Abstract
Biochar has a charcoal polycyclic aromatic structure which allows its long half-life in soil, making it an ideal tool for C sequestration and for adsorption of organic pollutants, but at the same time raises concerns about possible adverse impacts on soil biota. Two biochars were tested under laboratory-controlled conditions on Eisenia andrei earthworms: a biochar produced at low temperature from wine tree cuttings (WTB) and a commercial low tar hardwood lump charcoal (HLB). The avoidance test (48-h exposure) showed that earthworms avoid biochar-treated soil with rates higher than 16 t ha−1 for HLB and 64 t ha−1 for WTB. After 42 days, toxic effects on earthworms were observed even at application rates (100 t ha−1) that are generally considered beneficial for most crops. The concentration of HLB and WTB required to kill half of earthworms’ population (LC50; 95 % confidence limits) in the synthetic OECD soil was 338 and 580 t ha−1, respectively. Accumulation of polycyclic aromatic hydrocarbons (PAH) in earthworms exposed to the two biochar types at 100 t ha−1 was tested in two soils of different texture. In biochar-treated soils, the average earthworm survival rates were about 64 % in the sandy and 78 % clay-loam soils. PAH accumulation was larger in the sandy soil and largest in soils amended with HLB. PAH with less than four rings were preferentially scavenged from the soil by biochars, and this behaviour may mask that of the more dangerous components (i.e. four to five rings), which are preferentially accumulated. Earthworms can accumulate PAH as a consequence of exposure to biochar-treated soils and transfer them along the food chain. Soil type and biochar quality are both relevant in determining PAH transfer.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biochar is increasingly being promoted by climate policy makers as a winning strategy to reduce use of fossil fuels, improve soil fertility, and remediate polluted soils (Anyika et al., 2015) and as a tool in the offset of global warming through soil C sequestration (DeLuca et al. 2006; Lehmann et al. 2011; Laird 2008). However, due to the potential introduction of contaminants (polycyclic aromatic hydrocarbons (PAH) and potentially toxic elements), biochar management scenarios and their impacts require further and comprehensive research before this practice can be extensively implemented (Sohi et al. 2008; Verheijen et al. 2010; Oleszczuk et al., 2013; Kuśmierz and Oleszczuk 2014). The biochar loading capacity (BLC), defined as the maximum amount of biochar, which can be safely added to soils without compromising soil functions, and which is strongly influenced by both soil and biochar properties (texture, pH, organic C, soil microbial biomass, etc.), has not yet been determined (Verheijen et al. 2010).
Biochar is, in fact, a polycyclic organic material which differs only in size from a class of atmospheric priority pollutants. The Second Report to Congress on the atmospheric deposition of pollutants to the Great Waters (United States Environmental Protection Agency 1987) introduced polycyclic organic matter (POM) in the list of priority pollutants. POM has predominantly a particulate form, and eight major categories of compounds have been defined by the Environmental Protection Agency (EPA) as POM constituents. The most common category is the PAH (Sorgi 2007; Liu et al. 2008). Due to the way it is produced—burning carbon-rich biomass at high temperatures and in an oxygen-limited environment (pyrolysis)—biochar contains a wide range of PAH. Major concern is usually restricted to six to 16 compounds, specified by US EPA (2002) and that of EU (2006), due to their carcinogenic, mutagenic, or teratogenic action through the formation of DNA adducts (Freddo et al. 2012). The PAH concentration in biochar is rather variable and depends mostly on pyrolysis temperature (Keiluweit et al. 2012). Several studies noted low concentrations of PAH content for Σ16 US EPA PAH (sum of the 16 most commonly identified PAH of environmental concern considered by US EPA) ranging between 0.06 to 0.15 mg kg−1, 0.08–8.7 mg kg−1, or 0.07–3.27 mg kg−1 (Nakajima et al. 2007; Freddo et al. 2012; Hale et al. 2012) and for Σ15 EU PAH of 0.2–5 mg kg−1 (Fabbri et al. 2013). However, Brown et al. (2006) reported cumulated values of PAH ranging from 3 to 28 mg kg−1 in synthetic wood char; Kloss et al. (2012) quantified values up to 33.7 mg kg−1 for Σ16 US EPA regardless of the type of processed feedstock and Jonker and Koelmans (2002) up to Σ13 PAH 45 mg kg−1 for charcoal.
Soil background levels and proposed limits for PAH are based on the total contaminant concentration and not on the bioavailable fraction, which would be more closely related to environmental risk (Semple et al. 2004; Hale et al. 2012). Few studies evaluated the bioavailable concentration of PAH in biochar and in soils amended with biochar. This lack of knowledge involves also the extent to which PAH fractions are associated to potential ecotoxicological implications (Oleszczuk et al. 2013). Waqas et al. (2015) investigated the availability of PAH fractions from sewage sludge biochar to tomato plants; however, many PAH are animal carcinogens and their lipophilic nature can result in bioaccumulation by soil biota.
Earthworms, the most common model organism among soil biota in ecotoxicological studies, have a relatively high body lipid content; therefore, accumulation of lipophilic toxicants have often been detected in their tissues. Many small mammals and birds pray on earthworms, and this contributes in the transport of contaminants from soil to food chains (Bergknut et al. 2007; Fagervold et al. 2010).
The interaction of earthworms with biochar has already attracted the attention of researchers, but the effects are contradictory and still not fully clarified (Lehmann et al. 2011; Weyers and Spokas 2011; Wang et al. 2011). Van Zwieten et al. (2010) observed in Ferrosol that earthworms preferred biochar-amended soil over the controls: this is indeed not surprising, given the extremely acid pH of the soil (pH = 4.20) and the well-known liming effect of biochar addition. Chan et al. (2008) reported that earthworms reacted differently to different types of biochar. Gomez-Eyles et al. (2013) observed a significant weight loss of earthworms in a hardwood biochar-amended soil contaminated with PAH, relative to the same soil without biochar.
Earthworms are particularly exposed to biochar toxicity because they may feed on microbes (Shan et al. 2013) which are generally more abundant on biochar surfaces. Topliantz et al. (2005) and Ponge et al. (2006) proposed that its ingestion may favour microbes on which earthworms depend for enzymatic digestion, possibly profiting from detoxifying or pH-ameliorating effects of the material. Weyers and Spokas (2011) found that biochar application rates larger than 67 t ha−1 had a negative impact on earthworm survival rates. These contrasting results highlight the need to elucidate the complex and combined effects of soil type, application rate, and feedstock used for biochar to predict the impact of biochar application on the soil biota and particularly on earthworms.
Chemical analysis alone is not sufficient for the estimation of the risk related to the utilisation of biochars as a soil fertiliser (Malara and Oleszczuk 2013). Biological tests, albeit not alternative to chemical analyses, may prove to be useful for estimating the potential risks. In addition, the application of biological tests permits the study of the possible interactions among various contaminants that provide ultimate evidence on the existence or absence of toxic effect on organisms.
The purposes of this research were as follows: (i) to verify the biochar potential toxicity to E. andrei, a typical earthworm indicator species; (ii) to test the effect of soil texture and biochar type on PAH bioaccumulation in E. andrei; and (iii) to quantify the potential limits of biochar addition without compromising earthworm survival.
Materials and methods
Soils
The Organisation for Economic Co-operation and Development (OECD) artificial soil (OECD 1984), a standard test substrate for terrestrial ecotoxicological studies, was employed in the avoidance and toxicity tests. The artificial soil was prepared by mixing 10 % (w/w) Sphagnum moss peat, 20 % (w/w) kaolinite clay, and 70 % (w/w) industrial quartz sand. The dry components of the artificial soils were mixed thoroughly in the correct proportions before water was added in order to achieve a moisture content of about 35 % dry weight (OECD 1984). The pH was then adjusted with calcium carbonate (CaCO3) to the optimal range (6.5–7.5), and the OECD soil was left to pre-equilibrate for 2 weeks at room temperature before starting the experiment.
Two natural soils were selected for the PAH accumulation experiment: (i) a sandy soil (Typic Udorthent; 89 % sand, 6 % silt, and 5 % clay; 2.3 % organic C; pH 7.5) and (ii) a clay-loam soil (Oxyaquic Eutrudept; 19 % sand, 50 % silt, and 21 % clay; 1.8 % organic C; pH 7.3). After removing the superficial layer and plant debris, the collected soils (5–20 cm in depth) were sieved to <2 mm and stored moist at 40 % of their water holding capacity and 4 °C before use. The PAH contents of the soils are reported in Table 1.
Biochars
Two different biochars were used for this study: a biochar produced at low temperature (<350 °C) from wine tree cuttings (WTB) with a traditional earth mould kiln and a low tar hardwood lump charcoal (HLB) industrially produced by gasification. The two biochars were ground and sieved to below 2 mm and wetted with distilled water prior to soil application. Main chemical characteristics of the two biochars are reported in Table 2.
Experimental model organism
E. andrei earthworms (Oligochaeta, Lumbricidae) were reared under standard culture conditions in a climate chamber at 20 °C with 12/12 h light/dark periods and 80 % relative humidity. Plastic containers were filled with a bedding of potting soil and peat, adjusted to pH 6.5–7.5. The cultures were regularly fed with poplar leaf litter plus vegetable scraps.
For this study, sexually mature adult earthworms (2 months old) with well-developed clitellum, with a length of approximately 7 cm and a wet weight between 200 and 400 mg, were used, as recommended by international guidelines (ISO 11268–2 1998; OECD 2010). At the beginning of the experiment, adult earthworms were transferred from the culturing media, washed, dried by blotting with a tissue, and weighed before being transferred to test pots.
Avoidance test
A two-chamber earthworm avoidance test was carried out as described by Loureiro et al. (2005). Briefly, a plastic square container (20 × 20 × 10 cm) was divided by way of a removable separator into two separate sections. One section of the test vessel was filled with 800 g of the OECD soil, the other with the same soil amended with different amounts of biochar. A geometric progression of increasing additional loads was examined: the seven treatments corresponded, on a field basis, to an equivalent load of 8, 16, 32, 64, 128, 256, and 1024 t ha−1 of biochar (considering 1.3 t m−3 of soil bulk density and 20 cm of incorporation depth). Five replicates were carried out for each experimental unit. Before starting the experiments, the artificial soil and the artificial soil plus biochar mixtures were brought to 40 % of their respective water holding capacities (WHC) with distilled water and were left to equilibrate for at least 24 h. After filling the container sections, the separator was removed and ten worms were placed on the centre line on the soil surface and the container lid closed. After 48 h, the communications between the two sections were again blocked by inserting the separator, and the worms in each test substrate were extracted and counted.
Dose–response toxicity test
The earthworms were acclimatised for 24 h under the same incubation conditions described above in the untreated artificial soil before the dose–response test.
The test was conducted using the standard OECD soil as previously described (OECD 1984) with an exposure period of 59 days. As in the previous experiment, a range of concentrations in geometric progression corresponding to 0, 8, 16, 64, 128, 256, and 1024 t biochar ha−1 was tested. The artificial soil and the artificial soils plus biochar mixtures were all brought to 40 % of their WHC and were left to equilibrate for at least 24 h. Pots were kept in an incubation chamber at constant temperature (20 ± 1 °C) and humidity, under 12/12 h light/dark cycles. Water content and pH were monitored throughout the experiment. The test was run with three replicates. After 59 days, the test pots were emptied and the surviving adults counted and weighed after 24 h depuration.
On the 60th day, earthworms were returned to the same vessels and fed with a mixture of uncontaminated poplar leaves and vegetable scraps to assess if lack of fresh food may have influenced their growth and survival. The experiment went on for another 72 days, during which period each individual organism from each exposure group was weighed at regular intervals to monitor changes in wet weight.
PAH accumulation experiment
In the PAH accumulation experiment, 30 specimens of E. andrei per pot were placed in 22 plastic pots, each filled with 2 kg of sandy or clay-loam soil (controls) or biochar-amended soils. Biochar-amended soils contained 42 g of biochar per kilogram of soil. This addition rate equates to a field application of 100 t ha−1 considering a soil incorporation up to 20 cm in depth.
Right before addition, biochar was mixed in a proportion of 1:1 by dry weight with wet minced poplar leaf litter, and the mixture was either added on top of the soil (litter placement) or mixed with the soil (soil incorporation). The pots were kept in an incubation chamber at constant temperature (20 ± 1 °C) and humidity, under 12 h light/dark cycles for 48 days. All pots were sealed with nylon gauze. Soil water loss was monitored by weighting pots at regular intervals, and humidity was kept at 40 % of the soil WHC throughout the experiment. The numbers of replicate treatments were as follows: three replicates for controls for each soil type and two replicates for treatments.
At the end of the PAH accumulation experiment, earthworms were carefully handpicked from the soil, after it had been spread out in a thin layer on a polyethylene sheet. They were counted and allowed to purge the gut content on moistened filter paper until casts were no longer released. They were then rinsed, blotted dry, killed with liquid nitrogen, homogenised, and stored at −20 °C for PAH analyses (Arnold and Hodson 2007).
PAH extraction and analyses
Before extraction, the earthworms were allowed to defrost. The extraction was performed using an Accelerated Solvent Extractor (ASE X-100, Dionex): ca. 1.5 g of sample was placed in the extractor cell together with 1.5 g of dried Na2SO4. A solution of surrogate standards of deuterated PAH (100 μL, 1.2 μg mL−1 Wellington L429-IS) was added for the evaluation of recovery rates. The extraction was performed in a mixture of dichloromethane/acetone 1:1 for trace analysis (Pestanal, Sigma-Aldrich) at 140 °C and 100 atm (three extraction cycles of 10 min). The extract (ca. 120 mL) was rotary-evaporated under reduced pressure to ca. 1 mL. In order to reduce the amount of lipids present, which can interfere with gas chromatography–mass spectrometry (GC–MS) analysis, the extract was purified using alkaline digestion by adding 20 mL of 6 M NaOH and letting the solution in contact for 18 h at ambient temperature. Extraction was then carried out with 3 × 20 mL of hexane.
PAH were extracted from soil and biochar similarly as from earthworms but using toluene 100 % instead of dichloromethane/acetone and avoiding the alkaline digestion which was not required.
The organic phase was rotary-evaporated under reduced pressure to ca. 1 mL and purified by column chromatography on Florisil activated at 180 °C for 12 h, eluting with 15 mL of dichloromethane. The eluate was evaporated to dryness by a gentle nitrogen stream and the residue dissolved in 2 mL of cyclohexane for trace analysis (Pestanal, Sigma-Aldrich) containing 100 μL of Pyrene-D (20 μg mL−1, Aldrich 490695) as internal standard (Martinez et al. 2004).
PAH analysis was carried out by a GC–MS system (Agilent 6890/5973 Inert, Agilent DB 5 ms UI capillary column 30 m × 0.25 mm i.d. × 0.25 μm film thickness) with helium as carrier gas. The system was equipped with an autosampler (Gerstel MPS2).
The GC oven temperature program started at 55 °C then was ramped to 200 °C at 25 °C/min, to 320 °C at 10 °C/min, and to 325 at 25 °C/min, with a final isothermal stage held for 10 min. The mass spectrometer operated in selective ion monitoring (SIM) mode. The following mass ions were used for the quantification of individual PAH: naphthalene (Nap, m/z 128), acenaphthene (Acp, m/z 153), fluorene (Fl, m/z 166), phenanthrene (Phe, m/z 178), anthracene (Ant, m/z 178), fluoranthene (Flt, m/z 202), pyrene (Pyr, m/z 202), benzo[a]anthracene (BaA, m/z 228), chrysene (Chr, m/z 228), benzo[b + k]fluorantene (B[b + k]F, m/z 252), benzo[a]pyrene (BaP, m/z 252), indeno[123-cd]pyrene (Ind, m/z 276), dibenzo[ah]anthracene (Dba, m/z 278), and benzo[ghi]perylene (BghiP, m/z 276). The PAH recoveries were calculated respectively by the following: napthalene-d8 (m/z 136, for Nap), acenaphtylene-d8 (m/z 160, for Acp and Fl), phenanthrene-d10 (m/z 188, for Phe and Ant), fluoranthene-d10 (m/z 212, for Flt and Pyr), benzo[a]anthracene-d12 (m/z 240, for BaA and Chr), benzo[b]fluoranthene-d12 (m/z 264, for B[b + k]F), benzo[a]pyrene-d12 (m/z 264, for BaP), indeno[1,2,3-cd]pyrene-d12 (m/z 288, for IND and DBA), and benzo[ghi]perylene-d12 (m/z 288, for BghiP).
Results obtained from the analysis of a certified soil material BNAs Clay Loam Soil (CRM 131-100) were used to ensure quality control of analyses (Table 1).
Bioconcentration factors (BAFs) were expressed in kilogram of soil per kilogram of worm and were calculated by dividing the concentration of PAH per kilogram of earthworm dry weight (Cew) by that in the dry soil (Csoil) as follows (Doctor et al. 2000):
Data analyses
Concentrations were based on oven-dried soil weight and expressed as mean ± standard deviation (SD). Lethal concentration that caused 50 % deaths (LC50) was evaluated with the Trimmed Spearman-Karber Method by a statistics software tool provided by the EPA Ecological Exposure Research Division (EERD–EPA 2014) (http://www.epa.gov/nerl/topics/chemicalsafety.html).
The non-parametric Kruskal–Wallis test was performed to examine the significance of difference between treatments (P < 0.05) in the PAH bioaccumulation experiment (Miller and Miller 1993). Data were statistically analysed by R software (R Development Core Team 2010).
Results
Avoidance test
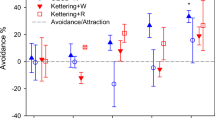
Figure 1 shows the average number of worms that remained in the biochar-treated soils after 48 h. All earthworms escaped from treatments with HLB at and above 256 t ha−1 and at 1024 t ha−1 for WTB. Elaboration of results showed that the biochar dose that resulted in a 50 % reduction of earthworm number was 45 t ha−1 for HLB and 122 t ha−1 for WTB. A significant decrease (−30 %) was already observed at 16 t ha−1 application rate in HLB treatment. The maximum biochar rate that produced non-observable effects (NOE) was equivalent to 8 and 16 t ha−1 in HLB and WTB, respectively.
Dose–response toxicity test
After 7 days, the concentrations required to kill half the members of the tested population (LC50; 95 % confidence limits) was 933 t ha−1 However, LC50 decreased with time (Fig. 2), and after 42 days, it reached 338 and 580 t ha−1 respectively for HLB and WTB and did not change significantly until the end of the experiment (59 days). All earthworms exposed to the highest rate (1024 t ha−1) of both biochars died within 3 weeks from the start of the experiment.
Following the OECD protocol, earthworms were not fed during this test, therefore earthworms that had survived lost weight in all treatments, as the organic component of the artificial soil (peat) cannot represent an adequate food source. No significant differences were found at lower biochar addition rate: the average weight loss per earthworm ranged from about 30 (control) to 25 % (16 t ha−1 biochar) of the initial wet weight. Conversely, a much larger (44 %) loss was observed in the 256 t ha−1 treatment.
At the end of the test, earthworms were put back in the same containers and fed with poplar litter plus food scraps. No more earthworms died during this further period of exposure. Cumulative weight gains per treatment, measured 72 days after regular feeding was resumed, were significantly lower in earthworms exposed to 256 t ha−1 biochar (Fig 3).
PAH accumulation experiment
An unexpectedly large mortality was observed in the biochar treatments. Biochars were added at a rate of 100 t ha−1, which corresponds to less than 30 % of the LC50 determined in the OECD soil for HLB. According to results obtained in the artificial soil toxicity test, this dose should have resulted in the loss of less than 10 % of the population. On the contrary, death rates reached 37.5 % in the sandy soil and 22.5 % in the clay-loam soil plus biochar (Table 3). In the controls, however, they were low and comprised between 1 and 4 % respectively in the sandy and clay-loam soils. Earthworms recovered from the controls were also much more viable than those found in both biochar treatments which had also acquired a lumpy and irregular shape (Supplementary materials: Figs. S1 and S2).
Considering the effect of the biochar type on the death rate, the difference with controls (mean of all soils, 2.8 %) was still highly significant: the largest mortality was caused by the HLB (39.1 %) compared to the WTB (19.2 %).
Earthworms accumulated PAH from biochar at about the same rate, either when applied at the soil surface or directly incorporated in soil (Fig. 4), so the epigeic behaviour of E. Andrei did not affect results. Surprisingly, in spite of the fact that HLB had a much larger concentration of cumulated PAH than WTB (6809 ng g−1 in HLB against 2288 ng g−1 in WTB), more PAH were taken up from the WTB than from the LHB biochar, showing a significant effect of biochar type on PAH bioaccumulation (Fig. 5). Concentrations of individual PAH compounds in earthworms, as function of soil type, are reported in the Supplementary materials (Figs. S3, S4, and S5).
Effect of biochar placement: mixed with litter and added at the soil surface (litter placement), or mixed with litter and incorporated in the soil (soil incorporation) on the cumulative PAH accumulation by Eisenia andrei. Values represented are the mean (n = 8) of both soils and both biochar types. Controls are the mean of six replicates. Bars represent standard error. Different letters show significant statistical difference (P < 0.05)
Effect of biochar type (WTB and HLB) on the cumulative PAH accumulation by Eisenia andrei. Values represented are the mean (n = 8) of both placement treatments and both soils tested. Controls are the mean of six replicates. Bars represent standard error. Different letters show significant statistical difference (P < 0.05)
Soil texture also had a significant impact on the cumulated PAH uptake, which was largest in the sandy soil (Fig. 6). Interaction with soil clay minerals evidently decreased PAH availability: earthworms took up less PAH from the clay-loam soil, notwithstanding that cumulated soil PAH concentrations in all treatments, included controls, were from 25 to 50 % larger in this soil than in the sandy soil.
Effect of soil texture on the cumulative PAH accumulation by Eisenia andrei. Values represented are the mean (n = 8) of both soil placements and both biochar types. Controls are the mean of six replicates. Bars represent standard error. Different letters show significant statistical difference (P < 0.05)
Compounds with less than four aromatic rings were either made less bioavailable by the presence of biochar or their concentrations in earthworm tissues were not significantly different from controls. On the contrary, accumulation of PAH with four or more rings followed biochar addition in the sandy soil. In the clay-loam soil, accumulation was observed only for four-ringed PAH, but was again depressed by biochar for PAH with five or more rings.
The cumulated concentrations of PAH with four or more rings (ΣPAH≥4) showed in the sandy soil an even more pronounced and significant accumulation, confirming that PAH were more readily absorbed by earthworms in the sandy soil and that more PAH were accumulated by earthworms from the WTB than from the HLB-treated soils (Fig. 7).
Effect of soil and biochar types on the accumulation of the PAH fraction with four or more rings by Eisenia andrei. Values represented are the mean (n = 4) of placement treatments. Controls are the mean of three replicates. Bars represent standard error. Different letters show significant statistical difference (P < 0.05)
Discussion
E. andrei is an epigeic species that prevalently lives in the organic-rich horizons or in compost and therefore might not be considered the best model organism to test toxicity in soil. However, it represents the standard species in most ecotoxicology studies (OECD 2010). We therefore decided to use the same organism for the different tests carried out in this work.
In our experiment on PAH accumulation, biochar was either mixed with the soil (soil incorporation) or placed at the top of the soil and mixed with the litter (litter placement). Our results concerning the litter placement treatments clearly showed that, in both cases, earthworms ingested biochar particles together with litter fragments and that they modified their feeding behaviour according to food availability. At the end of the experiment, the biochar placed at the top of the soil was clearly seen well mixed with the soil filling up borrowing holes (supplementary material). Charcoal is ingested by earthworms, together with soil particles, and when excreted, the charcoal/soil paste is stabilised by Van der Waals forces after drying and forms a dark-coloured humus (Hayes 1983). PAH accumulation was the same either when biochar has been placed on the top (litter placement) or when incorporated into the soil (Fig. 4).
The range of concentrations tested in this work is very wide, due to the geometric progression required by the OECD tests, and may seem too large to reflect actual field situations. However, as reported in the literature, agricultural yield increases were more frequently obtained at application rates up to 100 t C per hectare (Jeffery et al. 2011), corresponding to about 120 to 240 t biochar per hectare; therefore, the PAH accumulation experiment was carried out at a dose which represents a possible agronomic application rate. So far, BLCs have been determined keeping in mind potential yield effects for specific crops—or even individual application rate treatments—and tested over short periods. As stated by Verheijen et al. (2010), the BLC guidelines need to be developed considering not only the ‘per application’ rate, but also long-term cumulate rates (i.e. t ha−1 year−1 over 10 or 100 years), especially if biochar application is meant as a means to mitigate climate change. Considering all this and the persistence of biochar in soil, the range of concentrations examined does not exceed scenarios that would allow a sustainable use of biochar in agriculture.
In all experiments, biochar was pre-wetted before being added to the soil, and both soil and soil plus biochar mixtures were equilibrated at 40 % of their WHC, as a lower moisture of the soil caused by biochar addition was indicated as the main cause of avoidance by Li et al. (2011). In spite of this, earthworms showed a very clear tendency to avoid biochar-treated soils. Earthworm avoidance tests have a strong ecological implications: not only do they demonstrate sensitivity to hazardous compounds, but due to the mobility and sensitivity of earthworms, they also indicate that in the environment, the population of earthworms will be severely reduced or made less active in biochar-treated soils. This obviously does not apply to situations where soils are strongly polluted or very acidic or have some other undesirable characteristic for earthworms that can be partially mitigated by biochar addition (Van Zwieten et al. 2010). Ponge et al. (2006) reported that the earthworm Pontoscolex corethrurus, the organisms most responsible for the incorporation of charcoal into the topsoil aiding to the formation of stable humus in Terra Pretas, preferred a mixture of charcoal and soil compared to pure soil. However, the Terra Pretas actually contain only about 50 t ha−1 of charcoal C (Verheijen et al. 2010).
Liesch et al. (2010a) tested the toxicity of pine chip and poultry litter biochars on Eisenia fetida and attributed to increased soil pH earthworm mortality and their weight loss, which reached 100 % at 67.5 t ha−1 of poultry litter biochar, whereas mortality and weight loss with pine chip biochar did not differ from control treatments. In this work, at all times during the toxicity test, differences in water content among treatments did not exceed 7 % and pH values ranged between 6.9 and 7.7. Neither pH nor water contents can therefore be considered cause of avoidance or mortality.
Earthworm mortality
The much stronger toxicity of biochars observed in the two natural soils as compared to the synthetic OECD soil may have been caused by some kind of pathogen. However, earthworms in the controls did not appear to suffer from any disease, and mortality was very low in both control soils. Information in the literature is scarce, but Liesch et al. (2010b) found survival rates between 52 and 2 % for the application of different biochars. A number of PAH have an immunosuppressive effect in mice, and the degree of immunosuppression correlates with their carcinogenic potency (White et al. 1985). Either biochar made earthworms more susceptible to the pathogen, or it favoured its proliferation and transfer to earthworms. It has been shown that biochar provides a very good habitat for microorganisms, and this can mean that it could as well provide a favourable environment for some pathogens. It is also possible, however, that the much larger amount of organic matter contained in the OECD soil (about ten times that in the natural soils tested) has adsorbed and made less bioavailable any toxic compound (e.g. phenols) eventually released from biochar. The peat used to prepare the OECD artificial soil also provides organic matter not bound to mineral components and therefore potentially more active in the neutralisation of negative effects of biochar (e.g. by adsorption).
PAH accumulation
It is not likely that earthworms were affected by PAH accumulated during exposure. Cumulative concentrations of PAH in the two natural soils, 0.106 and 0.253 mg kg−1 in the sandy and clay-loam soils, respectively, were low and well within several concentration ranges previously reported for European background soils of non-polluted areas such as the following: forest 0.06–2.6 mg kg−1 (Krauss et al. 2000; UNEP 2002) and agricultural/rural 0.016–7.4 mg kg−1 (Jones et al. 1989; Wild and Jones 1995; Cousins et al. 1997; Krauss and Wilcke 2003). After addition of biochar, the concentrations increased to 0.329 mg kg−1 (WTB-amended) and 0.396 mg kg−1 (HLB-amended) in the sandy soil and 0.329 (WTB-amended) and 0.523 mg kg−1 (HLB-amended) in the clay-loam soil, but remained still well below limits suggested by EU guidelines. Bioaccumulation of PAH of four-ring size, however, occurred during the experiment in both soils and in the sandy soil, also for PAH of larger size. Albeit not very strong compared to other situations, this may still be of concern because of possible magnification through the food chain, particularly considering that most of the carcinogenic potential lays with the four to seven ring members of this class of pollutants. The average BAF (see supplementary materials), calculated with respect to the concentrations of each single PAH in the soil, was 24 in the sandy soil, compared to four in the clay-loam soil, but the tendency to accumulation varied widely among the different PAH ranging from 88-fold for benzo(b + k) fluoranthene to 2-fold for fluoranthene in the sandy soil only (see supplementary materials). No accumulation of benzo[b + k]fluoranthene, indeno[123-cd] pyrene, and dibenzo[ah]anthracene was observed in the clay-loam soil, where the strongest accumulation (18-fold) was found for benzo(a)anthracene. BAFs, calculated as the ratio between concentration of PAH in the earthworms and that in the corresponding soil, were correlated to the K ow (R 2 = 0.48) (Fig. 8).
Relationship between average (mean of all factors: soil type, biochar type, and placement) bioconcentration factors (BC = PAH concentration in earthworms/PAH concentration in biochar-amended soil) of the different PAH and the decimal logarithm of their octanol/water partition coefficients (log K ow)
Risk assessments of potential bioaccumulation based on the concentrations of extractable pollutants in earthworms are probably significantly underestimated. Irreversible binding of organic xenobiotic compounds to earthworm tissues can in fact strongly affect bioaccumulation results as demonstrated by Shan et al. (2010).
Biochar type
The two biochars employed have widely different characteristics derived from their different process conditions: WTB was produced through a slow process, low temperature in a traditional earth-mound kiln, whereas the HLB was produced industrially by gasification. WTB contained 2288 ng g−1 PAH, whereas HLB contained 6809 ng g−1. In spite of the much lower content, more PAH were accumulated in earthworms exposed to WTB. It is possible that PAH were more strongly retained by the more charred surface of the HLB and therefore made less available for sorption by the skin and gut tissues of the earthworms. In fact, with slow heating rate and temperatures, the pyrolysis results in an essentially amorphous matrix, whereas at higher rates and temperatures graphene sheets begin to grow (Amonette and Joseph 2009). Two main mechanisms are involved in PAH retention by biochar: (i) surface interactions and (ii) absorption into micropores. The latter explains the scavenging effect of both biochars towards low molecular weight compounds (Obst et al. 2009) through absorption, whereas aromatic sheets that are likely more abundant on the surface of HLB show highly favourable π−π interactions with the planar, larger PAH (Hale et al. 2012). The observed behaviour is also coherent with PAH absorption on activated carbon. Bansel and Goyal (2005) reported that PAH with lower molecular weight are adsorbed to a larger extent by activated C than are PAH with higher molecular weight. This indicates that cumulated PAH contents alone do not provide sufficient information on related potential environmental risks, but ecotoxicological test are still relevant to assess potential toxicity.
Conclusions
Application of large amounts of biochar to soils may not be a sustainable strategy for C sequestration as often claimed. Among the possible negative side effects, PAH accumulation along the food chain and toxicity for soil biota must be fully investigated before issuing guidelines on biochar maximum loads. Our results point out that negative effects on earthworms may be observed even at application rates of 100 t ha−1 that are considered beneficial for most crops.
The type of biochar also has a strong bearing on its source/sink behaviour for PAH: our work points out that the cumulated PAH content alone is not a good parameter to predict their environmental impact. The fraction of PAH from four to six rings, which features the most active carcinogenic compounds in this class, actually behaves differently than the low molecular weight members. The latter are preferentially scavenged from the soil by biochars, and their behaviour may mask the release of the more dangerous fraction. Surface properties may also be important in determining PAH bioavailability; this aspect needs to be further investigated, especially at field level, to devise processes that produce safer biochars for large-scale (agricultural) uses.
Finally, the type of soil, to which biochar is applied, can strongly affect transfer of the PAH fraction from four to six rings to soil biota, and different limits should therefore be issued for soils of different texture.
References
Amonette JE, Joseph S (2009) Characteristics of biochar: microchemical properties. In: Lehmann J, Joseph S (Eds) Biochar for Environmental Management: Science and Technology, Earthscan pp 37
Anyika C, Abdul Majid Z, Ibrahim Z, Zakaria MP, Yahya A (2015) The impact of biochars on sorption and biodegradation of polycyclic aromatic hydrocarbons in soils—a review. Environ Sci Pollut Res 22:3314–3341
Arnold RE, Hodson ME (2007) Effect of time and mode depuration on tissue copper concentrations of the earthworm Eisenia andrei, Lumbricus rubellus and Lumbricus terrestris. Environ Pollut 148:21–30
Bansel R C and M Goyal (2005) Activated Carbon Adsorption. pp 429–430. CRC Press ISBN: 978-0-8247-5344-3
Bergknut M, Sehlin E, Lundstedt S, Andersson PL, Haglund P, Tysklind M (2007) Comparison of techniques for estimating PAH bioavailability: uptake in Eisenia fetida, passive samplers and leaching using various solvents and additives. Environ Pollut 145:154–160
Brown RA, Kercher AK, Nguyen TH, Nagle DC, Ball WP (2006) Production and characterization of synthetic wood chars for use as surrogates for natural sorbents. Organic Geochem 37:321–333
Chan YK, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008) Using poultry litter biochars as soil amendments. Aust J Soil Res 46:437–444
Cousins IT, Kreibich H, Hudson LE, Lead WA, Jones KC (1997) PAHs in soils: contemporary UK data and evidence for potential contamination problems caused by exposure of samples to laboratory air. Sci Total Environ 203:141–156
DeLuca TH, MacKenzie MD, Gundale MJ, Holben WE (2006) Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci Soc Am J 70:448–453
Doctor PG, Gano KA, Lane NK (2000) Evaluation of a terrestrial food web model to set soil cleanup levels. In: Price FT, Brix KV, Lane NK (eds) “Environmental toxycology and risk assessement, vol 9, Recent Acheivements in Environmental fate and transport. American Society for testing and materials, West Conshohocken, PA
EERD–EPA (http://www.epa.gov/nerl/topics/chemicalsafety.html) Accessed 27 January 2014
EU (2006) European commission regulation No 1881/2006 of 19th December. Setting maximum levels for certain contaminants in foodstuffs, available at http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF Accessed 27 January 2014.
Fabbri D, Rombolà AG, Torri C, Spokas A (2013) Determination of polycyclic aromatic hydrocarbons in biochar and biochar amended soil. J Anal Appl Pyrol 103:60–67
Fagervold SK, Chai Y, Davis JW, Wilken M, Cornelissen G, Ghosh U (2010) Bioaccumulation of polychlorinated dibenzo-p-dioxins/dibenzofurans in E. Fetida from floodplain soils and the effect of activated carbon amendment. Environ Sci Technol 44:5546–5552
Freddo A, Cai C, Reid BJ (2012) Enviromental contextualisation of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ Pollut 171:18–24
Gomez-Eyles JL, Beesley L, Moreno-Jimenez E, Ghosh U, Sizmur T (2013) The potential of biochar amendments to remediate contaminated soils. In: Ladygina N, Rineau F (eds) Biochar and soil biota. CRC press, Boca Raton, pp 100–133
Hale SE, Lehmann J, Rutherford D, Zimmerman AR, Bachmann RT, Shitumbanuma V, O’Toole A, Sundqvist KL, Arp HPH, Cornelissen G (2012) Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ Sci Technol 46:2830–2838
Hayes MHB (1983) Darwin’s “vegetable mould” and some modern concepts of humus structure and soil aggregation. In: Satchell JE (ed) Earthworm ecology from Darwin to vermiculture. Chapman and Hall, London, pp 19–33
ISO 11268–2 (1998) Soil quality.effects of pollutants on earthworms (Eisenia fetida)-Part 2: Determination of effects on reproduction
Jeffery S, Verheijena FGA, van der Veldea MAC, Bastos A (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosys Environ 144:175–187
Jones KC, Stratford JA, Waterhouse KS, Vogt NB (1989) Organic contaminants in Welsh soils: polynuclear aromatic hydrocarbons. Environ Sci Technol 25:540–550
Jonker MTO, Koelmans AA (2002) Extraction of polycyclic aromatic hydrocarbons from soot and sediment: solvent evaluation and implications for sorption mechanism. Environ Sci Technol 36:4107–4113
Keiluweit M, Kleber M, Sparrow MA, Simoneit BRT, Prahl FG (2012) Solvent-extractable polycyclic aromatic hydrocarbon in biochar: influence of pyrolysis temperature and feedstock. Environ Sci Technol 46:9333–9341
Kloss S, Zehetner F, Dellantonio A, Hamid R, Ottner F, Liedtke V, Schwanninger M, Gerzabek MH, Soja G (2012) Characterization of slow pyrolysis biochars: effects of feedstocks and pyrolysis temperature on biochar properties. J Environ Qual 41:990–1000
Krauss M, Wilcke W, Zech W (2000) Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in forest soils: depth distribution as indicator of different fate. Environ Pollut 110:79–88
Krauss M, Wilcke W (2003) Polychlorinated naphthalenes in urban soils: analysis, concentrations and relation to other persistent organic pollutants. Environ Pollut 122:75–88
Kuśmierz M, Oleszczuk P (2014) Biochar production increases the polycyclic aromatic hydrocarbon content in surrounding soils and potential cancer risk. Environ Sci Pollut Res 21:3646–3652
Laird DA (2008) The charcoal vision: a win-win scenario for simultaneously producing bioenergy, permanently sequestring carbon, while improving soil and water quality. Agron J 100:178–181
Lehmann J, Rillig M, Thies J, Masiello C, Hockaday W, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Li D, Hockaday WC, Masiello CA, Alvarez PJJ (2011) Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol Biochem 43:1732–1737
Liesch AM, Weyers SL, Gaskin JW, Das KC, Gilkes RJ, Prakpongkep N (2010a) Impact of two different biochars on earthworm growth and survival. An Environ Sci 4:1–9
Liesch M, Weyers S, Gaskin J, Das KC, Gilkes RJ, Prakpongkep N (2010b) The effects of two different biochars on earthworm survival and microbial carbon levels. Proceedings of the 19th World Congress of Soil Science: Soil solutions for a changing world, Brisbane, Australia, 1–6 August 2010. Symposium 4.2.2 Soil and water - global change, 2010, p 67–70
Liu G, Niu Z, Niekerk DV, Xue J, Zheng L (2008) Polycyclic aromatic hydrocarbons (PAHs) from coal combustion: emissions, analysis and toxicology. Rev Environ Contam Tox 192:1–28
Loureiro S, Soares AMVM, Nogueira JA (2005) Terrestrial avoidance behavior tests as screening tool to assess soil contamination. Environ Pollut 138:121–131
Malara A, Oleszczuk P (2013) Application of a battery of biotests for the determination of leachate toxicity to bacteria and invertebrates from sewage sludge-amended soil. Environ Sci Pollut Res 20:3435–3446
Martinez E, Gros M, Lacorte S, Barceló D (2004) Simplified procedures for the analysis of polycyclic aromatic hydrocarbons in water, sediments and mussels. J Chromatogr A 1047:181–188
Miller JC, Miller JN (1993) Statistics for analytical chemistry, 3rd edn. Ellis Horwood Ltd, Chichester, p 233
Nakajima D, Nagame S, Kuramochi H, Sugita K, Kageyama S, Shiozaki T, Takemura T, Shirashi F, Goto S (2007) Polycyclic aromatic hydrocarbon generation behavior in the process of carbonization of wood. B Environ Contam Tox 79:221–225
Obst M, Grathwohl P, Cornelissen G, Kappler A, Nisch B, Hitchcock AP, Kalirai S, Reid M, Gocht T (2009) PAH Sorption to Black Carbon Particles: Spectromicroscopic Quantification and Mapping. Earth Environ Sci Canadian Light Source. 2009 Activity Report. http://www.lightsource.ca/about/pdf/activity_report_2009/4.pdf
OECD (1984) Test No. 207: Earthworm acute toxicity tests. OECD Publishing.
OECD (2010) Test No. 317: Bioaccumulation in terrestrial oligochaetes. OECD Publishing.
Oleszczuk P, Josko I, Kusmierz M (2013) Biochar properties regarding to contaminants content and ecotoxicological assessment. J Hazard Mat 260:375–382
Ponge JF, Topoliantz S, Ballof S, Rossic JP, Lavelle P, Betsche JM, Gaucher P (2006) Ingestion of charcoal by the Amazonian earthworm Pontoscolex corethrurus: a potential for tropical soil fertility. Soil Biol Biochem 38:2008–2009
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org (Accessed 01/08/2010). ISBN 3-900051-07-0
Shan J, Wang T, Gliangli C, Klumpp E, Ji R (2010) Bioaccumulation and bound-residue formation of a branched 4-nonylphenol isomer in the geophagous earthworm Metaphire guillelmi in a rice paddy soil. Environ Sci Technol 44:4558–4563
Shan J, Liu J, Wang Y, Yan X, Guo H, Li X, Ji R (2013) Digestion and residue stabilization of bacterial and fungal cells, protein, peptidoglycan, and chitin by the geophagous earthworm Metaphire guillelmi. Soil Biol Biochem 64:9–17
Semple KT, Doick KJ, Jones KC, Burauel P, Craven A, Harms H (2004) Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environ Sci Technol 38:229A–231A
Sohi SP, Lopez-Capel E, Bol R, Krull E (2008) Biochar, climate change and soil: A review to guide future research. CSIRO Land and Water Science Report 05/09, 64 pp
Sorgi K (2007) Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ Chem Lett 5:169–195
Topliantz S, Ponge JF, Ballof S (2005) Manioc peel and charcoal: a potential organic amendment for sustainable soil fertility in the tropics. Biol Fertil Soils 41:15–21
United Nations Environmental Programme (UNEP) (2002) Regionally based assessment of persistent toxic substances: Europe. UNEP, Geneva, Available from: http://www.chem.unep.ch/pts/regreports/regreports_copy(1).htm [Last Accessed 27 January 2014]
United States Environmental Protection Agency (1987) EPA −453/R-97-011 Office of Air Quality Planning and Standards, Research Triangle Park, NC27711, USA.
United States Environmental Protection Agency (2002) Polycyclic organic matter. Environmental Protec-tion Agency, Washington, DC, available at: http://www.epa.gov/ttn/atw/hlthef/polycycl.html [Last accessed 27 January 2014]
Van Zwieten L, Kimber S, Moriss S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Verheijen FGA, Jeffery S, Bastos AC, van der Velde M, Diafas I (2010) Biochar application to soils—a critical scientific review of effects on soil properties, processes and functions. EUR 24099 EN. Office for the Official Publications of the European Communities, Luxembourg, p 149
Wang F, Bu Q, Xia X, Shen M (2011) Contrasting effects of black carbon amendments on PAH bioaccumulation by Chironomus plumosus larvae in two distinct sediments: role of water absorption and particle ingestion. Environ Pollut 159:1905–1913
Waqas M, Li G, Khan S, Shamshad I, Reid BJ, Qamar Z, Chao C (2015) Application of sewage sludge and sewage sludge biochar to reduce polycyclic aromatic hydrocarbons (PAH) and potentially toxic elements (PTE) accumulation in tomato. Environ Sci Pollut Res 10.1007/s11356-015-4432-8.
Weyers SL, Spokas KA (2011) Impact of biochar on earthworm populations: a review. Appl Environ Soil Sci 2011:Article ID 541592, 12 p
White KLJ et al (1985) Immunosuppression by polycyclic aromatic hydrocarbons: a structure-activity relationship in B6C3F1 and DBA/2 mice. Immunopharmacology 9:155–164
Wild SR, Jones KC (1995) Polynuclear aromatic hydrocarbons in the UK environment: a preliminary source inventory and budget. Environ Pollut 88:91–108
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Rights and permissions
About this article
Cite this article
Malev, O., Contin, M., Licen, S. et al. Bioaccumulation of polycyclic aromatic hydrocarbons and survival of earthworms (Eisenia andrei) exposed to biochar amended soils. Environ Sci Pollut Res 23, 3491–3502 (2016). https://doi.org/10.1007/s11356-015-5568-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5568-2