Abstract
The Mar Piccolo of Taranto (Ionian Sea, Southern Italy) is a semi-enclosed and strongly polluted basin. For decades, it has been subjected to different anthropogenic impacts. These stressors caused severe sediments contamination with high concentration of different pollutants (PAHs, PCB, heavy metals). In order to assess the current status of sediments contamination, an ecotoxicological investigation combined with chemical analysis (heavy metals, PAH, and PCB) has been performed. In order to derive ecologically relevant conclusions, a multiorganisms and multiend-points approach has been applied, exposing organisms from different trophic levels to elutriate and whole sediment. The battery of bioassays consists of a microalgal growth inhibition test (Dunaliella tertiolecta), acute and sublethal assays (end-points: mortality, immobilization and swimming speed alteration) on crustaceans larvae and juveniles, and rotifers (Amphibalanus amphitrite, Artemia salina, Corophium insidiosum and Brachionus plicatilis), and embryotoxicity test on echinoderms (Paracentrotus lividus). Considering the high levels of sediment contamination highlighted from chemical analysis, an unexpected very low toxic effect was observed, even considering the sublethal end-point (larval swimming speed alteration). The results of this study suggest a very complex contaminants dynamic in the Mar Piccolo sediments that, despite a strong level of contamination, seems to not affect in a proportional manner the biological compartment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Mar Piccolo of Taranto (Ionian Sea, Southern Italy) represents an example of Mediterranean coastal marine ecosystem whose biological balances have been strongly modified by the anthropogenic development, in particular by harbor and industrial activities (petroleum refinery and an iron industry), maritime traffic (commercial and military), and urban wastewaters discharge (Calace et al. 2005; Buccolieri et al. 2006; Cardellicchio et al. 2008). Due to the strong pressure present on the area, the Mar Piccolo has been included in the list of contaminated Sites of National Interest (SIN), defined by specific statutory provisions on the basis of their characteristics, such as the quantity and hazardousness of pollutants, the extent of health and ecological risks, and the detriment to cultural and environmental heritage; criteria for identifying SIN were rationalized by the Italian Law no. 134/2012. Furthermore, the Mar Piccolo is a semi-enclosed basin, characterized by a scarce water circulation, thus promoting organic matter sedimentation that plays a key role in the transport and accumulation of contaminants in the sediments (Buccolieri et al. 2006; Cardellicchio et al. 2006, 2007). Sediments may act as a potential source of various chemical substances in aquatic system; in particular, heavily industrialized areas, such as Taranto, are a critical source of contaminants, like heavy metals, carcinogens and mutagens, and organic pollutants (PAH, PCB) which may accumulate in sediments via several pathways (Buccolieri et al. 2006; Cardellicchio et al. 2006, 2007; Di Leo et al. 2010). Furthermore, heavy metals can be absorbed from the water column onto fine particle surface, move thereafter toward sediments and affect the ecosystems through bio-accumulation and bio-magnification processes, potentially being toxic for environment and for human life (Annicchiarico et al. 2007).
The contamination of marine sediments represents a relevant environmental problem due to ecological role of this matrix into aquatic system, providing habitat and feeding for many aquatic organisms (Chapman et al. 2002; Magalhaes et al. 2007). Benthic organisms are potentially exposed to substances dissolved in the overlying water, in pore water or by direct contact to sea bottom (Burton 2002; Macken et al. 2008; Prato et al. 2010, 2012). In the past, sediments quality has been monitored mainly by chemical data acquisition. However, the complexity of sediment-contaminant interactions makes this approach insufficient to provide exact information about their potential effect on biota (Cairns 1990; Chapman et al. 1992; Davoren et al. 2005; García-Lorenzo et al. 2009; Prato et al. 2012). For this reason, many studies (Giller et al. 1998; Brohon et al. 2001; Frische 2003; Calace et al. 2005; Prato and Biandolino 2009; Prato et al. 2012) have proposed a monitoring approach based on the integration of biological and chemical measurements. Toxicity bioassays have been applied to monitor sediment quality, because living system responses are able to significantly integrate the complexity of contaminants mixture effect and to give information about their bioavailability (Novelli et al. 2003; Prato et al. 2012). Therefore, it results clear that a multispecies ecotoxicological approach is fundamental. In fact, the use of organisms representative of different trophic levels or habitats and having different sensitivity toward toxicants allows a complete evaluation of the potential contaminants toxicity, by considering several exposure routes and different end-points of effect (Burton 2002).
Species selection represents one of the most critical steps when setting up an experimental design for sediments monitoring (Bombardier and Bermingham 1999; Chapman et al. 2002; Davoren et al. 2005; Annicchiarico et al. 2007; Narracci et al. 2009; Prato et al. 2012). In detail, the battery of bioassays selected in this study consists of a microalgal growth inhibition test (Dunaliella tertiolecta), acute and sublethal assays (Immobilization and swimming speed alteration) on crustaceans and rotifers larvae (Amphibalanus amphitrite, Artemia salina and Brachionus plicatilis), a mortality test on amphipod (Corophium insidiosum), and an embryotoxicity test on echinoderms (Paracentrotus lividus). D. tertiolecta is a well-known and widely used model organism in algal bioassays because of its high sensitivity to several xenobiotics; moreover, this species can be easily maintained in laboratory cultures (US EPA 1974; Walsh 1983; Wong et al. 1995). Crustaceans and rotifers larvae are extensively used in ecotoxicological tests because of their ecological relevance and even because they are easy to maintain under laboratory conditions all year round (Sanchez-Fortùn et al. 1997; Nunes et al. 2006; Garaventa et al. 2010; Piazza et al. 2012). Sea urchins such as P. lividus are a well-known, sensitive, and reliable model organism. Toxicity bioassays (exposure of gametes and embryos to aqueous phase such as pore water) with this species are applied worldwide to assess and monitor sediment toxicity (Carr and Chapman 1995; US EPA 2000) and elutriate (Beiras 2002; Nendza 2002; Volpi Ghirardini et al. 2005). As regards amphipods, several species are successfully used in whole-sediment toxicity evaluation, because they represent one of the most sensitive taxa among benthic animals, and abundant and ecologically important component of soft-bottom estuarine and marine benthic communities. In particular, C. insidiosum was selected for this monitoring, because previous studies suggested its tolerance to non-contaminant variables (biotic and biotic) and sensitivity to toxicants (Prato and Biandolino 2006; Prato et al. 2012).
The aim of this study was to assess the current status of contamination of sediments from the Mar Piccolo of Taranto using an ecotoxicological approach based on the evaluation of sediment-associated effects on marine organisms and to evaluate these results on the light of those from chemical analysis (PAH, PCB, and heavy metals). In order to obtain ecologically relevant conclusions on sediments toxicity, a multiorganisms and multiendpoint approach was applied using a battery of bioassays with marine organisms belonging to different trophic levels, exposing them to both elutriate and whole sediment.
Materials and methods
Study area and sediments sampling
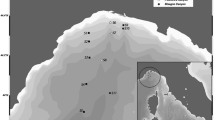
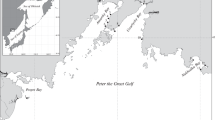
Mar Piccolo of Taranto (Ionian Sea, Southern Italy) is a semi-enclosed basin with a total surface area of 20.72 km2 structured in two shelves, “first inlet” and “second inlet,” which have a maximum depth of 13 and 10 m, respectively. Mar Piccolo can be compared to a brackish lake that communicates with the Mediterranean Sea through the Mar Grande. In fact, the first inlet is directly connected to the Mar Grande, whereas the second inlet is more internal and connected only to first one (Fig. 1). Salinity is influenced by the input of fresh water deriving from small tributary rivers and by freshwater springs called “citri.” The low hydrodynamic and the reduced water exchange with the nearby Mar Grande determine a high water stratification, mainly in summer.
Sediments were collected in June 2013 from eight stations (St.) located in the first inlet of Mar Piccolo (1A, 1D, 1I, 1E, 1 F, 1G, 1H, 1C) and three in the second one (2A, 2B, 2C) (Fig. 1 and Table 1). Sediments for ecotoxicological evaluation were sampled with a Van-Veen grab, by collecting the surface layer (0–5 cm). Those for chemical analysis were collected with a gravity corer model SW-104 (Magagnoli and Mengoli 1995). Sediments were maintained at 4 °C and analyzed within 1 week from their collection.
Chemical analyses
The top layer (0–2 cm) of core sediments was thoroughly mixed and sieved through a 2 mm mesh to remove any debris. Subsequently, a sediment aliquot was dried at 50 °C up to a constant weight, ground and homogenized in a mortar to a fine powder.
Total metals (Al, As, Cd, Cr, Cu, Fe, Hg, Li, Mn, Ni, Pb, and Zn) were determined by inductively coupled plasma atomic emission spectroscopy (ICP–AES) after acid digestion of sediments in a microwave oven. For digestion, 0.4 g of dried sample was put into a polytetrafluoroethylene vessel with 8 ml of nitric acid. For each digestion program, a blank was prepared with the same amount of acids. A microwave system (Multiwave 3000, Anton Paar, Austria) was used to accomplish sediments digestion according to US EPA test method 3051A (US EPA 2007a). An inductively coupled plasma atomic emission spectrometer (Optima 2100DV, PerkinElmer, USA) was used for As, Cd, Cr, Cu, Ni, Pb, and Zn analysis according to EPA test method 200.7 (US EPA 1994). Hg concentration was determined by cold vapor atomic absorption spectrometry (Analyst 100, PerkinElmer, USA) on to dried sediment sample following US EPA test method 245.1 (US EPA 1976).
The accuracy and precision of the analytical procedures have been checked by analyzing a certified marine reference sediment (PACS-2 marine sediment, National Research Council Canada). Analytical results indicate a good agreement between the certified and found values and metals recovery ranging from 93 % (Pb) to 111 % (Cd).
For organic pollutants analysis, another sample aliquot was air dried in the dark at room temperature for 48 h on hexane-rinsed aluminum foil and then finely ground in an agate mortar. The extraction was performed using a Microwave Sample Preparation System (Multiwave 3000, Anton Paar, Austria), in accordance with the EPA test method 3546 (US EPA 2007b) by the addiction of a 25 ml 1:1 acetone/hexane solvent mixture; samples were concentrated in a rotating evaporator (Rotavapor-R Buchi, CH), and the sulfur compounds were removed by soaking the extracts with activated copper powder.
Purification and fractionation were performed by eluting about 2–3 ml of the extracts through chromatography glass columns (with a length of 30 cm and an inner diameter of 10 mm) packed with Silica gel/Alumina/Florisil (4 + 4 + 1 g). The first fraction, containing PCBs, was eluted with 25 ml of n-hexane, whereas the second fraction, containing the PAHs, was eluted with 30 ml of 8:2 n-hexane/methylene chloride solvent mixture (Fossato et al. 1998, 2000).
The concentration of 14 US EPA priority pollutant PAHs (naphthalene (Naph), acenaphtene (Ace), fluorene (Fl), phenanthrene (Phe), anthracene (Ant), fluoranthene (Ft), pyrene (Py), benzo(a)anthracene (B[a]A), chrysene (Chy), benzo(b)fluoranthene (B[b]Ft), benzo(k)fluoranthene (B[k]Ft), benzo(a)pyrene B[a]Py, dibenzo(a,h)antracene (diB[a,h]A), and benzo(g,h,i)perylene B[g,h,i]Per) was analyzed with high-performance liquid chromatograph (PE 200, USA), coupled to a programmed fluorescence detector (HP 1046A, USA). The column used was a reverse-phase Supelcosil LC-PAH (L = 150 mm, d = 3 cm, 5 μm). Linear gradient elution was executed with acetonitrile-water mixture as mobile phase at a flow rate of 0.8 ml/min. The composition of the gradient started with 40 % of acetonitrile held constant for 4 min, and it was increased to 100 % in 11 min and then held constant for other 10 min. The column compartment was thermostated at 40 °C. A wavelength program was developed to enable the optimal detection of all compounds.
PCBs were analyzed by gas chromatography/mass spectrometry (GC/MS) (Agilent 7820A GC coupled with Agilent 5977E Series GC/MS). The software MassHunter was used for data analysis. The GC was equipped with a 30-m HP-5MS capillary column (0.25 mm ID, 0.25 μm film), and the GC conditions were the following: The GC inlet temperature was held at 280 °C, injection was performed in splitless mode, and carrier gas (helium) flow was kept at 1.8 ml/min. The initial oven temperature was 70 °C for 2 min and then ramped with 25 °C/min to 150 °C, and thereafter ramped with 3 °C/min until temperature reaches 220 °C, a final ramp with 3 °C/min until 280 °C and held for 10 min. Total runtime was 42 min. The MS Source temperature was maintained to 230 °C. Selected ion monitoring (SIM) acquisition mode was used. The identification of PAHs and PCBs was based on matching retention time, and the quantification was determined from calibration curve established for each compound by analyzing four external standard. Average coefficient of determination (R 2) of the calibration curves was greater than 0.99 for both PAHs and PCBs analysis, and the residual standard deviation (RSD) of the calibration factors was always less than 20 % (average of 10 %). The method detection limits (measured using the calibration curve method) ranged between 0.05 and 0.1 ng g−1 for PAHs and 0.05 ng g−1 for PCBs. Blanks were run for the entire procedure. Validation of the recovery and accuracy was carried out with IAEA-417 and IAEA-159 sediment sample certified reference materials.
Ecotoxicological evaluation
Elutriate tests
Elutriates were prepared according to EPA-823-F-01-023 method (US EPA 2001). In brief, 0.22-μm Filtered Natural Sea Water (FNSW) 37 ‰ salinity was added to 10-g subsamples of sediment in a 1:4 (w/v) ratio. The slurry was mixed for 1 h in dark conditions at 20 °C and then centrifuged at 1200×g for 15 min at 4 °C. The supernatant was collected and filtered through a 0.2-μm filter. Elutriate was then diluted using FNSW (“100 % elutriate” meaning not diluted elutriate) (Table 2). The assays were conducted within 24 h from elutriate preparation. As regards microalgal growth inhibition test, elutriate (and its dilutions) was prepared, by using F2 culture medium (Guillard and Ryther 1962) without EDTA (disodium ethylenediaminetetraacidic acid) to avoid its action as chelating agent.
Microalgae
The green microalga Dunaliella tertiolecta was obtained from the Institute of Marine Science culture collection. Algal cells were cultured in artificial seawater with F2 culture medium at 20 ± 0.5 °C with a 12–12-h light-dark period and light intensity of 6000–10,000 lx (Sbrilli et al. 1998). Toxicity tests have been performed according to the test method ISO 10253 (ISO 2006) modified as reported in ICRAM (2001) by using multiwell plates instead of glass flasks. The microalga D. tertiolecta was exposed to different elutriate dilutions (Table 2). Three replicates for each dilution, including the control, were prepared. After 72 h, culture growth was stopped using Lugol’s solution, and the algal growth inhibition was evaluated (referred to the control) by counting cells with a hemocytometer Thoma, using an inverted microscope.
Crustaceans and rotifers larvae
Stage II nauplii of the barnacle Amphibalanus amphitrite, Instar I larvae of the brine shrimp Artemia salina, and 24-h early stages of the rotifer Brachionus plicatilis were used. Crustaceans larvae were obtained in laboratory conditions as reported in Piazza et al. (2012) and Garaventa et al. (2010), whereas early stages of the rotifer were purchased from MicroBioTests, Inc. (Gent, Belgium), and obtained following the protocol of the manufacturer (Rotoxkit M protocol).
For bioassays setup, 10–15 organisms were placed in polystyrene 24 multiwell plates containing 1 ml/well of different FNSW elutriate dilutions (Table 2); A. amphitrite nauplii were incubated at 20 ± 0.5 °C and brine shrimps and rotifers at 25 ± 0.5 °C, all in dark conditions. Each treatment, including the control, was prepared in triplicates.
After 24 and 48 h, the percentages of immobilization (%I) and swimming speed alteration (%SSA) were evaluated. The number of immobile organisms is made by dead larvae (considering as dead the stationary larvae that don’t move any appendages for 10 s) and by “not-swimming” larvae, considering “not-swimming” those larvae that do not shift their barycentre but move their appendages (Garaventa et al. 2010).
The sublethal end point, swimming speed alteration, was evaluated using the Swimming Behaviour Recorder set to record organisms’ movement for three seconds in dark condition. The experimental setup for measuring swimming speed alteration evaluation has been described in Faimali et al. (2006) and Garaventa et al. (2010). Data were referred as % of Alteration of the swimming speed, normalized to the average swimming speed of the control (S = average swimming speed):
Echinoderms
Sea urchin gametes were collected, after KCl injection, from Paracentrotus lividus specimens reared under controlled conditions in a recirculating system as described in Fabbrocini and D’Adamo (2011). The embryotoxicity test was performed using the procedure reported in detail in Arizzi Novelli et al. (2002) and Volpi Ghirardini et al. (2005).
Briefly, 1 ml of fertilized egg suspension was added to 10-ml aliquots of elutriate and incubated in a dark room at 18 °C for 72 h. Samples were then preserved in concentrated buffered formalin, and the percentage of plutei with normal development was determined by counting 200 larvae. Artificial Sea Water (ASW) was used for the negative control and copper as the reference toxicant for the positive controls. Three replicates were performed for each elutriate dilution and for controls (Table 2). Two independent trials were carried out; for each trial, all elutriate samples were simultaneously tested in order to use the same pool of gametes.
Whole sediment test
Specimens of Corophium insidiosum were collected from a clean site of the Gulf of Taranto, using a 0.5-mm sieve. Once in laboratory, the organisms were placed in aerated glass containers with their native sediment and were acclimated for 3–4 days, before the tests (Prato et al. 2012).
Ten-day sediment toxicity test was performed, following the standard guides for conducting acute sediment toxicity tests with marine–estuarine amphipods, with some modifications (ASTM 1992; SETAC 1993). Briefly, 20 young adults (2–4 mm) were randomly selected and introduced into a 1-L glass beaker containing approximately 2 cm of sediment and 750 ml of filtered seawater (0.45 μm). During the 10-day exposure, no food was added to the test chambers. At the end of the test, the contents of each beaker were sieved, and the survivors were counted. Specimens were considered living, if movement was exhibited after gentle stimulation. In order to obtain an acceptable measure of the sediment acute toxicity tests, a negative control test (with sediment collected in an unpolluted area) and a positive control reference test (using CdNO3 as a reference toxicant) were conducted.
Data analysis
Immobilization test was considered valid if the percentage of affected organisms in the control was less than 10 % (US EPA 2002). The validity criterion for embryotoxicity test was a percentage of normal plutei in the control greater than 70 % (Volpi Ghirardini and Arizzi Novelli 2001) and that of the whole-sediment test was 15 % mortality in the control (Prato et al. 2012). The algal growth inhibition tests were considered valid if the control cell density increased at least by a factor 16 after 72 h (ISO 10253 2006).
Median lethal, inhibition, and effective concentrations (LC50/IC50/EC50), and related 95 % confidence limits (CL) were calculated using Spearman-Karber analysis (Finney 1978). In order to check differences versus the control, one-way analysis of variance (ANOVA), followed by Student Newman–Keuls (SNK) test pair-wise comparison of each sediment dilutions over the control, was performed.
Results
Chemical analysis
The results of chemical analysis of sediments from the Mar Piccolo of Taranto are reported in Table 3. Concentrations of inorganic and organic pollutants were compared to Italian regulatory limits for marine sediments (D.M. 260/2010) and to the effects range-low (ERL) and effects range-median (ERM) reported in sediment quality guideline (Long et al. 1995) to investigate the eco-toxicological implications. ERL defines the chemical concentrations below which the probability of toxicity and other effects on benthic biota are minimal. Differently, the ERM represents the mid-range above which adverse effects are more likely, although not always expected. Within the interval between the two values negative effects would occasionally occur (Long et al. 1995). Contaminants exceeding threshold limits are reported in gray in Table 3. With respect to the Italian regulatory limits (D.M. 260/2010), exceeding concentrations were observed for most of heavy metals as Hg, Cr, Ni, Pb, and Cd. PAHs showed a very high concentration in the first inlet (ranging from 1646 μg kg−1 d.w. in St. 1A to 4690 μg kg−1 d.w in St.1H). The PCBs exceeded legal limits in both inlets reaching values of two orders of magnitude higher than the limit (for instance, St.1I 1045 μg kg−1 d.w. vs. 8 μg kg−1 d.w.—D.M. 260/2010).
Ecotoxicological evaluation
Elutriate tests
For all the model organisms and end-points, only results obtained exposing organisms to undiluted elutriates (100 % elutriate) are reported. Results of the one-way ANOVA and post hoc comparison (100 % elutriate vs. control) for all species, end-points, and stations are reported in Table 5.
Microalgae
Algal growth inhibition for D. tertiolecta exposed to undiluted elutriate is shown in Fig. 2. Unfortunately, due to algal culture problems, it was not possible to analyze sediments 1C and 1H and those from the second inlet. The test validity criterion was satisfied; in fact, the algal density in the control after 72 h reached the concentration of 2.09 × 105 ± 4.43 × 103 cell/ml, starting from an initial inoculum in the order of 2 × 103 cell/ml. A high variability of growth inhibition among stations is evident, with effect percentages ranging from 6 to 39 %. As reported in Table 4, the algal growth inhibition was significantly affected (compared to the control, p < 0.01) in five stations (1A, 1D, 1C, 1E, 1F), but none of the sediments elutriates were able to affect the growth of at least 50 % thus not allowing the IC50 value calculation (Table 4).
Crustacean larvae and rotifers
Immobilization percentages (%I) calculated exposing crustaceans (A. salina and A. amphitrite) and rotifers (B. plicatilis) to undiluted sediment elutriates for 24 and 48 h are reported in Fig. 3. Test validity criterion was achieved with the following %I values after 48 h: A. salina 3.7 ± 1.6, A. amphitrite 0, and B. plicatilis 8.27 ± 3.7. After 24 h, the percentage of immobilization was lower than 30 % for all tested species and for all sediments; therefore, it was not possible to calculate the EC50 values (Table 4). After 48 h, an increase of immobilization percentages was observed in several stations, especially 1A, 1C, 2A, 1G, 2B, and 2C for A. amphitrite and 2C for B. plicatilis (Fig. 3), while A. salina shows a trend similar to that obtained after 24 h of exposure to elutriates. Despite an effect enhancement, not even after this prolonged exposure time, it was possible to calculate EC50 values for all sediments for these model organisms (Table 4).
As reported in Table 5, for this end-point, A. salina does not show any significant response (in comparison with the control) for all stations at both exposure times. On the contrary, A. amphitrite and B. plicatilis show for some sampling stations significant effects at different exposure times (1A, 1D, 1I, 1F, and 1G for A. amphitrite and 1F for B. plicatilis; Table 5).
The results of the swimming speed alteration test (% SSA) obtained exposing crustaceans (A. salina and A. amphitrite), and rotifers to undiluted sediment elutriate are reported in Fig. 4.
This sublethal end-point was significantly altered (in comparison with the control, p < 0.01) for all species and for all sediments collected at both exposure times (Table 5). However, after 24 h (Fig. 4), the percentage of alteration of swimming speed was lower than 50 % for all species; therefore, it was not possible to calculate EC50 values (Table 4). After 48 h (Fig. 4), an increase of swimming speed alteration was observed for all species, in particular, A. amphitrite shows, for several stations (1D, 1C, 1E, 1H, 1G), a speed alteration between 50 and 70 % with respect to control, thus allowing the EC50 value calculation, as reported in Table 4.
Considering brine shrimps (A. salina) and the rotifer B. plicatilis, the swimming speed alteration percentages are lower than 50 % for all sampling stations even after 48 h. Furthermore, for three elutriates (1F, 2A, and 2B), an enhancement of swimming speed was observed after 48 h of exposition with respect to 24 h (Fig. 4).
It is important to highlight that sediments collected in station 1C, 1E, and 1H caused an hormetic effect for B. plicatilis response (Calabrese and Baldwin 2001), resulting in an increase of swimming speed respect to control, both after 24 and 48 h (Fig. 4).
Echinoderms
Sea urchin embryotoxicity results are reported in Fig. 5. The test validity criterion was fully satisfied with control showing a percentage of normal plutei equal to 96.5 ± 2.54. For all tested sediments, a high toxic effect is evident, with significant percentages of abnormally developed embryos versus the control (Table 5). Most of the results of embryotoxicity on sediment tested sediments allowed to calculate the EC50 values (Table 4).
Whole sediment
Crustacean juveniles
A mortality above 15 % (precisely, 3.5 ± 0.17) occurred in the negative control. All results obtained for the positive controls were within the range of previously reported values for this species (Annicchiarico et al. 2007; Prato and Biandolino 2006; Prato et al. 2010, 2012). The results of acute toxicity test with C. insidiosum exposed to whole sediment for 10 days are reported in Fig. 6. Only sediments from stations 1D, 1I, 1C, and 1E caused a mortality higher than the test validity criteria (15 % mortality in the control) however with mortality percentages lower than 30 %, thus not allowing an LC50 values calculation (Table 4).
Discussion
The potential environmental risk of sediments from Mar Piccolo is mainly due to the strong contamination in the area caused by industrial settlements and harbor activities (Buccolieri et al. 2006; Cardellicchio et al. 2006 and 2008; Annicchiarico et al. 2007; Di Leo et al. 2010). The impact of these anthropic activities is confirmed by PAHs and PCBs chemical analysis, displaying concentrations that cover a range from 157 to 4690 μg kg−1 d.w. and from 83 to 1045 μg kg−1 d.w., respectively (Table 3). Sediments from the first inlet resulted to be more contaminated than those from the second one (PCBs of 602 μg kg−1 d.w. vs. 106 μg kg−1 d.w; PHAs 2368 μg kg−1 d.w vs. 347 μg kg−1 d.w.). This result confirms what reported by Cardellicchio et al. (2007) that identified a lower PAHs and PCBs contamination in the second inlet, placed far from industrial and harbor influence and characterized by water stratification phenomena of lower intensity respect to the first inlet (Cardellicchio et al. 2006).
In this study, PAH concentrations resulted to be lower than those reported by Cardellicchio et al. (2007) in the same area and also than those registered by La Rocca et al. (1996) in the Lagoon of Venice (65–48,000 μg kg−1 d.w.) but decisively greater than those reported for other low to moderately contaminated Mediterranean coastal areas (Caricchia et al. 1993; Gogou et al. 2000; Orecchio et al. 2010; Di Leonardo et al. 2014). Also, the average current PCBs concentration resulted to be to a certain extend decreased compared to what previously reported for Mar Piccolo, with 2–1684 μg kg−1 (Cardellicchio et al. 2007), and also to what registered in different geographic areas such as Venice Lagoon with 6–1590 μg kg−1 d.w (Frignani et al. 2001) and Alexandrian Harbour with 0.9–1211 μg kg−1 (Barakat et al. 2000).
Heavy metals concentration in sediments analyzed in this survey resulted to be very high, except for Cu and Zn (Table 3). In particular Hg and Pb showed the highest levels for both inlets with results comparable to those reported by Cardellicchio et al. (2006) that identified level of Hg of 2.62 and 0.66 mg kg−1 d.w. and a concentration of Pb of 111 and 45 mg kg−1 d.w. in the first and second inlet, respectively.
For an objective evaluation of sediments pollution and in order to observe obtained ecotoxicological data in the light of the overall chemical contamination, threshold limits imposed by the Italian law (D.M. 260/2010) for heavy metals and organic pollutants (PAHs and PCBs) were reported (Table 3). Most of the investigated chemicals were beyond threshold limits, and PAHs and PCBs were considerably above them; in fact, PCBs reached values of two orders of magnitude higher than the limit (St. 1I 1045 μg kg−1 d.w. vs. 8 μg kg−1 d.w.—D.M. 260/2010).
Chemical results confirmed that sediments from Mar Piccolo are strongly contaminated; nevertheless, the obtained biological responses highlighted an unexpected low toxic effect for model organisms exposed to both elutriates and whole sediments.
Considering results obtained by exposing the green alga D. tertiolecta to elutriates, the obtained effect, even if significantly different from the control for all tested sediments except 1I and 1G (Table 5), resulted to be so slight that it was never possible to calculate the IC50 values (Fig. 2, Table 4). Moreover, a high variability of the growth inhibition percentages among stations was observed (from 6 to 39 %; Fig. 2); this finding is in agreement with what reported by Prato et al. (2012) exposing D. tertiolecta to elutriate from sediments collected from other sites in Mar Piccolo; furthermore, Prato et al. (2012) identified D. tertiolecta as the more sensitive species among those tested (T. fulvus, M. galloprovincialis, and C. insidiosum).
Considering Crustaceans (A. salina and A. amphitrite) and Rotifers (B. plicatilis) exposed to sediments elutriate, it was possible to appreciate different sensitivity levels among species and among end-points.
The acute toxicity test showed that none of the immobilization results obtained with A. salina significantly differed from controls (Table 5) after both 24 and 48 h of exposure. The rotifers showed a similar low acute response even if, with this model organism, significant differences in immobilization percentages (compared to control) in stations 1D, 1E, and 2C (Table 5) were found, but despite this, none of the immobilization test with B. plicatilis allowed to derive EC50 values (Table 4). In fact, all the immobilization percentages resulted to be below 50 %; actually, they generally reached a maximum of 20 %. Among larval stages exposed to sediment elutriates, A. amphitrite nauplii resulted to be the most sensitive in terms of acute response at both exposure times (24 and 48 h; Fig. 3). A significant toxic effect compared to controls was found for sediments from all stations except 1I, 1E, and 1H, but the level of effect was too low to calculate EC50 values; in fact, the highest percentage of immobilization, after 48 h of exposure to elutriates, was observed in stations 1C, 2A, and 2B (43.68 ± 9.68, 43.37 ± 4.41, and 43.35 ± 6.03, respectively) with values never exceeding the 50 %.
Results obtained with the swimming speed alteration test (sublethal end-point) with A. salina, A. amphitrite, and the rotifer B. plicatilis (Fig. 4) were useful to better highlight the slight toxic effect associated to the investigated sediments. Among the model organisms exposed to elutriate, A. amphitrite nauplii resulted to be the most sensitive even considering the sublethal end-point, confirming barnacle nauplii as useful model organisms in toxicity testing (Faimali et al. 2006; Piazza et al. 2012). SSA test results after 48 h of exposure of A. amphitrite to sediment elutriates allowed to calculate EC50 values for most of the investigated sediment (1D, 1C, 1E, 1H, 1G, 2B; Table 4).
The low level of toxicity is pointed out also by results obtained with B. plicatilis (Fig. 4); in fact, sediments from stations 1C, 1E, and 1H after 24 h of exposition caused an increase in rotifers swimming speed. This phenomenon is well-known in toxicology, and it is defined as hormesis; it occurs at low levels of toxicity when many biological systems display an overcompensation response, which results in a stimulation effect (Calabrese and Baldwin 2001). A similar behavior was reported by Garaventa et al. (2010) for the same model organism exposed to different toxic compounds. Between the evaluated end-points (acute and sublethal), the behavioral one (swimming speed alteration) confirmed to be a very useful tool to identify toxic effects when the level of toxicity is low (Faimali et al. 2006; Garaventa et al. 2010). Recently, many bioassays on sediment elutriate were conducted using larvae of marine organisms (Geffard et al. 2002, 2003, 2004; ASTM 1992; Nascimento et al. 2000; Losso et al. 2004; Onorati and Mecozzi 2004; Pelosi and Franchi 2003; Mueller et al. 2003; Beiras et al. 2003; Cheung et al. 2003; Davoren et al. 2005; Faimali et al. 2006), but only few authors reported study on elutriate sediment toxicity assessment by the use of behavioral responses such as the swimming speed (Chapman et al. 2002; Faimali et al. 2006). A. salina and B. plicatilis seem not to be appropriate model organisms for the screening of low toxicity sediments, resulting to be less sensitive than A. amphitrite both considering acute and behavioral end-points (Table 4). A similar observation was reported by Davoren et al. (2005) exposing A. salina larvae to pore water and elutriate, showing that this crustacean has too low sensitivity against the environmental matrix.
As regards embryotoxicity test, all sediments caused medium/high level of acute toxicity in sea urchin, and a high variability was observed among sediments, but this does not seem to be correlated with chemical contamination; the results do not allow to identify differences in toxic effect between the two inlets of the Mar Piccolo, and in fact, the average embryotoxic effect is 50.06 ± 11.60 in the first inlet and 50.95 ± 6.27 in the second one (Table 6). P. lividus revealed to be the most sensitive organism among the tested ones. The high sensitivity pointed out in this study by sea urchin, compared with others marine organisms exposed to elutriate, confirmed the choice of P. lividus as good biological model and a sensitive tool for evaluating the sediment biological quality of contaminated sediments (Volpi Ghirardini et al. 2005; Fabbrocini et al. 2010; D’Adamo et al. 2014).
The results obtained exposing microalgae D. tertiolecta, larvae of the crustaceans A. salina and A. amphitrite, and the rotifer B. plicatilis to aqueous elutriates indicated that sediments form Mar Piccolo, despite the high level of contamination highlighted by chemical analysis (Table 3), are not able to cause relevant toxic effect on the model organisms.
Considering metals toxic effect, the low toxicity exerted by sediments could be due to the negative values of redox potential reported for Mar Piccolo sediments (Cardellicchio et al. 2006) that may influence metal behavior in benthic sediment changing oxidation state of ligand, capable of completing the metal. Furthermore, the same authors pointed out that in strongly anoxic sediment like those from Mar Piccolo, sulfide production via bacterial sulfate would buffer pore-water metal concentrations.
For what concern organic micropollutants, a possible explanation of the highlighted low toxic effect could be that PAHs and PCBs have a low affinity for the water phase and therefore are not available in the elutriate matrix. The absence of correlation between the contamination levels and the toxic effect has been already observed in a previous study by Volpi Ghirardini et al. (2005). The authors exposed P. lividus to elutriates from sediments collected in different sites in the Lagoon of Venice and observed a low toxic effect associated to one sediment collected in the Industrial area of Porto Marghera characterized by high level of PAHs contamination (560 μg kg−1); authors supposed that this finding could be due to the industrial origin of the sediment and to the richness in pollutants with poor bioavailability in the water phase.
For that, it is possible to speculate that the toxic effects exerted by elutriate matrix were so low compared to detected chemicals contamination, because of a low bioavailability of contaminants such as PAH and PCB within the elutriate phase. Similar results were also observed in the whole-sediment test with the amphipod C. insidiosum. It is well known that when using a battery of bioassays for sediment assessment, the use of sediment reworker species is recommended for the high ecological relevance of whole sediment testing (Picone et al. 2008). Knowledge about the use of amphipods for sediment toxicity assessment is very wide. Moreover, a lot of studies indicated that this species is a good biological model for sediment toxicity evaluation, because amphipods come into contact with the compounds through both physical contact and ingestion of sediment material (Prato and Biandolino 2006, 2009; Annicchiarico et al. 2007; Narracci et al. 2009; Prato et al. 2010, 2012). In our study, C. insidiosum showed high tolerance toward the sediments from Mar Piccolo with a mortality around 25 % in St. 1C and 1E and never exceeding 15 and 20 % for the other stations that are the mortality percentages of the control reported by the protocol for test validity (Prato et al. 2010, 2012); the same authors previously observed a similar general mortality trend in the investigated stations (Prato et al. 2012). In 2010, Prato et al. (2010)compared the toxicity of two sediments collected in the Livorno harbor (Ligurian Sea), characterized by a heavy metal contamination lower than that found in this study with one sediment from the first Inlet of the Mar Piccolo. The results indicated that the sediments from Livorno, even if less contaminated than those analyzed in this study (Table 3), caused a significantly higher mortality of C. insidiosum (48 and 58 %) than that obtained in the present study. These results support the hypothesis that the contaminants present in sediments from Mar Piccolo (Table 3) are poorly bioavailable.
The results obtained with ecotoxicological tests, even if of low magnitude, do not allow to clearly separating the two inlets on the basis of the effect. In fact, the “inlet effect” seems to be species and end-point dependent (Table 6).
Conclusion
The results obtained in this study confirmed the importance of coupling ecotoxicological investigations with chemical ones during sediment contamination monitoring; in fact, the analytical concentration of contaminants in sediment alone provides little information as to the possible biological effects of the contaminants (Chapman et al. 1998). The use of ecotoxicological bioassays is an important complementary tool to evaluate the synergistic effect of contaminants mixture and also to evaluate the ability of sediments to hold contaminants and reduce their release in the water column. The results of this study suggest very complex contaminants dynamic in the Mar Piccolo sediments that despite a strong level of contamination seems to not affect in a proportional manner the biological compartment. This finding needs to be better addressed integrating the obtained chemical and ecotoxicological data with other results obtained during the recent and future Ritmare project surveys in the Mar Piccolo, such as contaminants accumulation in organisms, biomarkers, and responses at community levels. In fact, community level responses and ecotoxicological approaches are complementary, and the use of combined approaches of different disciplines is fundamental to achieve a good evaluation of an ecosystem health status (Martinez-Haro et al. 2015). It would be important to integrate all the chemical and biological data within a quantitative weight of evidence (WOE) approach (Regoli et al. 2014). In fact, a multidisciplinary approach is fundamental in chronically polluted sites as Mar Piccolo where the low sediment-associated toxic effect identified in this study may be altered by changes in sediment physicochemical characteristics (redox potential, pH, water stratification, dissolved oxygen) or by anthropic alterations as dredging activities that can remobilize contaminants affecting their mobility and bioavailability (Bocchetti et al. 2008).
References
Annicchiarico C, Biandolino F, Cardellicchio N, Di Leo A, Giandomenico S, Pratoe E (2007) Predicting toxicity in marine sediment in Taranto Gulf (Ionian Sea, Southern Italy) using Sediment Quality Guidelines and a battery biomassa. Ecotoxicology 16:239–246
Arizzi Novelli A, Argese E, Tagliapietra D, Bettiol C, Volpi Ghirardini A (2002) Toxicity of tributyltin and triphenyltin towards early life stages of Paracentrotus lividus (Echinodermata: Echinoidea). Environ Toxicol Chem 21(4):859–864
ASTM (1992) Standard guide for conducting 10-d static sediment toxicity tests with marine and estuarine amphipods. In Annual Book of ASTM Standards, Water and Environmental Technology 11.04, E1367-92. ASTM, Philadelphia
Barakat AO, Kim M, Qian Y, Wade TL (2000) Organochlorine pesticides and PCBs residues in sediments of Alexandria Harbour, Egypt. Mar Pollut Bull 44:1421–1434
Beiras R (2002) Comparison of methods to obtain a liquid phase in marine sediment toxicity bioassays with Paracentrotus lividus sea urchin embryos. Arch Environ Contam Toxicol 42:23–28
Beiras R, Bellas J, Fernandez N, Lorenzo JI, Cobelo-Garcia A (2003) Assessment of coastal marine pollution in Galicia (NW Iberian Peninsula); metal concentrations in seawater, sediments and mussels (Mytilus galloprovincialis) versus embryo-larval bioassays using Paracentrotus lividus and Ciona intestinalis. Mar Environ Res 56(4):531–553
Bocchetti R, Fattorini D, Pisanelli B, Macchia S, Oliviero L, Pilato F, Pellegrini D, Regoli F (2008) Contaminant accumulation and biomarker responses in caged mussels, Mytilus galloprovincialis, to evaluate bioavailability and toxicological effects of remobilized chemicals during dredging and disposal operations in harbour areas. Aquat Toxicol 89:257–266
Bombardier M, Bermingham N (1999) The SED-TOX index: toxicity-directed management tool to assess and rank sediments based on their hazard-Concept and application. Environ Toxicol Chem 18(4):685–698
Brohon B, Delolme C, Gourdon R (2001) Complementarity of bioassays and microbial activity measurements for the evaluation of hydrocarbon-contaminated soils quality. Soil Biol Biochem 33(7):883–891
Buccolieri A, Buccolieri G, Cardellicchio N, Dell’Atti A, Di Leo A, Maci A (2006) Heavy metals in marine sediments of Taranto Gulf (Ionian Sea, Southern Italy). Mar Chem 99:227–235
Burton AG Jr (2002) Sediment quality criteria in use around the world. Limnology 3:65–75
Cairns J Jr (1990) The genesis of biomonitoring in aquatic ecosystems. The environmental professional. USA 12:169–176
Calabrese EJ, Baldwin LA (2001) Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci 22:285–291
Calace N, Ciardullo S, Petronio BM, Pietrantonio M, Abbondanzi F, Campisi T, Cardellicchio N (2005) Influence of chemical parameters (heavy metals, organic matter, sulphur and nitrogen) on toxicity of sediments from the Mar Piccolo (Taranto, Ionian Sea, Italy). Microchem J 79:243–248
Cardellicchio N, Buccolieri A, Giandomenico S, Lerario VL, Lopez L, Pizzulli F (2006) Distribution and occurence of polycyclic aromatic hydrocarbons (PAHs) in sediments from the Mar Grande and gulf of Taranto (Ionian Sea, Southern Italy). Ann Chim 96(1–2):51–64
Cardellicchio N, Buccolieri A, Giandomenico S, Lopez L, Pizzulli F, Spada L (2007) Organic pollutants (PAHs, PCBs) in sediments from the Mar Piccolo in Taranto (Ionian Sea, Southern Italy). Mar Pollut Bull 55:451–458
Cardellicchio N, Buccolieri A, Di Leo A, Giandomenico S, Spada L (2008) Levels of metals in reared mussels from Taranto Gulf (Ionian Sea, Southern Italy). Food Chem 107:890–896
Caricchia AM, Chiavarini S, Cremisini C, Martini F, Morabito R (1993) PAHs, PCBs, and DDE in the Northern Adriatic Sea. Mar Pollut Bull 26(10):581–583
Carr RS, Chapman DC (1995) Comparison of methods for conducting marine and estuarine sediment porewater toxicity tests Extraction, storage and handling techniques. Arch Environ Contam Toxicol 28:69–77
Chapman PM, Swartz RC, Roddie B, Phelps HL, Van den Hurk P, Butler R (1992) An international comparison of sediment toxicity tests in the North Sea. Mar Ecol Prog Ser 91:253–264
Chapman PM, Wang F, Janssen C, Persoone G, Allen HE (1998) Ecotoxicology of metals in aquatic sediments: binding and release, bioavailability, risk assessment, and remediation. Can J Fish Aquat Sci 55:2221–2243
Chapman PM, Ho KT, Munns WR Jr, Solomon K, Weinstein MP (2002) Issues in sediment toxicity and ecological risk assessment. Mar Pollut Bull 44:271–278
Cheung KC, Wong MH, Yung YK (2003) Toxicity assessment of sediments containing tributyltin around Hong Kong harbour. Toxicol Lett 137(1–2):121–131
D. M. 260/2010 Regolamento recante i criteri tecnici per la classificazione dello stato dei corpi idrici superficiali, per la modifica delle norme tecniche del decreto legislativo 3 aprile 2006, n.152, recante norme in materia ambientale, predisposto ai sensi dell’articolo 75, comma 3, del medesimo decreto legislativo. “Ministero dell’ambiente e della tutela del territorio e del mare”. Gazzetta Ufficiale, 8 Novembre 2010 (italian)
D’Adamo R, Specchiulli A, Cassin D, Botter M, Zonta R, Fabbrocini A (2014) The effect of floods on sediment contamination in a microtidal coastal lagoon: the Lagoon of Lesina, Italy. Arch Environ Contam Toxicol 67:297–309
Davoren M, Illeabhain NS, Ohalloran J, Hartl MGJ, Sheehan D, Obrien NM et al (2005) A test battery approach for the ecotoxicological evaluation of estuarine sediments. Ecotoxicology 14:741–755
Di Leo A, Cardellicchio N, Giandomenico S, Spada L (2010) Mercury and methylmercury contamination in Mytilus galloprovincialis from Taranto Gulf (Ionian Sea, Southern Italy): risk evaluation for consumers. Food Chem Toxicol 48:3131–3136
Di Leonardo R, Mazzola A, Tramati CD, Vaccaro A, Vizzini S (2014) Highly contaminated areas as sources of pollution for adjoining ecosystems: the case of Augusta Bay (Central Mediterranean). Mar Pollut Bull 89:417–426
Fabbrocini A, D’Adamo R (2011) Gamete and embryos of sea urchins (P. lividus Lmk 1816) reared in confined conditions: their use in toxicity bioassays. Chem Ecol 27(2):105–115
Fabbrocini A, Di Stasio M, D’Adamo R (2010) Computerized sperm motility analysis in toxicity bioassays: a new approach to pore water quality assessment. Ecotoxicol Environ Saf 73:1588–1595
Faimali M, Garaventa F, Piazza V, Magillo F, Greco G, Corrà C, Giacco E, Gallus L, Falugi C (2006) Swimming speed alteration of larvae of Balanus amphitrite as behavioural end-point for laboratory toxicological bioassays. Mar Biol 149:87–96
Finney DJ (1978) Statistical method in biological assay, 3rd edn. Charles Griffin & Co. Ltd, London
Fossato VU, Campesan G, Dolci F, Stocco G (1998) Trends in chlorinated hydrocarbons and heavy metals in sediments of Venetian canals. Rapp Comm Int Mer Medit 35:258–259
Fossato VU, Campesan G, Craboledda L, Dolci F, Stocco G (2000) Organic micropollutants and trace metals in water and suspended particulate matter. In: Lasserre P, Marzollo A (ed) Venice Lagoon Ecosystem, Unesco/Murst. vol 1 p 8
Frignani M, Bellucci LG, Carraro C, Raccanelli S (2001) Polychlorinated biphenyls in sediments of the Venice Lagoon. Chemosphere 43:567–575
Frische T (2003) Ecotoxicological evaluation of in situ bioremediation of soils contaminated by the explosive 2,4,6-trinitrotoluene (TNT). Environ Pollut 121(1):103–113
Garaventa F, Gambardella C, Di Fino A, Pittore M, Faimali M (2010) Swimming Speed alteration of Artemia sp. and Brachionus plicatilis as a sub-lethal behavioural end-point for ecotoxicological surveys. Ecotoxicology 19:512–519
García-Lorenzo ML, Martinez-Sánchez MJ, Perez-Sirvent C, Molina J (2009) Ecotoxicological evaluation for the screening of areas polluted by mining activities. Ecotoxicology 18:1077–1086
Geffard A, Geffard O, His E, Amiard JC (2002) Relationships between metal bioaccumulation and metallothionein levels in larvae of Mytilus galloprovincialis exposed to contaminated estuarine sediment elutriate. Mar Ecol Prog Ser 233:131–142
Geffard O, Geffard A, His E, Budzinski H (2003) Assessment of the bioavailability and toxicity of sediment-associated polycyclic aromatic hydrocarbons and heavy metals applied to Crassostrea gigas embryos and larvae. Mar Pollut Bull 46:481–490
Geffard O, Budzinski H, LeMenach K (2004) Chemical and ecotoxicological characterization of the “Erika” petroleum: bio-tests applied to petroleum water-accommodated fractions and natural contaminated samples. Aquat Living Resour 17(3):289–296
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30:1389–1414
Gogou A, Bouloubassi I, Stephanou EG (2000) Marine organic geochemistry of the Eastern Mediterranean: aliphatic and polyaromatic hydrocarbons in Cretan Sea superficial sediments. Mar Chem 68:265–282
Guillard RRL, Ryther JH (1962) Studies on marine planktonic diatoms, I. Cyclotella nana Hustedt and Detonula confervaceae (Cleve) Gran. Can J Microbiol 8:229–239
ICRAM (2001) Metodologie analitiche di riferimento. Ministero dell’Ambiente e della Tutela del Territorio. Servizio Difesa Mare, Roma
ISO 10253 (2006) Water quality - Marine algal growth inhibition test with Skeletonema costatum and Phaeodactylum tricornutum
La Rocca C, Conti L, Crebelli R, Crochi B, Iacovella N, Rodriguez F, Turrio Baldassarri L, Di Domenico A (1996) PAHs content and mutagenicity of marine sediments from the Venice lagoon. Ecotoxicol Environ Safe 33(3):236–245
Law no. 134/2012 (conversion law of Decree 83 dated June 22, 2012)
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:8–97
Losso C, Novelli AA, Picone M, Marchetto D, Pessa G, Molinaroli E, Ghetti R, Ghirardini AV (2004) Evaluation of surficial sediment toxicity and sediment physico-chemical characteristics of representative sites in the Lagoon of Venice (Italy). J Mar Syst 51(1–4):281–292
Macken A, Giltrap M, Foley B, McGovern E, McHugh B, Davoren M (2008) An integrated approach to the toxicity assessment of Irish marine sediments: validation of established marine bioassays for the monitoring of Irish marine sediments. Environ Int 34:1023–1032
Magagnoli A, Mengoli M (1995) Carotiere a gravità “SW-104” per carote di sedimento e acqua di fondo di grande diametro e minimo disturbo. Brevetto C.N.R. N. 3730/89. R. T. n. 27, Istituto per la Geologia Marina del C.N.R. – Bologna (Italian)
Magalhaes C, Coasta J, Teixeira C, Bordalo AA (2007) Impact of trace metals on denitrification in estuarine sediments of the Douro River estuary. Portugal. Mar Chem 107:332–341
Martinez-Haro M, Beira R, Bellas J, Capela R, Coelho JP, Lopes I, Moreira-Santos M, Reis-Henriques AM, Ribero R, Santos MM, Marques JC (2015) A review on the ecological quality status assessment in aquatic systems using community based indicators and ecotoxicological tools: what might be the added value of their combination? Ecol Indic 48:8–16
Mueller DC, Bonner JS, McDonald SJ, Autenrieth RL, Donnelly KC, Lee K, Doe K, Anderson J (2003) The use of toxicity bioassays to monitor the recovery of oiled wetland sediments. Environ Toxicol Chem 22(9):1945–1955
Narracci M, Cavallo RA, Acquaviva MI, Prato E, Biandolino F (2009) A test battery approach for ecotoxicological characterization of Mar Piccolo sediments in Taranto (Ionian Sea, Sothern Italy). Environ Monit Assess 148:307–314
Nascimento A, Smith DH, Pereira SA, Sampaio de Araujo MM, Silva MA, Mariani AM (2000) Integration of varying responses of different organisms to water and sediment quality at sites impacted and not impacted by the petroleum industry. Aquat Ecosyst Health Manag 3:449–458
Nendza M (2002) Inventory of marine biotest methods for the evaluation of dredged material and sediments. Chemosphere 48:865–883
Novelli AA, Losso C, Ghetti PF, Volpi Ghirardini A (2003) Toxicity of heavy metals using sperm cell and embryo toxicity bioassays with Paracentrotus lividus (Echinodermata: echinoidea): comparisons with exposure concentrations in the lagoon of Venice, Italy. Environ Toxicol Chem 22(6):1295–1301
Nunes BS, Carvalho FD, Guilhermino LM, Van Stappen G (2006) Use of the genus Artemia in ecotoxicity testing. Environ Pollut 144:453–462
Onorati F, Mecozzi M (2004) Effects of two diluents in the Microtox-toxicity bioassay with marine sediments. Chemosphere 54(5):679–687
Orecchio S, Cannata S, Culotta L (2010) How building an underwater pipeline connecting Libya to Sicilian coast is affecting environment: polycyclic aromatic hydrocarbons (PAHs) in sediments; monitoring the evolution of the shore approach area of the Gulf of Gela (Italy). J Hazard Mater 181:647–658
Pelosi S, Franchi M (2003) Multiple survey of environmental conditions in Varano lagoon (south Italy). Quim Nov. 26(6):789–794
Piazza V, Ferioli A, Giacco E, Melchiorre N, Valenti A, Del Prete F, Biandolino F, Dentone L, Frisenda P, Faimali M (2012) A standardization of Amphibalanus (Balanus amphitrite) (Crustacea, Cirripedia) larval bioassay for ecotoxicological studies. Ecotoxicol Environ Safe 79:134–138
Picone M, Bergamin M, Alessandra AN, Noventa S, Delaney E, Barbanti A, Volpi Ghirardini A (2008) Evaluation of Corophium orientale as bioindicator for Venice Lagoon: sensitivity assessment and toxicity-score proposal. Ecotoxicol Environ Safe 70:174–184
Prato E, Biandolino F (2006) Monocorophium insidiosum (Crustacea, Amphipoda) as a candidate species in sediment toxicity testing. Bull Environ Contam Toxicol 77(1):1–9
Prato E, Biandolino F (2009) Factors influencing the sensitivity of Gammarus aequicauda population from Mar Piccolo in Taranto (Southern Italy). Ecotoxicol Environ Safe 72:770–774
Prato E, Bigongiari N, Barchigiani C, Biandolino F (2010) Comparison of amphipods Corophium insidiosum and C. orientale (Crustacea: Amphipoda) in sediment toxicity testing. J Environ Sci Health Part A 45:1461–1467
Prato E, Parlapiano I, Biandolino F (2012) Evaluation of a bioassays battery for ecotoxicological screening of marine sediments from Ionian Sea (Mediterranea Sea, Southern Italy). Environ Monit Assess 184:5225–5238
Regoli F, Pellegrini D, Cicero AM, Nigro M, Benedetti M, Gorbi S, Fattorini D, D’Errico G, Di Carlo M, Nardi A, Gaion A, Scuderi A, Giuliani S, Romanelli G, Berto D, Trabucco B, Guidi P, Bernardeschi M, Scarcelli V, Frenzilli G (2014) A multidisciplinary weight of evidence approach for environmental risk assessment at the Costa Concordia wreck: integrative indices from Mussel Watch. Mar Environ Res 92:96–104
Sanchez-Fortùn S, Sanz F, Santa-Maria A, Ros JM, De Vicente ML, Encinas MT, Vinagre E, Barahona MV (1997) Acute sensitivity of three age classes of Artemia salina larvae to seven chlorinated solvents. Bull Environ Contam Toxicol 59:445–451
Sbrilli G, Limberti A, Caldini G, Corsini A (1998) Metodologia di saggio algale per il controllo dei corpi idrici e delle acque di scarico. ARPAT Firenze pp 1–191(Italian)
SETAC (1993) Guidance document on sediment toxicity assessment for freshwater and marine environments. In: Hill IR, Matthiessen P, Heinbach F (eds) Workshop on Sediment Toxicity Assessment, Renesse, The Netherlands, 8–10 November 1993, SETAC-Europe
US EPA (1976) Method 245.1 Mercury (Manual Cold Vapor Technique) Approved for NPDES and SDWA (Issued 1974)
US EPA (1994) Method 200.7 Determination of metals and trace elements in water and wastes by inductively coupled plasma-atomic emission spectrometry
US EPA (2000) Estuarine and coastal marine waters: bioassessment and biocriteria Technical guidance. US EPA 822-B-00-024
US EPA (2001) Methods for collection, storage and manipulation of sediments for chemical and toxicological analyses: technical manual. Office of Water. EPA-823-F-01-023
US EPA (2002) Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. U.S. Environmental Protection Agency Office of Water (4303T). EPA-821-R-02-012 pp 275
US EPA (2007a) Method 3051A, Microwave assisted acid digestion of sediments, sludges, soils and oils, Revision 1 pp 30
US EPA (2007b) Method 3546, Microwave extraction. pp 13
US EPA (1974) Quality criteria for water, office of water and hazardous materials. US EPA, Washington DC
Volpi Ghirardini A, Arizzi Novelli A (2001) A sperm cell toxicity test procedure for the Mediterranean species Paracentrotus lividus (Echinodermata:Echinoidea). Environ Technol 22:439–445
Volpi Ghirardini A, Arizzi Novelli A, Tagliapietra D (2005) Sediment toxicity assessment in the Lagoon of Venice (Italy) using Paracentrotus lividus (Echinodermata: Echinoidea) fertilization and embryo bioassays. Environ Int 31:1065–1077
Walsh GE (1983) Cell death and inhibition of population growth of marine unicellular algae by pesticides. Aquat Toxicol 3:209–214
Wong YS, Tamj NFY, Lau PS, Xue XZ (1995) Toxicity of marine sediments in Victoria harbor, Hong Kong. Mar Pollut Bull 31:464–470
Acknowledgments
This work has been supported by RITMARE (Ricerca Italiana per il MARE) Flagship Project, a National Research Programme funded by the Italian Ministry of University and Research (MIUR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Rights and permissions
About this article
Cite this article
Costa, E., Piazza, V., Gambardella, C. et al. Ecotoxicological effects of sediments from Mar Piccolo, South Italy: toxicity testing with organisms from different trophic levels. Environ Sci Pollut Res 23, 12755–12769 (2016). https://doi.org/10.1007/s11356-015-5471-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5471-x