Abstract
Green synthesis of silver nanoparticles (AgNPs) using Hybanthus enneaspermus extract at room temperature that act as a reducing agent as well as capping agent has been investigated. The synthesized AgNPs were characterized by UV–visible spectroscopy, X-ray diffraction (XRD), Fourier transform infrared (FTIR), zeta potential, and dynamic light scattering (DLS) transmission electron microscopy (TEM) and energy-dispersive X-ray (EDX). The silver surface plasmon resonance was observed at 420 nm in the UV–visible spectrum. XRD peaks were observed at 2θ values in 38.20°, 44.40°, 64.60°, and 77.50° which are indexed as (111), (200), (220), and (311) bands of face-centered cubic (fcc) structures of silver. FTIR revealed the AgNPs were capped with plant compounds of alcohol, phenols, carbonyl, amines, and amide functional groups. TEM image shows that the particles were of spherical, hexagonal, and triangular in shape, and the size range was 16–26 nm. Further, DLS exhibits the average size of 25.2 nm and the zeta values were measured (−27.1 mV) which proves the stability of the AgNPs. The conversion of Ag+ ions into Ag0 was calculated using inductively coupled plasma atomic emission spectroscopy (ICP-MS) and was found to be 96 %. The biosynthesized AgNPs showed the larvicidal activity with the LC50 values of 17.24 and 13.12 mg/L against the fourth-instar larvae of Anopheles subpictus and Culex quinquefasciatus, respectively. The GC-MS analysis of the plant extract showed that 39 bioactive phytochemical compounds have been found to possess a wide range of activities, which may help in the protection against incurable diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the major public health problems in developing countries is vector-borne diseases. Especially, mosquito vectors are solely responsible for transmitting diseases such as malaria, dengue, Chikungunya, Japanese encephalitis, and lymphatic filariasis. These diseases pose a major problem in disease-prevalent countries and are presently rampant owing to amplify globalization, urbanization, and global warming (Joseph et al. 2011; Simonsen and Mwakitalu 2013). Culex mosquitoes are painful and persistent biters and are responsible for filariasis. Lymphatic filariasis is a neglected tropical disease. More than 1.3 billion people in 72 countries worldwide are threatened by lymphatic filariasis, commonly known as elephantiasis. Over 120 million people are currently infected, with about 40 million disfigured and incapacitated by the disease (WHO 2012). Anopheles stephensi is responsible for the transmission of malaria in urban regions of India (Rahman et al. 1989). In India, malaria is still the most important cause of morbidity and mortality with approximately two to three million new cases rising every year (Sharma et al. 2009). Biological control is used as an alternative to currently employed larvicides for minimizing the mosquito population which provides an effective and environmentally friendly approach to bring down mosquitoes’ population under bottom level. Unfortunately, the mosquitoes developed resistance against the chemical larvicides (Cadavid-Restrepo et al. 2012; Chenniappan and Ayyadurai 2012).
Nanotechnology is a branch of science that is growing day by day and building an impact in various aspects of human life and manufacturing a new material at a nanolevel (Ahluwalia et al. 2014; Albrecht et al. 2006). The nanotechnology areas include the development and the study of materials with unique properties arising from their nanoscale dimensions (Bhattacharya and Gupta 2005). In recent years, nanotechnology is gaining profoundness owing to its increase of vital role in most dynamic areas of research in modern science (Sharma et al. 2009). The progress in this area largely depends on the ability to synthesize nanoparticles from various materials in different sizes and shapes, as well as to their effective inclusion into complex structures (Yu 2007). The metal nanoparticles have been synthesized by using several methods, including chemical, physical, electrochemical, irradiative, photochemical, and biological techniques (Kathiravan et al. 2014). Metal nanoparticles have attractive optical and electrical properties associated to the quantum size effect and their areas such as optics, optoelectronics, catalysis, nanostructure fabrication, and chemical or biochemical sensing (Qi et al. 2004). Numerous scientists reported that chemical methods and synthesis of nanoparticles employ toxic chemicals as reducing agents, organic solvents, or non-biodegradable agents. It is dangerous to the environment and biological systems (Lee et al. 2008; Njagi et al. 2011).
Nowadays, biological resources such as bacteria, fungi, and plants could be used to synthesize nanoparticles (Ramamurthy et al. 2012). The potentials and promises of plant system in the biologically assisted synthesis of metal nanoparticles called “green synthesis” have now become a key in nanotechnology research (Jha et al. 2009). In green synthesis approach, use of the plant has offered a reliable, simple, nontoxic, and ecofriendly approach (Narayanan and Sakthivel 2010; Satishkumar et al. 2009; Suman et al. 2013a, b). Overview of silver nanoparticle (AgNP) preparation by green synthesis approaches, including mixed valance polyoxometallates, polysaccharides, tollens, and irradiation methods was reported by (Sharma et al. 2009). The green synthesis methods are low cost and efficient and generally lead to the formation of crystalline nanoparticles with a variety of sizes. This depends on nature of plant extract concentration, pH, and temperature and incubation time of synthesis reaction (Noruzi et al. 2014). The effectiveness of AgNPs obtained via green synthesis is synergistically better in therapeutic activity (Iravani 2011). The synthesis of nanoparticles using green synthesis is potentially advantageous over microorganisms due to the ease of biohazards and culture of microbes (Nalawade et al. 2014).
Hybanthus enneaspermus belongs to family Violaceae. The plant is used medicinally in Ayurveda, Siddha, and other traditional system. The plant is commonly named as “Orithal thaamarai” in Tamil and Rathnapurusha in Sanskrit. In Ayurveda, it is known as Sthalakamala. The fruit is used to treat scorpion stings (Narayanan and Sakthivel 2010; Raveendra Retnam and John De Britto 2007). It is an herbaceous plant or shrub distributed in the tropical and subtropical areas of the globe. The plant is used as an aphrodisiac, demulcent, cardiotonic, diuretic, anti-convulsant, and anti-malarial. It is applied to treat urinary infections, diarrhea, leucorrhoea, dysuria, inflammation, and male sterility (Boominathan et al. 2003; Hemalatha et al.,2003) Pharmacologically, the plant is reported to possess anti-microbial, anti-inflammatory, anti-tussive, anti-plasmodial, anti-convulsant, and free scavenging activities (Weniger et al. 2004). The components of the plant dipeptide alkaloid (aurantiamide acetate), triterpene (isoarborinol), and β-sitosterol are previously isolated from H. enneaspermus (Tripathy et al. 2009).
Hence, the present study was aimed to investigate the bioactive components present in the H. enneaspermus and biosynthesis of the AgNPs and to test the larvicidal effect.
Material and methods
Collection and identification of plant material
Fresh plant material was collected from Paradarami Village, Gudiyattam Taluk, and Vellore District of Tamil Nadu, India, during 2013. The taxonomic identification was made by Dr. Mujeera Fatima, Department of Plant Biology and Biotechnology, Nandanam Government Arts College, Chennai, India. The voucher specimen was numbered and maintained in our research laboratory and deposited in our university botany herbarium.
Preparation of plant extract for GC-MS analysis
The newly collected plant material of H. enneaspermus was washed thoroughly in tap water, shade dried, and powdered in a blender. The respective plant powder 70 g was filled in the thimble and extracted successively with methanol using a Soxhlet extractor for 10 h. The extracts were concentrated using a rotary flash evaporator and preserved at 5 °C in an airtight bottle until further use.
GC-MS analysis
GC-MS analysis was carried out with an SHIMADZU QP 2010T which composed of an auto sampler and gas chromatography interfaced to a mass spectrometer (GC-MS) instrument employing the following condition: capillary column −624 ms (30 m × 0.32 mm × 1.8 m) operating in an electronic mode at 70 eV; helium (99.99 %) was used as the carrier gas at a constant flow of 1.491 mL/min and injection volume of 1.0 mL, injector temperature of 140 °C, and ion source temperature of 200 °C. The oven temperature was programmed from 45 °C. Mass spectra were taken at 70 eV.
Synthesis of AgNPs
The fresh part of H. enneaspermus broth solution was prepared by taking 250 mg of thoroughly washed and finely cut parts of H. enneaspermus in a 250-mL Erlenmeyer flask along with 100 mL of sterilized double-distilled water, and the mixture was boiled at 60 °C for 5 min. The extract was filtered with Whatman filter paper no. 1 and stored at −10 °C for further experiments. The 3 mL of freshly prepared plant extract was added to 40 mL of 1 mM AgNO3 aqueous solution (6.79 mg of AgNO3 was added in 40 mL of Milli-Q water) at room temperature for 30 min.
Characterization of AgNPs
AgNP synthesis using H. enneaspermus extract was confirmed by UV–visible spectrophotometer (Schimadzu 1600). Further, the reaction mixture was centrifuged at 60,000×g for 40 min and the resulting pellet was dissolved in deionized water and filtered through Whatman filters (0.45 μm). An aliquot of this filtrate containing AgNPs was used for further characterization techniques. X-ray diffraction (XRD) measurement of H. enneaspermus of reduced AgNPs was carried out on films of the respective solution drop coated onto a glass substrate on a Philip PW 1830 instrument operating at a voltage 40 kV and a current of 30 mA with CuK 1 radiation. For Fourier transform infrared (FTIR) measurement, dry powder of the nanoparticles obtained in the following manner was prepared by centrifuging the synthesized AgNP solution at 10,000 rpm for 20 min. The solid residue containing AgNP solution was dispersed in sterile deionized water for three times to remove the unattached biological impurities. The pure residue was then dried perfectly in an oven overnight at 70 °C. The powder obtained was subjected to FTIR measurement carried out on a PerkinElmer Spectrum one instrumental resolution of 4 cm−1 in KBr pellets. The surface charge in terms of zeta potential and particle size distribution of the colloidal nanoparticles was determined using Malvern Zetasizer Nano Series (Malvern). Final concentration after reduction of Ag+ ions was determined using inductively coupled plasma atomic emission spectroscopy (ICP-MS) using Agilent 7700, Japan. For transmission electron microscopic studies, 25 μL of sample was sputter on carbon-coated copper transmission electron microscopy (TEM) grids. TEM measurements were performed on a JEOL model 1200EX instrument operated at an accelerating voltage of 120 kV and later with an XDL 3000 powder.
Larvicidal bioassay
One gram of aqueous aerial extract was first dissolved in 100 mL of distilled water (stock solution). From the stock solution, 100 mg/L was prepared with dechlorinated tap water for a bioassay test of plant extract. The larvicidal activity was assessed following as per the method of Rahuman et al. (2000). For the bioassay test, larvae were taken in five batches of 20 in 249 mL of water and 1.0 mL of aqueous plant extract concentration. Control was set up with dechlorinated tap water. The numbers of dead larvae were counted after 24 h of exposure, and the percentage of mortality was reported from the average of five replicates. The experimental media in which 100 % mortality of larvae occurs alone were selected for dose–response bioassay. Synthesized AgNP toxicity tests were conducted using a multiconcentration test, consisting of a control and different concentrations of nanoparticles. Each test was performed by placing 20 mosquito larvae into 200 mL of sterilized double-distilled water with nanoparticles into a 250-mL beaker (Borosil). The nanoparticle solutions were diluted using double-distilled water as a solvent according to the desired concentrations (50, 40, 30, 20, and 10 mg/L).
Dose–response bioassay
Based on the preliminary screening results, crude extract of H. enneaspermus and synthesized AgNPs were subjected to dose–response bioassay for larvicidal activity against the larvae of Anopheles subpictus and Culex quinquefasciatus. Different concentrations ranging from 50 to 250 mg/L (aqueous plant extracts) and 10 to 50 mg/L (for synthesized AgNPs) were prepared for larvicidal activity. The numbers of dead larvae were counted after 24 h of exposure, and the percentage of mortality was reported from the average of five replicates.
Statistical analysis
The average larval mortality data were subjected to probit analysis (FORTRAN) for calculating LC50. Other analyses at 95 % fiducial limit of upper confidence limit and lower confidence limit values were calculated following Reddy et al. (1992). Results with p < 0.05were considered to be statistically significant.
Result and discussion
In the present study, the larvicidal activity of H. enneaspermus aqueous extract and the synthesized AgNPs were observed against the fourth-instar larvae of Anopheles subpictus (LC50 = 117.83 and 126.59 mg/L) and against the fourth-instar larvae of C. quinquefasciatus (LC50 = 17.24 and 13.12 mg/L), respectively (Tables 1 and 2). It has been reported that leaf extracts of Ocimum canum, Ocimum sanctum, and Rhinacanthus nasutus have been found to be only moderately toxic against the larvae of Aedes aegypti with LC50 values ranging between 99.42 and 81.56 ppm (Kamaraj et al. 2008). The mosquito larvicidal activity of UV irradiation-induced AgNPs was found to decrease the survival of fourth-instar larvae of Aedes aegypti by 88 % after 24 h of exposure at 1-ppm concentration (Sap-Iam et al. 2010). Earlier authors reported that the larvicidal effect of aqueous crude leaf extracts, silver nitrate, and synthesized AgNPs of Mimosa pudica showed that the highest mortality was found in synthesized AgNPs against the larvae of Anopheles subpictus (LC50 = 08.89, 11.82, and 0.69 ppm) and against the larvae of C. quinquefasciatus (LC50 = 09.51, 13.65, and1.10 ppm) (Marimuthu et al. 2010). The synthesized AgNPs using Ammannia baccifera showed that the highest larvicidal efficacy with the LC50 values of 29.54 mg/L for Anopheles subpictus and 22.32 mg/L for C. quinquefasciatus (Suman et al. 2013a, b). The larvicidal activity of synthesized AgNPs utilizing an aqueous extract of Eclipta prostrate and the crude aqueous extract was observed against C. quinquefasciatus (LC50 = 27.49 and 4.56 mg/L; LC90 = 70.38 and 13.14 mg/L) and against Anopheles subpictus (LC50 = 27.85 and 5.14 mg/L; LC90 = 71.45 and 25.68 mg/L), respectively (Rajakumar and Rahuman 2011). Synthesized AgNPs using Tinospora cordifolia extract was tested against the larvae of Anopheles subpictus (LC50 = 6.43 mg/L) and against the larvae of C. quinquefasciatus (LC50 = 6.96 mg/L) (Jayaseelan et al. 2011).
This biological reduction of AgNPs would be a boon for the development of clean, nontoxic, and environmentally acceptable green approach to produce AgNPs involving organisms even ranging to higher plants. The formed AgNPs are highly stable and have significant mosquito larvicidal activity of Anopheles subpictus and C. quinquefasciatus. This is the first report on the larvicidal activity of synthesized AgNPs from H. enneaspermus. The use of plants can also be suitably scaled up for large-scale synthesis of nanoparticles in a controlled manner according to their size, shape, and dispersity. Moreover, the use of plants in the process of nanoparticle synthesis is more beneficial than other processes since the nanoparticles are produced extracellularly. Recently, syntheses of AgNPs by using plant extracts are getting more popular (Li et al. 2007; Song and Kim 2009).
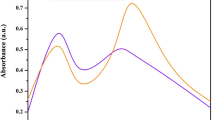
UV–vis spectroscopy was ascertained to check the formation and stability of AgNPs in aqueous solution (Fig. 5). In the current study, AgNPs were rapidly formed after the addition of H. enneaspermus extract; the reaction mixture turned dark brown (Fig. 1a) from light green after 15 min of incubation. The appearance of dark brown indicates the formation of the AgNP formation (Fig. 1a). It is well known that the optical absorption spectra of metal nanoparticles were dominated by surface plasmon resonances (SPRs) that shift to longer wavelengths with increasing particle size due to the capping agent (Brause et al. 2002). Also, it is well recognized that the absorbance of AgNPs mainly depends upon the size and shape (Rao and Trivedi 2006). Figure 1b, c shows SPR for the sample solution to occur at the wavelength of 430 nm (Fig. 1b) in 15 min and 432 nm (Fig. 1c) after 24 h which confirms the presence of AgNPs in the prepared solution. A narrow absorption peak of UV–visible range indicates that the distribution of smaller-sized nanoparticles will be higher in the mixture (Maqusood Ahamed et al. 2011). The SPR absorbance is sensitive to the nature, size, and shapes of particles present in the solution, and also, it depends upon their inner particle distance and the surrounding media (Nazeruddin et al. 2014).
The XRD pattern of the H. enneaspermus plant extract AgNPs is shown in Fig. 2. Four sharp peaks are observed at 2θ values in 38.20°, 44.40°, 64.60°, and 77.50° which are indexed as (1 1 1), (2 0 0), (2 2 0), and (3 1 1) bands of face-centered cubic (fcc) structures of silver(JCPDS File No: 03-0921). The peak corresponding to (111) plane is more intense than other planes, suggesting it as a predominant orientation. No extra diffraction peaks in XRD pattern are observed, revealing that synthesized AgNPs are essentially pure. Similar values of diffraction peaks for nanosilver have been reported by others (Satishkumar et al. 2009; Vimala et al. 2009; Ashok et al. 2010). The XRD pattern clearly demonstrated that the AgNPs were crystalline in nature and the average crystal size was found to be 12.4 nm calculated by the Scherrer equation (Sheny et al. 2011).
In order to determine the possible bio-reducing functional groups involved in reduction process and their unique interactions with AgNPs, FTIR analysis was performed. The band intensities of infrared spectra of AgNPs are depicted in Fig. 3. The broad spectrum peak at 3420 cm−1 corresponds to strong stretching vibrations of the hydroxyl functional group (Priyadarshini et al. 2013). Less-intense peak at 2920 and 2851 cm−1 could be assigned to the presence of secondary amines and C–H stretching vibrations (Ahluwalia et al. 2014). The bands at 1038 and 1636 cm−1 correspond to the –N–H and carbonyl (C–O–) stretching vibrations in amide linkages (amide I and amide II) of protein. The band at 1384 cm−1 was mainly attributed to the amide bands, −C–N stretching vibrations of proteins. The spectrum supports the presence of alcohol, phenols, carbonyl, and amines (both aromatic and aliphatic) and amide functional groups in the synthesis of AgNPs (Suresh et al. 2011). Hence, it may be assumed that these functional groups are responsible for the reduction, capping, and stabilization of synthesized AgNPs.
The zeta potential is commonly used to find out the stability of colloidal systems Jayaseelan et al. 2013; Sun et al. 2007). The zeta potential of nanoparticles was evaluated in water as dispersant. Generally, a suspension that exhibits an absolute zeta potential less than 20 mV is considered unstable and will result in precipitation of particles from solution (Sun et al. 2006), whereas the absolute zeta potential higher than 20 mV is stable (Badawy et al.,2010). In this study, zeta potentials of AgNPs were −27.1 mV indicating the stability of AgNP suspensions (Fig. 4a). Particle size determination was carried out; the particle size range was found to be 5.84 to 61.21 nm, and the Z-average was found to be 25.2 nm (Fig. 4b).
The morphology and size of the synthesized AgNPs were determined by TEM image. The particles formed were spherical, hexagonal, and triangular in shape, and the size range was 16–26 nm (Fig. 5a). The presence of the elemental silver can be identified by the EDX analysis, which indicated the reduction in silver ions to elemental silver in the reaction mixture. The EDX spectrum illustrated the presence of strong metallic Ag signals along with weak oxygen and carbon peak, which may be originating from the biomolecules that are bound to the surface of nanosilver particles (Fig. 5b). ICP-MS analysis showed the final AgNP solution prepared using H. enneaspermus extract containing 96 % of Ag0.
The results of the GC/MS analysis showed that 39 compounds (Table 3) were present in the methanol extract of H. enneaspermus. These compounds were identified through mass spectrometry attached with GC. The mass spectra of these compounds were matched with those found in the NIST/NBS spectral database and the data are given. The fragmentation pattern of the major compound (retention time 45.808) was found to be dodecanoic acid, 1,2,3-propa. The plant is already reported to contain propane,1,1,3-triethoxy-, phenol,4,6-di(1,1-dimethyl)-2-methyl, 1,14-tetradecanediol, phytol, 2-pepridionne, N-[4-bromo-nbutyl]-, cedran-diol, 8S, 14- and 2H-pyran, 2-(7-heptadecynyloxy)tetrahydro reported to possess anti-cancer, hepatoprotective, anti-inflammatory, and anti-microbial and inhibition of parasitic growth (Anand and Gokulakrishnan 2012).
Conclusion
In the present study, for the first time, green synthesis of AgNPs using aqueous H. enneaspermus was attempted. The physical property of synthesized nanoparticle was characterized using relevant techniques. The formed AgNPs are highly stable and had significant mosquito larvicidal activity against Anopheles subpictus and C. quinquefasciatus. These synthesized AgNPs have the potential to be used as an ideal ecofriendly approach for the vector control programs.
References
Ahluwalia V, Kumar J, Sisodia R, Shakil NA, Walia S (2014) Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind Crop Prod 55:202–206
Albrecht MA, Evans CW, Raston CL (2006) Green chemistry and the health implications of nanoparticles. Green Chem 8:417–432
Anand T, Gokulakrishnan K (2012) Phytochemical analysis of Hybanthus enneaspermus using UV, FTIR and GC-MS. IOSR J Pharm 2:520–524
Ashok B, Bhagyashree J, Ameeta Ravi K, Smita Z (2010) Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf B: Biointerfaces 80:45–50
Bhattacharya D, Gupta RK (2005) Nanotechnology and potential of microorganisms. Crit Rev Biotechnol 25:199–204
Boominathan R, Devi BP, Mandal SC (2003) Evaluation of antitussive potential of Ionidium suffruticosam Ging. (Violaceae) extract in albino mice. Phytother Res.17: 838
Brause R, Moeltgen H, Kleinermanns K (2002) Characterization of laser-ablated and chemically reduced silver colloids in aqueous solution by UV/VIS spectroscopy and STM/SEM microscopy. Appl Phys B Lasers Opt 75:711–716
Cadavid-Restrepo G, Sahaza J, Orduz S (2012) Treatment of an Aedes aegypti colony with the Cry11Aa toxin for 54 generations results in the development of resistance. Mem Inst Oswaldo Cruz 107:74–79
Chenniappan K, Ayyadurai N (2012) Synergistic activity of Cyt1A from Bacillus thuringiensis subsp. israelensis with Bacillus sphaericus B101 H5a5b against Bacillus sphaericus B101 H5a5b-resistant strains of Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res 110:381–388
El Badawy AM, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM (2010) Impact of environmental conditions (pH, ionic strength, and) electrolyte type on the surface charge and aggregation of silver nanoparticles suspensions. Environ Sci Technol 44:1260–1266
Hemalatha S, Wahi AK, Singh PN, Chansouria JPN (2003) Anti convulsant and free radical scavenging activity of Hybanthus enneaspermus A preliminary screening. Indian J Tradit Knowl 2:383–388
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650
Jayaseelan C, Rahuman AA, Rajakumar G, Vishnu Kirthi A, Santhoshkumar T, Marimuthu S, Bagavan A, Kamaraj C, Zahir AA, Elango G (2011) Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant, Tinospora cordifolia Miers. Parasitol Res 109:185–194
Jayaseelan C, Ramkumar R, Rahuman AA, Perumal P (2013) Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind Crop Prod 45:423–429
Jha AK, Prasad K, Prasad K, Kulkarni AR (2009) Plant system: nature’s nanofactory. Colloids Surf B: Biointerfaces 73:219–223
Joseph B, Sujatha S, Anusha JR (2011) Bioactivity of Hemidesmus indicus on human pathogenic bacteria and Culex quinquefasciatus (Diptera: Culicidae). Res J Med Plant 5:613–620
Kamaraj C, Rahuman AA, Bagavan A (2008) Antifeedant and larvicidal effects of plant extracts against Spodoptera litura (F.), Aedes aegypti L. and Culex quinquefasciatus Say. Parasitol Res 03:325–331
Kathiravan V, Ravi S, Ashok kumar S (2014) Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc 121:88–93
Lee KJ, Kamala-Kannan S, Sub HS, Seong CK, Lee GW (2008) Biological control of Phytophthora blight in red pepper (Capsicum annuum L.) using Bacillus subtilis. World Microbial Biotechnol 24:1139–1145
Li S, Shen Y, Xie A, Yu X, Qiu L, Zhang L, Zhang Q (2007) Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem 9:852–858
Maqusood Ahamed MA, MajeedKhan MKJ, Siddiqui MS, AlSalhi AA, Salman (2011) Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Phys E 43:1266–1271
Marimuthu S, Rahuman AA, Govindasamy R, Thirunavukkarasu S, Arivarasan VK, Chidambaram J, Asokan B, Zahir AA, Elango G, Chinnaperumal K (2010) Evaluation of green synthesized silver nanoparticles against parasites. Parasitol Res 108(6):1541–1549
Nalawade P, Mukherjee P, Kapoor S (2014) Biosynthesis, characterization and antibacterial studies of silver nanoparticles using pods extract of Acacia auriculiformis. Spectrochim Acta a Mol Biomol Spectrosc 121–124
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156:1–13
Nazeruddin GM, Prasad NR, Prasad SR, Shaikh YI, Waghmare SR, Adhyapak P (2014) Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Ind Crop Prod 60:212–216
Njagi EC, Huang H, Stafford L, Genuino H, Galindo HM, Collins JB, Hoag GE, Suib SL (2011) Biosynthesis of iron and silver nanoparticles at room temperature using aqueous Sorghum bran extracts. Langmuir 27:264–271
Noruzi M, Zare D, Khoshnevisan K, Davoodi D (2014) Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc 79:121–124
Priyadarshini S, Gopinath V, Meera Priyadharsshini N, MubarakAli D, Velusamy P (2013) Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids Surf B 102:232–237
Qi L, Xu Z, Jiang X, Hu C, Zou X (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339:2693–700
Rahman SJ, Sharma SK, Rajagopal R (1989) Manual on entomological surveillance of vector borne diseases. NICD, New Delhi
Rahuman AA, Gopalakrishnan G, Ghouse BS, Arumugam S, Himalayan B (2000) Effect of Feronia limonia on mosquito larvae. Fitoterapia 71:553–555
Rajakumar G, Abdul Rahuman A (2011) Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop 118:196–203
Ramamurthy CH, Kumar MS, Suyavaran VS, Mareeswaran R, Thirunavukkarasu C (2012) Evaluation of antioxidant, radical scavenging activity and polyphenolics profile in Solanum torvum L. fruits. J Food Sci 77:907–913
Rao CRK, Trivedi DC (2006) Biphasic synthesis of fatty acids stabilized silver on particle size. Mater Chem Phys 99:354–360
Raveendra Retnam K, John De Britto A (2007) Antimicrobial activity of a medicinal plant Hybanthus enneaspermus (Linn.) F. Muell. Nat Product Radiance 6:366–368
Reddy PJ, Krishna D, Murty US, Jamil K (1992) A microcomputer FORTRAN program for rapid determination of lethal concentrations of biocides in mosquito control. Comput Appl Biosci 8:209–213
Sap-Iam N, Homklinchan C, Larpudomlert R, Warisnoicharoen W, Sereemaspun A, Dubas ST (2010) UV irradiation induced silver nanoparticles as mosquito larvicides. J Applied Sci 10(23):3132–3136 (ISSN 1812–5654)
Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS (2009) Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf 73:332–338
Sharma VK, Ria AY, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interf Sci 145:83–96
Sheny DS, Mathew J, Philip D (2011) Phytosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim Acta A 79:254–262
Simonsen PE, Mwakitalu ME (2013) Urban lymphatic filariasis. Parasite Res 112:35–44
Song JY, Kim BS (2009) Bioproc Biosyst Eng 32:79–84
Suman TY, Radhika Rajasree SR, Kanchana A, Elizabeth SB (2013a) Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf B 106:74–78
Suman TY, Elumalai D, Kaleena PK, Radhika Rajasree SR (2013b) GC-MS analysis of bioactive components and synthesis of silver nanoparticle using Ammannia bacciffera aerial extract and its larvicidal activity against malaria and filariasis vectors). Indu Crops Prod 47:239–245
Sun YP, Li XQ, Cao J, Zhang WX, Wang HP (2006) Characterization of zero-valent iron nanoparticles. Adv Colloid Interf Sci 120:47–56
Sun YP, Li XQ, Cao J, Zhang WX, Wang HP (2007) A method for the preparation of stable dispersion of zero-valent iron nanoparticles. Physicochem Eng Asp 308:60–66
Suresh AK, Pelletier DA, Wang W, Broich ML, Moon JW, Gu B, Allison DP, Joy DC, Phelps TJ, Doktycz MJ (2011) Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater 7:2148–2152
Tripathy S, Sahoo SP, Pradhan D, Sahoo S, Satapathy DK (2009) Evaluation of anti-arthritic potential of Hybanthus enneaspermus. Afr J Pharm Pharmacol 3:611–614
Vimala K, Samba Sivudu K, Murali Mohan Y, Sreedhar B, Mohana Raju K (2009) Controlled silver nanoparticles synthesis in semi-hydrogel networks of poly (acrylamide) and carbohydrates: a rational methodology for antibacterial application. Carbohydr Polym 75:463–471
Weniger B, Lagnika L, Vonthron-Sénécheau C, Adjobimey T, Gbenou J, Moudachirou M, Brun R, Anton R, Sanni A (2004) Evaluation of ethnobotanically selected Benin medicinal plants for their in vitro antiplasmodial activity. J Ethnopharmacol 90:279
World Health Organization (2012) Lymphatic filariasis. http://www.who. int/mediacentre/factsheets/fs102/en/
Yu DG (2007) Formation of colloidal silver nanoparticles stabilized by Na+-poly (gamma-glutamic acid)-silver nitrate complex via chemical reduction process. Colloids Surf B: Biointerfaces 59:171–178
Acknowledgments
The authors are grateful to the Management Sathyabama University for providing the necessary facilities and also wish to acknowledge Centre for Ocean Research, Sathyabama University, for helping out with ICP-MS analysis and Center for Nanoscience and Nanotechnology, Sathyabama University, Jeppiaar Nagar, Chennai, in analyzing the samples by XRD AND EDX.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Suman, T.Y., Rajasree, S.R.R., Jayaseelan, C. et al. GC-MS analysis of bioactive components and biosynthesis of silver nanoparticles using Hybanthus enneaspermus at room temperature evaluation of their stability and its larvicidal activity. Environ Sci Pollut Res 23, 2705–2714 (2016). https://doi.org/10.1007/s11356-015-5468-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5468-5