Abstract
Oxidative stress by increased production of reactive oxygen species has been implicated in pesticides toxicity. This study focused on the toxicological effects of chlorpyrifos, an organophosphate insecticide and abamectin, a biocide each alone or in combination on antioxidant status, and oxidative stress biomarkers in brain and kidney. Animals were divided into four groups. The first group was used as control while groups 2, 3, and 4 were treated with chlorpyrifos (CPF; 14.9 mg/kg BW), abamectin (ABM; 30 mg/kg BW), and chlorpyrifos plus abamectin, respectively. Rats were treated daily with the tested compounds by oral gavages for 30 days. Results revealed that thiobarbituric acid-reactive substances (TBARS) levels were significantly increased in brain and kidney due to insecticides administration. On the other hand, reduced glutathione (GSH) and protein contents in addition to the activities of antioxidant enzymes, alkaline phosphatase (ALP), and acetylcholinesterase (AChE) were significantly decreased in rat organs. A significant induction in lactate dehydrogenase (LDH) activity, urea, and creatinine levels were also observed. The response was more pronounced in rats treated with both CPF and ABM. Results showed that the used insecticides had the propensity to cause significant oxidative damage in rat brain and kidney which is associated with marked perturbations in antioxidant defense system. It can be concluded that antioxidant enzymes can be used as potential biomarkers of toxicity associated with pesticides exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human are potentially exposed to pesticides either directly, as workers in green-houses and in agriculture, or indirectly, via food consumption. In addition, it is likely that a significant amount of these pesticides and their metabolites reach rivers and estuaries via run-off from farmland that are potentially toxic to wildlife (El-Shenawy 2010). Pesticides are ubiquitous in the environment and have positive environmental and public health impact. Moreover, their usage helps in improving human nutrition value. Insecticides used in the present study are combination of organophosphorus insecticide and biocide that is developed to control a wide variety of pests. These chemicals have been used incidentally in large amounts and have also been largely involved in progressive pollution and health hazards. The pesticide used in the present study belongs to two different classes of insecticides, organophosphate (chlorpyrifos; CPF) and biocides (abamectin; ABM). Organophosphate compounds (Ops) are occasionally used indiscriminately in large amounts causing environmental pollution (Kalender et al. 2005; Cemek et al. 2010; Abdel-Daim, 2014). Organophosphate residues have been detected in soil, water bodies, vegetables, grains, and other food products (John et al. 2001). Chlorpyrifos, (O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate) a conventional organophosphorous insecticide, is widely used in agriculture and animal farms (USEPA, 1986). Chlorpyrifos interferes with the acetyl cholinesterase enzyme, which is necessary for normal nerve transmission (NRA, National Registration Authority 2000). Oxidative stress is known to cause a disruption of the oxidant-antioxidant balance leading to alterations of cellular macromolecules, disturbance in cell function and eventually can lead to cell death (Sies 1991; Kehrer 1993).
Abamectin, an analog of ivermectin, is derived from the soil bacterium Streptomyces avermitilis (Campbell et al. 1983; Agarwal 1998), consisting of avermectins mixture. It is a macrocyclic lactone disaccharide, a member of the avermectins family which is widely used as an antiparasitic drug in agricultural and domestic animals. Abamectin was widely employed to control insects and mites of a wide range of agricultural products such as fruits, vegetables, and ornamental crops (Lankas and Gordon 1989). It is also formulated into commercial baits for control of ants and cockroaches (Campbell 1989). Abamectin acts as an insecticide by interfering with the nervous system of the insects leading to paralysis. It exerts its antiparasitic activity through activation of glutamate-gated chloride channel present in the invertebrate nervous system (Cully et al. 1994). Abamectin is a highly effective and generally well tolerated microfilaricide that may soon become an essential component of many public health initiatives to interrupt transmission of lymphatic filarial infection in an effort to eliminate it globally (Brown et al. 2000). In addition, kidney function parameters were highly affected by abamectin (Eissa and Zidan 2009; El- Shafey et al. 2011). Recent studies indicate that the pesticides toxicity may be associated with the enhanced production of ROS (Bagchi et al. 1995; Verma et al. 2007). Also, the production of ROS has been proposed as a mechanism by which xenobiotics and pathological conditions may produce oxidative stress and induce various tissue damages (Oncu et al. 2002; Yu et al. 2008). Studies carried out on adult rats describing oxidative stress induced by insecticide mixture in soft tissues remain scarce. Therefore, the present study was designed to evaluate the toxic effect of an organophosphorus insecticide; chlorpyrifos and a biocide; abamectin singly or jointly on lipid peroxidation, antioxidant enzymes, and acetyl cholinesterase and kidney functions biomarker in male rats.
Materials and methods
Chemicals
Chlorpyrifos technical grade 98 % was obtained from El-Watanya Company, Egypt. Abamectin (1.8 % ABM, vabcomic), a mixture containing a minimum of 80 % avermectin B1 a (5-O-1 1 demethylavermectin A1 a) and a maximum of 20 % avermectin B1 b (5-O-demethyl-25-de-(1-methylpropyl)-25-(1–1 methylethyl) avermectin A1 a), was purchased from Chema Industries Company. All other reagents used were of analytical reagent grade.
Experimental design
Twenty male Wister rats weighing 150–170 g were obtained from the animal house of the Faculty of Medicine, Alexandria University, Alexandria, Egypt. Animal handling and experimental design procedures were approved by Research Ethical Committee of the Faculty of Medicine, Alexandria University, Alexandria, Egypt and the protocol conforms to the National Institutes of Health guidelines. Animals were housed five per cage and kept on commercial diet and provided tap water ad libitum. The animal room was maintained at 21–24 °C and 40–60 % relative humidity with 12-h light–dark cycles, the light cycle coinciding with the day light hours. After 2 weeks of acclimation, the groups were assigned at random to one of the following treatments: group 1 served as control and received corn oil (5 ml/kg BW), while group 2 and 3 were treated with chlorpyrifos (CPF; 14.9 mg/kg BW = 1/10 LD50) and abamectin (ABM; 30 mg/kg = 1/10 LD50), respectively. Group 4 received both chlorpyrifos and abamectin (1/10 LD50 for both CPF and ABM). Chlorpyrifos and abamectin doses were given according to the previous studies of Aly et al. (2010) and Eissa and Zidan (2009), respectively. Animals were treated daily with the tested compounds by oral gavages for 30 days.
Samples and parameters measured
Blood samples
Blood samples were taken by cardiac puncture and allowed to stand for 30 min at room temperature to clot before being centrifuged at 3000×g for 15 min. Serum was obtained by centrifugation and stored at–60 °C. Serum samples were used for the determination of urea and creatinine according to the methods described by Patton and Crouch (1977) and Henry et al. (1974), respectively.
Tissue preparation
Rats of each group were killed by decapitation at the end of the treatment period. Kidney and brain tissues were weighed, cut and homogenized (10 % w/v) separately in ice-cold 1.15 % KCl–0.01 mol/l sodium potassium phosphate buffer (pH 7.4) in a Potter-Elvehjem type homogenizer. The homogenate was centrifuged at 10,000 g for 20 min at 4 °C, and the resultant supernatant was used for different enzyme assays.
Biochemical analysis
Thiobarbituric acid-reactive substances (TBARS) were measured in the homogenate using the method of Ohkawa et al. (1979). Reduced glutathione (GSH) content was measured after reaction with 5,5′-dithiobis-(2-nitrobenzoic acid) using the method of Ellman (1959). Superoxide dismutase activity (SOD; EC 1.15.1.1) was determined according to Misra and Fridovich (1972). The assay procedure involves the inhibition of epinephrine auto-oxidation in an alkaline medium to adrenochrome, which is markedly inhibited by the presence of SOD. The enzyme catalase (CAT; EC 1.11.1.6) converts H2O2 into water. The CAT activity was measured spectrophotometrically at 240 nm by calculating the rate of degradation of H2O2, the substrate of the enzyme (Aebi 1984). Glutathione S-transferase (GST; EC 2.5.1.18) activity was determined according to Habig et al. (1974) using para-nitrobenzyl chloride as a substrate. Acetylcholinesterase (AChE; EC 3.1.1.7) activity was estimated in brain homogenate using acetylcholine iodide as a substrate according to the method of Ellman et al. (1961). Alkaline phosphatase (ALP; EC 3.1.3.1) activity was determined according to Principato et al. (1985). Lactate dehydrogenase (LDH; EC 1.1.1.27) was determined by the method of Cabaud and Wroblewski (1958). ALP and LDH were determined in freshly prepared homogenates. Protein content was determined using the method described by Lowry et al. (1951). Folin and Ciocalteus phenol reagent was used to develop the blue color that was measured spectrophotometrically at 750 nm. Bovine serum albumin was used as a standard.
Statistical analysis
Data were analyzed according to Steel and Torrie (1981). Statistical significance of the difference in values of control and treated samples was calculated by (F) test at 5 % significance level. Data of the present study were statistically analyzed by using Duncan’s multiple range test (SAS 1986).
Results
None of the rats treated with chlorpyrifos, abamectin or their combination, showed signs of morbidity or mortality during the study.
Renal biomarkers
The biochemical markers of renal damage were significantly elevated in all treated groups, where the levels of serum urea (29.8, 23.9, 32.8 %) and creatinine (31.3, 27.7, 38 %) were significantly increased in rats treated with CPF, ABM, and their combination as compared with the control (Table 1). This means that chlorpyrifos had strong effect than Abamectin while their combination is more effective.
Lipid peroxidation and reduced glutathione content
Data of TBARS measured in rat kidney and brain is presented in Fig. 1. A significant increase (p < 0.05) in TBARS concentrations was evident in kidney (41.9, 38, 47.7) and brain (33.9, 35.7, 38.9) of rats exposed to CPF, ABM, or CPF plus ABM as compared to control. On the other hand, effect of CPF, ABM, and their combination on GSH content in rat kidney (32.96, 30.63, 39.80 %) and brain (23.93, 26.18, 32.19) homogenate was presented in Fig. 2. A significant reduction was observed in GSH content exposed to CPF, and ABM alone while more pronounced effect was observed in rats treated with both CPF plus ABM.
Antioxidant enzyme activities
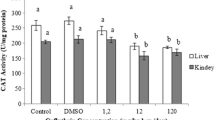
Data concerning kidney and brain antioxidant enzyme activities (GST, SOD, and CAT) are presented in Figs. 3, 4, and 5. A significant reduction was observed (p < 0.05) in the activity of GST activity in rat kidney by 37.4, 38.7, and 43 %, and in rat brain by 36.3, 41.3, and 44.5 % after treatment with CPF, ABM, and their combination, respectively. Also, SOD activity was significantly decreased by 29.3, 22.9, and 32.1 % in rat kidney, and 26.86, 22.82, and 32.36 % in rat brain, respectively. In addition, CAT activity was significantly reduced by 28.1, 24.9, and 30.4 in rat kidney and 26.9, 30.5, and 37.9 in rat brain of male rats treated with CPF, ABM, and their combination, respectively. It is clear that the activities of measured antioxidant enzymes were more affected by CPF than ABM while the combination of CPF and ABM was more obvious.
Acetylcholinesterase activity
A significant decrease in AChE activity (p < 0.05) by 37.7, 32.5, and 41 % was observed in rat brain homogenate in all rats exposed to CPF, ABM, or their combination (Fig. 6) as compared to control.
Lactate dehydrogenase, alkaline phosphatase activities, and protein content
Results indicated that LDH activity was significantly (p < 0.05) increased by 26.8, 25.6, and 36.1 % in rat kidney and 26.3, 25.9, and 31 % in rat brain treated with CPF, ABM, and their combination as compared to control, respectively. On the other hand, the activity of ALP was significantly decreased by 29.7, 25.7, and 33.2 in rat kidney and by 30.2, 35, and 39.6 in rat brain treated with CPF, ABM, and their combination as compared to control, respectively. Also, protein content was reduced significantly in rat kidney by 22.1, 15.6, and 29.9 % and in rat brain by 22.64, 20.95, and 25.87 in rats treated with CPF, ABM, and CPF + ABM as compared to control (Table 2). It was observed that the combination of CPF + ABM is more effective.
Discussion
There is no data concerning the influence of insecticide mixture on the activity of antioxidant enzymes of rat brain and kidney, and for this reason, it is very difficult to compare our results with those reported by other authors. Renal dysfunction was clearly evidenced in CPF and ABM and CPF + ABM-treated groups as revealed by the elevation in serum urea and creatinine (Table 1). In agreement with the previous studies, renal dysfunction was occasioned in animals treated with ABM, methomyl, and diazinon insecticides (Eissa and Zidan 2009; El-Demerdash et al. 2013; El-Demerdash and Nasr 2014). The increase in urea concentration in the current study may be due to its effect on liver function (data not shown), as urea is the end-product of protein catabolism, and/or referred to kidney dysfunction. The increase in urea and creatinine indicates diminished ability of the kidney to filter these waste products and excrete them in the urine. An association between hyperuricemia and renal injury has also been reported by Feig et al. (2006), where uric acid-mediated arteriolopathy and interstitial inflammation suggest mechanisms that would exacerbate or potentiate progressive renal functional decline after injury.
Oxidative stress occurs due to the imbalance between ROS and antioxidants, resulting in impairment in cellular functions and leading to potential tissue damage. Elevated levels of TBARS, the end products of lipid peroxidation, and decreased levels of GSH, which is an important redox regulator, are important biomarkers of oxidative stress induced by pesticides (Mansour et al. 2009). Lipid peroxidation (LPO) has been suggested as one of the molecular mechanisms involved in pesticide-induced toxicity (Kehrer 1993). The increased lipid peroxidation evidenced in kidney and brain of rats treated with chlorpyrifos, abamectin, and their combination is in agreement with several authors who reported that insecticides and their degradation products may act on membranes, oxidizing its lipid components and enhancing free radicals production during their exposure (Oncu et al. 2002; Verma et al. 2007; Aly et al. 2010; El-Demerdash 2011; 2012). Also, El-Shenawy (2010) reported that insecticides, ABM, and OP may induce oxidative stress in isolated rat hepatocytes and disturb membrane structure. Moreover, Li et al. (2013) suggested that avermectin (AVM) exposure caused a significant increase in LPO in brain tissue of pigeon. The increase in LPO following AVM exposure may be attributed to the induction of ROS which enhances the oxidation of polyunsaturated fatty acids.
Reduced glutathione acts as a non-enzymatic antioxidant which scavenges free radicals resulting from oxidative metabolism and escaping decomposition by antioxidant enzymes (Leve De and Kaplowitz 1991). During the metabolic action of GSH, its sulfhydryl group is oxidized into disulfide compound (Meister and Anderson 1983). GSH depletion is important biomarker of oxidative stress due to its utilization for conjugation and/or its participation as an antioxidant in neutralizing free radicals produced by insecticides and maintaining the intracellular redox balance in mammalian cell (Lu 1999). These effects have been previously observed by other authors in vitro and in vivo (Banerjee et al. 1999; Maran et al. 2009; El-Shenawy 2010; El-Demerdash 2011). Moreover, GSH also participates in the detoxification of xenobiotics as a substrate for the enzyme GST. Fetoui et al. (2009) have demonstrated that the depletion of intracellular sulfhydryl groups by insecticides is the prerequisite for ROS generation. So, the significant decrease in GSH content observed in the present study could lead to increased susceptibility to free radical damage.
Conjugation of electrophilic substrates to the thiol group of GSH is catalyzed by glutathione S-transferases producing less toxic forms and also reducing lipid peroxides (Mansour and Mossa 2009). In the present study, the marked inhibition in GST activity indicates insufficient conjugation of electrophiles and detoxification of these species. GST is one of enzyme systems involved in the detoxification of OP insecticides to non-toxic products or by rapidly binding and very slowly turning over the insecticide (Ranjbar et al. 2002). In consistent with the present results, Kale et al. (1999) and El-Demerdash (2007) reported a significant decrease in GST activity after in vivo and in vitro treatment with cypermethrin and lambda-cyhalothrin, respectively. Similarly, GST inhibition has been documented to occur under other oxidative stress conditions (Goel et al. 2005; Mansour and Mossa 2009). Our results demonstrate that GST is a part of adaptive response of rat organ cells to oxidative stress after CPF and/or ABM treatments.
SOD and CAT are the most important defense mechanisms against toxic effects of oxygen metabolism. SOD catalyzes the conversion of superoxide radical to hydrogen peroxide while CAT converts the later into water. These antioxidant enzymes can, therefore, alleviate the toxic effects of ROS (Mansour and Mossa 2009; Abdel-Daim et al., 2015). SOD and CAT activities were significantly reduced in rats treated with CPF, ABM, and their combination. In agreement with the present results, El-demerdash (2011) reported that a significant reduction in the activity of SOD and CAT antioxidant enzymes was observed due to the oxidative damage induced by a mixture of organophosphate and pyrethroid insecticides. Also, Li et al. (2013) demonstrated that the activities of SOD and CAT in the cerebellum and optic lobe of treatment groups were reduced with the increase in concentration of avermectin. Moreover, El-Sheikh and Galal (2015) found that emamectin benzoate (EB), an avermectin insecticide, reduced SOD activity in male rats. Since insecticides produced excessive ROS, the counter balancing effect of the antioxidant enzymes is lost (Banerjee et al. 1999; Seth et al. 2001). Thus, the inhibition of SOD and CAT enzymes involved in free radical removal led to the accumulation of superoxide, which promoted lipid peroxidation and modulation of DNA, altered gene expression, and cell death (Calviello et al. 2006).
Lactate dehydrogenase (LDH) was recognized as a potential marker for assessing the toxicity of xenobiotics. The changes in the dehydrogenase activity in insecticide-treated rats may be due to severe cellular damage, leading to increased release of dehydrogenase that impaired carbohydrate and protein metabolism (Sivakumari et al. 1997). The elevation of lactate also indicated metabolic disorders and a clear response against energy depletion. Sancho et al. (1998) observed the same response when European eels were exposed to fenitrothion (OP). Also, our results were consistent with El-Demerdash (2011; 2012) who reported that LDH activity was significantly increased in rat kidney and brain treated with a mixture of fenitrothion and lambda-cyhalothrin in vitro. Alkaline phosphatase is a biochemical parameter and sensitive index to changes due to pesticide toxicity. Phosphatase is an important and critical enzyme in the biological processes. It is responsible for detoxification, metabolism, and the biosynthesis of energetic macromolecules which are required for various essential functions. ALP is membrane-bound biomarker enzyme, and its decrease in kidney and brain tissues might be due to leakage of the enzyme into the blood as a result of tissue necrosis (Yarbrough et al. 1982). Also, vepacide may interfere with ALP leading to biochemical impairment of cellular functions and tissue lesions and an increased permeability of plasma membrane (Rahman and Siddiqui 2004). Protein is one of the main cellular components susceptible to damage by free radicals. The observed reduction in protein content is in agreement with Shakoori et al. (1990), El-Demerdash (2012), and El-Sheikh and Galal (2015). Protein depression was mainly due to excessive loss through nephrosis and/or due to the reduction of protein synthesis or increased proteolytic activity or degradation (Shakoori et al. 1990; Chatterjea and Shinde 2002).
AChE activity is a standard biomarker of organophosphate pesticide. The marked inhibition in brain AChE activity of rats treated with CPF, ABM, and CPF plus ABM is in agreement with Okahashi et al. (2005) and Feng et al. (2008) who reported that treatment with fenitrothion and trichlorfon, cholinesterase inhibitors, caused a significant reduction in AChE activity. Inhibition of AChE causes an increase in acetylcholine content at sites of cholinergic transmission in the body. Also, the inhibition of AChE is the most plausible explanation for much of the symptomatology following OP intoxication (Yamashita et al. 1997). Actually, the use of AChE inhibition as biomarker to assess the toxic effects of organophosphates has been studied for a wide range of species and many different xenobiotics and is a well-accepted index of organophosphates toxicity both in vivo and in vitro (Sanchez-Hernandez and Walker 2000; Adedara et al. 2015). Also, Ma et al. (2014) reported that ABM exposure inhibited AChE activity in freshwater snail Physa acuta. ABM acts as an insecticide by affecting the nervous system and paralyzing insects (El-Shenawy 2010). So, the decreased AChE might be referred to the increase in lipid peroxidation observed in the present study.
Conclusion
In conclusion, organophosphate, chlorpyrifos and biocide, abamectin each alone or in combination had toxic effects on rat brain and kidney. CPF had the capability to induce lipid peroxidation, perturbations in antioxidant defense system and biochemical parameters more than ABM. A stronger insecticidal effect of CPF and ABM mixture was also observed. The results indicated a risk of organ damage during exposure to a combination of insecticides.
References
Abdel-Daim MM (2014) Synergistic protective role of ceftriaxone and ascorbic acid against subacute diazinon-induced nephrotoxicity in rats. Cytotechnology. doi: 10.1007/s10616-014-9779-z
Abdel-Daim MM et al. (2015) Synergistic ameliorative effects of sesame oil and alpha-lipoic acid against subacute diazinon toxicity in rats: haematological, biochemical and antioxidant studies. Can J Physiol Pharmacol. doi: 10.1139/cjpp-2015-0131
Adedara IA et al (2015) Influence of diphenyl diselenide on chlorpyrifos-induced toxicity in Drosophila melanogaster. J Trace Elem Med Biol 32:52–59
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126, 3rd Ed, Lippincott-Raven publishers, Philadelphia
Agarwal AK (1998) Avermectin. In: Wexler P (ed) Encyclopedia of toxicology. Academic, San Diego, CA, pp 89–90
Aly N, EL-Gendy K, Mahmoud M, El-Sebae AK (2010) Protective effect of vitamin C against chlorpyrifos oxidative stress in male mice. Pest Biochem Physiol 97:7–12
Bagchi D, Bagchi M, Hassoum EA, Stohs SJ (1995) In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 104:129–140
Banerjee BD et al (1999) Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol Lett 107:33–47
Brown RK, Ricci FM, Ottesen EA (2000) Ivermectin: effectiveness in lymphatic filariasis. Parasitology 121:S133–S146
Cabaud PC, Wroblewski F (1958) Calorimetric measurement of lactate dehydrogenase activity of body fluids. J Clin Pathol 30:234–236
Calviello G et al (2006) DNA damage and apoptosis induction by the pesticide Mancozeb in rat cells: involvement of the oxidative mechanism. Toxicol Appl Pharmacol 211:87–96
Campbell WC (1989) Use of ivermectin in dogs and cats in ivermectin and Abamectin. Springer, New York, pp 245–259
Campbell WC et al (1983) Ivermectin: a potent antiparasitic agent. Science 221:823–828
Cemek M et al (2010) Protective roles of vitamin E (α-tocopherol), selenium and vitamin E plus selenium in organophosphate toxicity in vivo: a comparative study. Pest Biochem Physiol 96:113–118
Chatterjea MN, Shinde R (2002) Text book of medical biochemistry, 5th ed. Jaypee Brothers. Medical Publishers Ltd., New Delhi, p 317
Cully DF et al (1994) Cloning of an avermectin–sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature (Lond) 371:707–711
Eissa FI, Zidan NA (2009) Haematological, biochemical and histopathological alterations induced by Abamectin and Bacillus thuringiensis in Male Albino rats. Aus J Basic Appl Sci 3(3):2497–2505
El- Shafey AAM et al (2011) Some physiological and biochemical effects of Oshar extract and Abamectin Biocide on male albino rats. J Am Sci 7(12):254–261
El-Demerdash FM (2007) Lambda-cyhalothrin-induced changes in oxidative stress biomarkers in rabbit erythrocytes and alleviation effect of some antioxidants. Toxicol in Vitro 21:392–397
El-Demerdash FM (2011) Lipid peroxidation, oxidative stress and acetylcholinesterase in rat brain exposed to organophosphate and pyrethroid insecticides. Food Chem Toxicol 49:1346–1352
El-Demerdash FM (2012) Cytotoxic effect of fenitrothion and lambda-cyhalothrin mixture on lipid peroxidation and antioxidant defense system in rat kidney. J Environ Sci Health B47:262–268
El-Demerdash FM, Nasr HM (2014) Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J Trace Elem Med Biol 28:89–93
El-Demerdash FM et al (2013) Kidney antioxidant status, biochemical parameters and histopathological changes induced by methomyl in CD-1 mice. Exp Toxicol Pathol 65:897–901
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ellman GL et al (1961) A new rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
El-Sheikh EA, Galal AAA (2015) Toxic effects of sub-chronic exposure of male albino rats to emamectin benzoate and possible ameliorative role of Foeniculum vulgare essential oil. Environ Toxicol Pharmacol 39(3):1177–1188
El-Shenawy NS (2010) Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytes. Toxicology in Vitro 24(4):1148–1157
Feig DI et al (2006) Serum uric acid: a risk factor and a target for treatment? J Am Soc Nephrol 17:S69–73
Feng T et al (2008) Effects of trichlorfon and sodium dodecylsulphate on antioxidant defense system and acetylcholinesterase of Tilapia nilotica in vitro. Pest Biochem Physiol 92:107–113
Fetoui H, Garoui EM, Zeghal E (2009) Lambda-cyhalothrin-induced biochemical and histopathological changes in the liver of rats: ameliorative effect of ascorbic acid. Exp Toxicol Pathol 61:189–196
Goel A, Dani V, Dhawan DK (2005) Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos induced toxicity. Chem Biol Interact 156:131–140
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Henry RJ, Cannon DC, Winkelman W (1974) Clinical chemistry principals and techniques, 11th edn. Happer and Row Publishers, New York, p 1629
John S et al (2001) Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J Nutr Biochem 12:500–504
Kale M et al (1999) Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. Toxicol Lett 105:197–205
Kalender S et al (2005) Diazinon-induced hepatotoxicity and protective effect of vitamin E on some biochemical indices and ultra structural changes. Toxicology 211:197–206
Kehrer JP (1993) Free radicals as mediator of tissue injury and disease. Crit Rev Toxicol 23:21–48
Lankas GR, Gordon LR (1989) Toxicology. In: Campbell WC (ed) Ivermectin and abamectin. Springer, New York
Leve De L, Kaplowitz N (1991) Glutathione metabolism and its role in hepatotoxicity. Pharmacol Therap 52:287–305
Li M et al (2013) Antioxidant response and histopathological changes in brain tissue of pigeon exposed to avermectin. Ecotoxicology 22:1241–1254
Lowry OH et al (1951) Protein measurement with the Folin Phenol Reagent. J Biol Chem 193:269–275
Lu CS (1999) Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 13:1169–1183
Ma J et al (2014) Biochemical responses to the toxicity of the biocide abamectin on the freshwater snail Physa acuta. Ecotoxicol Environ Saf 101:31–35
Mansour SA, Mossa AH (2009) Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pest Biochem Physiol 93:34–39
Mansour SA, Mossa AH, Heikal TM (2009) Effects of methomyl on lipid peroxidation and antioxidant enzymes in rat erythrocytes: in vitro studies. Toxicol. Indust Health 25:557–563
Maran E et al (2009) Effects of four carbamate compounds on antioxidant parameters. Ecotoxicol Environ Saf 72:922–930
Meister A, Anderson ME (1983) Glutathione. Ann Rev Biochem 52:711–760
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
NRA, National Registration Authority (2000) The NRA review of chlorpyrifos, vol. 1
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Okahashi N et al (2005) Lack of evidence for endocrine disrupting effects in rats exposed to fenitrothion in utero and from weaning to maturation. Toxicology 206:17–31
Oncu M et al (2002) Nephrotoxicity in rats induced by chlorpyrifos-ethyl and ameliorating effects of antioxidants. Hum Exp Toxicol 21:223–230
Patton CJ, Crouch SR (1977) Spectrophotometric and kinetics investigation of the Berthelot reaction for determination of ammonia. Anal Chem 49:464–469
Principato GB et al (1985) Characterization of the soluble alkaline phosphatase from hepatopancreas of Squilla mantis L. Comp Biochem Physiol B 985:801–804
Rahman MF, Siddiqui MK (2004) Biochemical effects of vepacide (from Azadirachta indica) on Wistar rats during subchronic exposure. Ecotoxicol Environ Saf 59(3):332–339
Ranjbar A, Pasalar P, Abdollahi M (2002) Induction of oxidative stress and acetylcholinesterase inhibition in organophosphorous pesticide manufacturing workers. Hum Exp Toxicol 21:179–182
Sanchez-Hernandez JC, Walker CH (2000) In vitro and in vivo cholinesterase inhibition in Lacertides by phosphonate- and phosphorothioate-type organophosphates. Pest Biochem Physiol 67:1–12
Sancho E et al (1998) Liver energy metabolism of Anguilla anguilla after exposure to fenitrothion. Ecotoxicol Environ Saf 41:168–175
SAS (1986) Statistical analysis system. SAS User’s Guide: Statistics, version 5 Edition SAS Inst., Inc, Cary, NC, USA
Seth V, Banerjee BD, Chakravorty AK (2001) Lipid peroxidation, free radical scavenging enzymes, and glutathione redox system in blood of rats exposed to propoxur. Pest Biochem Physiol 71:133–139
Shakoori et al (1990) Toxic effects of talastar, a new synthetic pyrethroid, on blood and liver of rabbit. Pak J Zool 23:289–300
Sies H (1991) Oxidative stress: introduction. In: Sies H (ed) Oxidative stress: oxidants and antioxidants, vol 23. Academic, San Diego, CA, USA, pp 21–48
Sivakumari K et al (1997) Cypermethrin toxicity: sublethal effect of enzyme activities in a fresh water fish, Cyprinus carpio var. Communis. J Environ Biol 18:121–125
Steel RGD, Torrie JH (1981) Principle and procedure of statistics, 2nd edn. A Biometrical Approach Mc Gvaus-Hill Booh Company, New York, US
USEPA (1986) United State environmental protection agency. Ambient Water Quality Criteria for Chlorpyrifos, Report, US, Washington, DC
Verma RS, Mnugya A, Srivastava N (2007) In vivo chlorpyrifos induced oxidative stress: attenuation by antioxidant vitamins. Pest Biochem Physiol 88:191–196
Yamashita M, Tanaka J, Ando Y (1997) Human mortality in organophosphate poisoning. Vet Hum Toxicol 39:84–85
Yarbrough JD, Chambers JE, Robinson KM (1982) Effect of chronic exposures to pesticides on animal system. In: Chambers JE, Yarbrough YD (eds) Alterations in liver structure and function resulting from chronic insecticide exposure. Raven, New York, pp 25–59
Yu F et al (2008) Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamin C and E. Exp Toxicol Pathol 59:415–423
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Nasr, H.M., El-Demerdash, F.M. & El-Nagar, W.A. Neuro and renal toxicity induced by chlorpyrifos and abamectin in rats. Environ Sci Pollut Res 23, 1852–1859 (2016). https://doi.org/10.1007/s11356-015-5448-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5448-9