively. The air-dried wetland and forest soil samples were sent to the National Instrumentation Center for Environmental Management for elemental analysis of C, N, and S. The results of the elemental analysis, ignition loss (Korea Ministry of Environment (KMOE) 2011), pH (KMOE 2009), and particle size (Korea Ministry of Environment (KMOE) 2011) are summarized in Table 1.
The homogenized soils were then placed in acrylic plastic pots (10 cm diameter × 15 cm height) with a 1-cm diameter hole centered at 3 cm above the bottom. The hole was covered with a screen to prevent soil loss, and the outlet was sealed with a rubber septum.
A laboratory experiment was carried out in three growth chambers where the ambient temperatures were kept constant at 20, 25, and 30 °C throughout the experiment. Two pots with wetland soil (wetland pots) and two pots with forest soil (forest pots) were placed in each chamber. To investigate the effects of nitrogen deposition on the soil, two pots (one wetland pot and one forest pot in each growth chamber) received 0.02 g of dissolved NH4NO3 (aq) every month. The amount of the NH4NO3 input was calculated based on the estimated amount of total nitrogen deposition in Asia, which is 5000 mg N m−2 years−1 (Galloway et al. 2004), and the surface area (approximately 80 cm2) of each pot.
The moisture contents of the soils were kept constant by frequently adding deionized (DI) water to the soil surface, except for two-week drying intervals. Maintaining a constant moisture content value for two months was defined as a “wet” treatment, and having a two-week dry period in the middle of a “wet” treatment was defined as a “dry” treatment. The soil samples with the constant water content were classified as the wet group, and the rest of the samples were classified as the dry group. At the end of each treatment (wet or dry), the leachate was collected from the outlet 24 h after adding 500 mL of DI water to each pot. The differences in the parameter values between the beginning and the end of each treatment were denoted as Δ. The second wet and dry treatments were carried out consecutively in the same manner.
Instrumental analysis
Aliquots (50 mL) of the outflow samples were filtered using Whatman GF/CTM glass fiber filters (GE Healthcare, UK), and stored in a refrigerator at 2 °C until measurement. The samples were brought to room temperature before analyses of the dissolved organic carbon (DOC), specific UV absorbance (SUVA), and excitation–emission matrix (EEM) fluorescence spectroscopy (Nguyen et al. 2010; Westerhoff et al. 2001).
The DOC concentrations of the samples (mg C/L) were measured using a Shimadzu V-CPH analyzer. Based on repeated measurements, the relative precision of the DOC measurements was less than 3 %.
The absorption measurements were performed using an Agilent 8453 UV–Visible Spectrophotometer with a 1-cm width quartz cuvette. The specific ultraviolet absorbance (SUVA) values of the samples were calculated from the ratio of the UV absorbance at 254 nm to the DOC concentration multiplied by 100.
The fluorescence EEMs were obtained using a Hitachi Fluorescence Spectrometer F-7000 at 700 V with a xenon lamp. The analyte was put into a standard 1-cm quartz cuvette for scanning the emission spectra from 290 to 540 nm in 1-nm increments in response to each excitation wavelength from 250 to 400 nm at a 5-nm increment. The excitation and emission slits were adjusted to 5 nm with a response time of 0.5 s. The scanning speed of the instrument was set at 2400 nm/min. To eliminate the inner filter effect from the fluorescence measurements, all of the samples were diluted so that the maximum absorbance of the entire EEM scan was less than 0.05 (Hua et al. 2010). The fluorescence response to a blank solution (Milli-Q water) was subtracted from the fluorescence EEM of each sample. A “zero triangle” was also added to the EEMs where the excitation wavelength was larger than the emission wavelength (Nguyen et al. 2013; Stedmon et al. 2003). The region of the “zero triangle” was placed between the excitation wavelengths of 355–400 nm and the emission wavelengths of 320–378 nm. The EEM data were exported to an Excel file. The humification index (HIX) was calculated from the ratio of the region with an emission wavelength of 435 to 480 nm to that with an emission wavelength of 300 to 345 nm at an excitation wavelength of 254 nm, following the approach described by Zsolnay et al. (1999). The fluorescence index (FI) and biological index (BIX) were calculated following the approach described by Birdwell and Engel (2010). The ratio of the emission intensity at 450 nm to that at 500 nm, with the excitation wavelength of 370 nm, was assigned as FI. Similarly, the ratio of the emission intensity at 380 nm to that at 430 nm, with the excitation wavelength of 310 nm, was assigned as BIX.
PARAFAC modeling
PARAFAC models three-way data based on the assumption of the trilinearity of the fluorescence spectra by minimizing the sum of the squares of the residuals, as shown in Eq. (1) as follows:
where x ijk is one element of the three-way data array, with dimensions I, J, and K. For the EEMs, the element x ijk represents the fluorescence intensity of sample i measured at an emission wavelength j and an excitation wavelength k. The value of a if is directly proportional to the concentration of the f th fluorophore in the i th sample (defined as scores). The values of b j f and c kf are the estimates of the emission and excitation spectra, respectively, for the f th fluorophore. F represents the total number of components in the PARAFAC model, and i j k is the residual element, which represents the variability not accounted for by the model (Stedmon et al. 2003).
For this study, the PARAFAC modeling was performed using MATLAB 7.6 (MathWorks, Natick, MA, USA) with the N-way toolbox (Andersson and Bro 2000). A total of 72 EEMs (two sites × two nitrogen deposition conditions × three temperatures × three leachate collection times × two cycles) from the forest and the wetland soils under three different temperature conditions, with or without NH4NO3 deposition, were loaded on an N-way program for the PARAFAC analysis. The appropriate number of components was determined primarily based on the following three diagnostic tools: (1) core consistency, (2) non-zero loading, and (3) the split-half method (Murphy et al. 2013). Two outliers were identified as having leverages much greater than the median value, and excluding these outliers produced a notably different modeling result (Stedmon and Bro 2008). A total of 70 EEMs were used to produce three representative PARAFAC components. The component scores indicated the relative concentrations of the groups. Therefore, it was reasonable to estimate the concentrations of the three representative components as being proportional to the corresponding scores, although the values might not have represented the actual concentration of any of the particular fluorophores (Stedmon and Bro 2008).
Statistical analyses
The analyses of variance (ANOVA) were performed using SPSS 20 (IBM Corp., USA). The significance of the nitrogen deposition or temperature variation to the changes in the DOC was determined based on the two-way ANOVA test with 10–12 samples for each analysis unit, namely the wet forest, dry forest, wet wetland, or dry wetland group. A general linear model was selected for the analysis. A principal component analysis (PCA) with all seven of the dependent variables was performed using the principal function in the psych library of the open source language R.
Results and discussion
Variations in the DOC
The changes in the DOC normalized by the initial DOC (at the beginning of each treatment group) over two months are summarized in Fig. 2. Due to large differences in the DOC changes between the forest and wetland, the two soil groups were analyzed separately. As shown in Fig. 2a, a two-week drying period for both the forest and wetland dry groups resulted in significant increases in the DOC over two months, whereas the corresponding wet groups did not change. This observation agrees with the results from previous studies (Clark et al. 2006; Lundquist et al. 1999), indicating that rewetting after drying or a drought period is accompanied by a large increase in the DOC, and its magnitude depended on the length of the drying period.
The changes in the DOC normalized by the initial DOC (time zero of each group) over two months showing the drying-rewetting effect (a), nitrogen deposition effect (b) (the upward diagonal and solid bar correspond to without and with the monthly addition of 0.02 g NH4NO3, respectively), and temperature effect (c) (the upward diagonal, checker board, and solid bar correspond to 20, 25, and 30 °C, respectively). Green represents the forest, and orange represents the wetland. The bar heights show the averages, and error bars correspond to the standard errors
Each of the dry and wet groups was sub-grouped into those with and without nitrogen deposition (Fig. 2b) to see whether increasing the amount of nitrogen deposition with fertilizer would affect the microbial activities and DOC levels. Similarly, sub-grouping into different temperature conditions (Fig. 2c) was performed to compare the DOC changes with respect to the incubation temperature. A series of two-way ANOVA tests were conducted for the dry forest, wet forest, dry wetland, and wet wetland groups (Table 2) with 10–12 sample datasets (two nitrogen deposition conditions × three temperatures × two cycles—outliers) for each group. The significance of the effects of the nitrogen deposition and temperature on the ∆DOC/DOC0 was not as prominent as the drying-rewetting effect shown in Fig. 2a. Except for the dry forest group, adding 0.02 g/month of NH4NO3 did not affect the ∆DOC/DOC0 based on p < 0.05 as the criterion for discrimination. This result suggests a counterargument to the previous reports by Fog (1988) and Guggenberger (1994) that the nitrogen deposition enhances the DOC, resulting from the reduced lignolytic activity. On the other hand, the temperature variations were only effective for the dry wetland group (Table 2). These different response levels to the environmental conditions might be derived from the intrinsic sensitivities of the primary microorganisms utilizing carbon in the forest and the wetland. Davidson and Janssens (2006) have shown that the overall temperature sensitivities of soil carbon decomposition were collectively governed by abiotic constraints, such as soil aggregate formation, adsorption onto mineral surfaces, and soil water contents, as well as biotic constraints. Therefore, both microbial processes and abiotic changes should be taken into account as the driving factors for the increase in DOC under various environmental conditions.
The underlying mechanisms that could explain the increase in the DOC associated with the drying-rewetting treatment include, but are not limited to, (1) the physical disruption of the soil aggregate, resulting in the exposure of its interior organic matter to air, water, and microorganisms for oxidation, dissolution, and degradation; (2) the breakdown of the recalcitrant or colloidal organic matter by aerobic metabolism (Canfield 1994), thus providing more labile organic carbon to the pore water; and (3) the increased soil respiration rate (Cook and Allan 1992), inducing an increase in the microbial biomass and its decay. It could be explained that the increased DOC with the drying-rewetting treatment resulted from processes such as physical disruption and chemical oxidation of soil aggregates. However, the involvement of the microbial processes and their significance need more data to be confirmed.
Fluorescence EEM analysis using the PARAFAC method
Microbial respiration and metabolism leave optical footprints in the fluorescence spectroscopy, from which the chemical structures and conformations of the dissolved organic matter could be translated. In this regard, the EEM fluorescence spectroscopy could provide clues about the extent of the microbial activities based on the positions and the relative intensities of the characteristic peaks (Fellman et al. 2010; Birdwell and Engel 2010). Recently, PARAFAC applications for the fluorescence EEMs have shown promising results for the quantitative analysis of the major DOM constituents that are closely related to microbial processes as well as the origins (Hall et al. 2005; Stedmon and Markager (2005), and Hur and Cho 2012).
In this study, a total of 70 sample EEMs were imported to the N-way toolbox to be systematically deconvoluted into three representative components (Fig. 3) of the DOM constituents. The appropriateness of the number of components was validated using the split-half method, with two sets of a random selection of 35 EEMs (Fig. 3d–f).
As depicted in Fig. 3, the prominent peaks of component 1 (C1) exhibited the greatest red-shift at Ex/Em ratios of approximately 250/460 and 340/460 nm in comparison to the red-shift observed for the EEMs of component 2 (C2) and component 3 (C3). C1 may represent refractory terrestrial humic-like substances because the degree of red-shift indicates the extent of the conjugated or condensed compounds in the DOM (Stedmon et al. 2003). Based on the previous research of EEMs, the C2 and C3 EEMs in this study may correspond to marine humic-like substances and amino acids, respectively (Baghoth et al. 2011).
The component scores obtained from the PARAFAC indicated the relative concentrations of the corresponding components. The changes in the ratio of two distinct components (C1 and C3) in response to the dry treatment (Fig. 4a) indicated the significant impact of drying and rewetting on the fluorophore compositions, which is similar to the ∆DOC/DOC0 results. However, unlike the ∆DOC/DOC0 analysis results, the fluorophore composition of the dry wetland did not show much contrast to that of the wet wetland. In the dry forest soil, the proportion of the terrestrial humic-like component was reduced to a greater extent than that of the amino acids (i.e., ΔC1/C3 < 0). In contrast, the wet forest soil exhibited the opposite trend (i.e., ΔC1/C3 > 0) on average. The dependency of the trend on the dryness could indicate that the dry forest soil released a relatively large amount of autochthonous organic matter to the leachate, while the wet forest soil might have had a more intensive humification process. However, such a dependency on the dryness was not applicable to the wetland soils (Fig. 4a). Although the effects of the nitrogen deposition (Fig. 4b) and temperature variation (Fig. 4c) were not as significant as the drying and rewetting effect (Fig. 4a), the ΔC1/C3 responded with more sensitivity to the different sites, nitrogen deposition, and temperature under wet treatment conditions than under dry treatment conditions, which was the opposite of the trends found from the ∆DOC/DOC0 analysis.
The changes in the C1/C3 over two months, showing the drying-rewetting effect (a), nitrogen deposition effect (b) (the upward diagonal and solid bar correspond to without and with the monthly addition of 0.02 g NH4NO3, respectively), and temperature effect (c)(upward diagonal, checker board, and solid bar correspond to 20, 25, and 30 °C, respectively). Green represents the forest, and orange represents the wetland. The bar heights show the averages, and error bars correspond to the standard errors
Principle component analysis
Traditionally, a fluorescence EEM is used to track the various origins of the fluorescent DOM components by prominent peak picking (Birdwell and Engel 2010; Chen et al. 2003) or by calculating the characteristic indices, such as the humification index (HIX), fluorescent index (FI), and biological index (BIX), etc. (Fellman et al. 2010). The HIX indicates the degree of the humification, which is often considered as an indicator of the maturity of the DOM (Lu et al. 2000). The FI is related to the source of the constituting DOM, and it is qualitatively described as the ratio of the microbial to terrestrially derived DOM (Fellman et al. 2010). The BIX indicates the degree of autochthonous organic matter that is freshly introduced from a microbial or biological origin (Birdwell and Engel 2010). The calculated indices in this study are summarized in the supporting information (Table S1), and they were included in the input file for the PCA study (Fig. 5a).
aThe loadings of PC1 and PC2 for ∆DOC/DOC0, ∆C1(%), ∆C3(%), ∆SUVA, ∆HIX, ∆FI, and ∆BIX. A comparison of the distributions of the scores (PC1, PC2) for the 46 sample data between the wet vs. dry groups (b), with vs. without nitrogen deposition (c), 20 vs. 25 vs. 30 °C groups (d), forest vs. wetland groups (e); Fig. 5a is overlaid in b–e to identify the most closely correlated parameters with the groups of sample data
To investigate any linear correlations between the changes in the DOC and the other optical parameters of the DOM, a PCA was conducted with the ∆DOC/DOC0, ∆C1(%), ∆C3(%), ∆SUVA, ∆HIX, ∆FI, and ∆BIX values. A total of 46 (two dryness conditions × two sites × two nitrogen deposition conditions × three temperatures × two cycles—two outliers) sample data were used for this analysis. The major principal components, PC1 and PC2, explained approximately 43 and 29 % of the total variance, respectively. The loadings of PC1 and PC2 for each input parameter are depicted in Fig. 5a. Although PC1 and PC2 were mathematically produced parameters without considering any physical or biochemical implications of the input variables, the correlation between the principal components and each input variable (Fig. 5a) provides insight into the inter-dependency of the DOC with the other optical parameters of the DOM. Disregarding the ± sign of the slope corresponding to each parameter in Fig. 5a, the ∆C1(%), ∆C3(%), and ∆BIX values are closely related to PC1, and the ∆SUVA, ∆FI, and ∆DOC/DOC0 values are closely related to PC2. The ∆HIX is a unique parameter that has almost equal contributions from PC1 and PC2. If the large variations associated with PC1 and PC2 reflect the distinctive nature of their corresponding groups of parameters, then these PCA analysis results suggest that the first group parameters (i.e., ∆C1(%), ∆C3(%), and ∆BIX) and the second group parameters (i.e., ∆DOC/DOC0, ∆SUVA, and ∆FI) are complementary to each other. In addition, assuming the similarity within the same parameter group, the PARAFAC analysis results may provide useful information about the degree of freshly produced autochthonous organic matter (i.e., ∆BIX), and the changes in the DOC may indirectly explain the degree of aromatic compounds in the DOM (i.e., ∆SUVA).
The score plots of the paired groups are presented in Fig. 5b–e to highlight the effects of each environmental factor. Figure 5b clearly shows the effects of the dry treatment, which separated the dry group from the wet group by the PC2 values. This result agrees well with the previous results of a DOC change that is closely correlated with PC2, being affected by drying and rewetting. In contrast, the dryness does not affect the PC1 scores as much as the PC2 scores, which also matches well with the previous results of the PARAFAC analysis where only the dry forest group was distinguishable from the rest of the groups. Similarly, as indicated in the DOC analysis and PARAFAC results, nitrogen deposition (Fig. 5c) and temperature increase (Fig. 5d) do not induce any trends in PC1 and PC2. On the other hand, Fig. 5e indicates mixed trends of the principal components in response to the different sites. On the positive PC1 plane (i.e., wet group), more forest group data lie on the positive PC2; whereas on the negative PC1 plane (i.e., dry group), more forest group data lie on the negative PC2. These mixed trends with site variations might be partly explained by the ∆HIX, which is fairly well aligned with the forest group data (Fig. 5e).
Conclusions
In this study, we investigated the effects of soil drying-rewetting, nitrogen deposition, and temperature rise on the DOC change and the composition of the fluorescent or UV-active DOM in forest and wetland soil leachate. Among the tested environmental factors, a two-week-long drying period followed by rewetting resulted in the greatest impact on the changes in the measured parameter values. The DOC was sensitive not only to the dry treatment but also to the other two environmental factors. Nitrogen deposition to the dry forest soil induced a noticeable increase in the DOC. The temperature increase of the dry wetland soil resulted in significant variations in DOC changes. The PARAFAC analysis successfully deconvoluted the fluorescence spectra into three different fluorescent DOM components. The dry forest soil accumulated more amino acid type DOM than humic-like substances, while the wet forest soil group showed the opposite trend. A PCA with the seven parameters (∆DOC/DOC0, ∆C1(%), ∆C3(%), ∆SUVA, ∆HIX, ∆FI, and ∆BIX) produced an effective representation of the system with two principal components and provided insight into how each parameter would respond to the environmental factors. This study, using PARAFAC and other multivariate analysis methods, has shown that drying-rewetting is a crucial environmental factor that drives changes in the DOC and the fluorescent DOM compositions. The results of this study are expected to provide a thorough understanding of how the size and the characteristics of the DOM pool respond to various scenarios of climate change, cultivation practices, and water resource management.
References
Aber JD (1992) Nitrogen cycling and nitrogen saturation in temperate forest ecosystems. Trends Ecol Evol 7(7):220–224
Andersson CA, Bro R (2000) The N-way toolbox for MATLAB. Chemom Intell Lab Syst 52(1):1–4
Baghoth SA, Sharma SK, Amy GL (2011) Tracking natural organic matter (NOM) in a drinking water treatment plant using fluorescence excitation-emission matrices and PARAFAC. Water Res 45:797–809
Birch HF (1960) Nitrification in soils after different periods of dryness. Plant and Soil XII, no 1:81–96
Birdwell JE, Engel AS (2010) Characterization of dissolved organic matter in cave and spring waters using UV–vis absorbance and fluorescence spectroscopy. Org Geochem 41:270–280
Cai Y, Wang X, Ding W, Tian L, Zhao H, Lu X (2013) Potential short-term effects of yak and Tibetan sheep dung on greenhouse gas emissions in two alpine grassland soils under laboratory conditions. Biol Fertil Soils 49:1215–1226
Canfield EE (1994) Factors influencing organic carbon preservation in marine sediments. Chem Geol 114(3–4):315–329
Chen HYH, Shrestha BM (2012) Stand age, fire and clearcutting affect soil organic carbon and aggregation of mineral soils in boreal forests. Soil Biol Biochem 50:149–157
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003) Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technology 37(24):5701–5710
Cho KH, Kim JH (1994) Distribution of aquatic macrophytes in the littoral zone of lake Paltandho, Korea. Korea J Ecol 17(4):435–442
Clark JM, Chapman PJ, Heathwaite AL, Adamson JK (2006) Suppression of dissolved organic carbon by sulfate induced acidification during simulated droughts. Environ Sci Technology 40(6):1776–1783
Cook BD, Allan DL (1992) Dissolved organic carbon in old field soils: total amounts as a measure of available resources for soil mineralization. Soil Biol Biochem 24:585–594
Davidson EA, Jassens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Fellman JB, Hood E, Spencer RGM (2010) Fluorescence spectroscopy opens new window into dissolved organic matter dynamics in freshwater ecosystems: a review. Limnol Oceanogr 55(6):2452–2462
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63(3):433–462
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vorosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Guggenberger G (1994) Acidification effects on dissolved organic matter mobility in spruce forest ecosystems. Environ Int 20(1):31–41
Hall GJ, Clow KE, Kenny JE (2005) Estuarial fingerprinting through multidimensional fluorescence and multivariate analysis. Environ Sci Technol 39(19):7560–7567
Hua B, Veum K, Yang J, Jones J, Deng BL (2010) Parallel factor analysis of fluorescence EEM spectra to identify THM precursors in lake waters. Environ Monit Assess 161:71–81
Hur J (2011) Microbial changes in selected operational descriptors of dissolved organic matters from various sources in a watershed. Water Air Soil Pollut 215:465–476
Hur J, Cho J (2012) Prediction of BOD, COD, and total nitrogen concentrations in a typical urban river using a fluorescence excitation-emission matrix with PARAFAC and UV absorption indices. Sensors 12:972–986
Kalbitz K, Meyer A, Yang R, Gerstberger P (2007) Response of dissolved organic matter in the forest floor to long-term manipulation of litter and throughfall inputs. Biogeochemistry 86:301–318
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162
Korea Ministry of Environment (KMOE) 2009 Standard methods of analyzing contaminated soils, KMOE Notice 2009–255
Korea Ministry of Environment (KMOE) 2011 Standard methods of analyzing riverine or lake sediments, KMOE Notice 2011–92
Leenheer JA, Croue JP (2003) Characterizing aquatic dissolved organic matter. Environ Sci Technol 37:18A–26A
Lu XQ, Hanna JV, Johnson WD (2000) Source indicators of humic substances: an elemental composition, solid state 13C CP/MAS NMR and Py-GC/MS study. Appl Geochem 15:1019–1033
Lundquist EJ, Jackson LE, Scow KM (1999) Wet-dry cycles affect dissolved organic carbon in two California agricultural soils. Soil Biol Biochem 31:1031–1038
Murphy KR, Stedmon CA, Graeber D, Bro R (2013) Fluorescence spectroscopy and multi-way techniques. PARAFAC, Analytical Methods 5:6557–6566
Nguyen H-M, Hur J, Shin H-S (2010) Changes in spectroscopic and molecular weight characteristics of dissolved organic matter in a river during a storm event. Water Air Soil Pollut 212:395–406
Nguyen HV-M, Lee M-H, Hur J, Schlautman MA (2013) Variations in spectroscopic characteristics and disinfection byproduct formation potentials of dissolved organic matter for two contrasting storm events. J Hydrol 481:132–142
Ogawa H, Amagai Y, Koike I, Kaiser K, Benner R (2001) Production of refractory dissolved organic matter by bacteria. Science 292:917–920
Palomo L, Meile C, Joye SB (2013) Drought impacts on biogeochemistry and microbial processes in salt marsh sediments: a flow-through reactor approach. Biogeochemistry 112:389–407
Stedmon CA, Bro R (2008) Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial. Limnol Oceanogr Methods 6:572–579
Stedmon CA, Markager S (2005) Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol Oceanogr 50:686–697
Stedmon CA, Markager S, Bro R (2003) Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar Chem 82:239–254
Westerhoff P, Chen W, Esparza M (2001) Fluorescence analysis of a standard fulvic acid and tertiary treated wastewater. J Environ Qual 30:2037–2046
Zsolnay A, Baigar E, Jimenez M, Steinweg B, Saccomandi F (1999) Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 38:45–50
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST) (Grant Nos. 2013R1A1A1058884 and 2009-0083527).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
• The manuscript has not been submitted to more than one journal for simultaneous consideration.
• The manuscript has not been published previously (partly or in full).
• A single study is not split up into several parts to increase the quantity of submissions and submitted to other journals.
• No data have been fabricated or manipulated (including images) to support its conclusions.
• There is no plagiarism.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kim, EA., Nguyen, H.VM., Oh, H.S. et al. Influence of soil conditions on dissolved organic matter leached from forest and wetland soils: a controlled growth chamber study. Environ Sci Pollut Res 23, 5203–5213 (2016). https://doi.org/10.1007/s11356-015-5740-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5740-8