Abstract
In recent years, the number of cases of heavy metal contamination has increased worldwide, leading to reports on environmental pollution and human health problems. Phytoremediation can be potentially used to remove heavy metal from contaminated sites. This study determined heavy metal concentrations in the biomass of plant species growing on a multi-metal-contaminated site. Seven plant species and associated rhizospheric soil were collected and analyzed for heavy metal concentrations. While plant Cu, Zn, Cd, Ni, Pb, As, and Ba concentrations ranged from 8.8 to 21.1, 56.4 to 514.3, 0.24 to 2.14, 1.56 to 2.76, 67.8 to 188.2, 0.06 to 1.21, and 0.05 to 0.62 mg kg−1, respectively, none of the plants was identified as hyperaccumulators. Those in the rhizospheric soil ranged from 10.5 to 49.1, 86.2 to 590.9, 0.32 to 2.0, 3.6 to 8.2, 19.1 to 232.5, 2.0 to 35.6, and 85.8 to 170.3 mg kg−1, respectively. However, Zn, Cd, Pb, and As concentrations in the soil outside the rhizosphere zone were 499.0, 2.0, 631.0, and 48.0 mg kg−1, respectively. Senecio brasiliensis was most effective in translocating Cu, Cd, and Ba. The most effective plant for translocating Zn and Pb was Baccharis trimera and, for element As, Dicranopteris nervosa and Hyptis brevipes. Heavy metal and metalloid levels in spontaneous plants greatly exceeded the upper limits for terrestrial plants growing in uncontaminated soil, demonstrating the higher uptake of heavy metal from soil by these plants. It is concluded that naturally occurring species have a potential for phytoremediation programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, cases involving heavy metal contamination have increased worldwide causing more environmental pollution and human health problems (Govindasamy et al. 2011; Gall et al. 2015). However, in response to these problems, over the last few years, many studies have been performed to find plant species that can accumulate these elements in root and shoot tissues as a low-cost strategy to remediate these areas (Davari et al. 2015; Grison et al. 2015; Ma et al. 2015; Santos et al. 2014; Vamerali et al. 2015).
It is thus necessary to clean up metal-contaminated soils in order to minimize their impact on ecosystems and risks to human health (Ali et al. 2013; Gall et al. 2015). This is a challenging job with respect to cost and technical complexity (Barceló and Poschenrieder 2003; Gall et al. 2015). According to Ali et al. (2013), high cost, labor-intensive, irreversible changes in soil properties and disturbance of native soil microflora are a few of the characteristics of physical and chemical methods that limit their use. Chemical methods can also create secondary pollution problems.
Research is needed to develop a cost-effective, highly efficient, and environmentally friendly remediation method for the decontamination of heavy metal-polluted soils and water. One such novel approach is phytoremediation, which is considered a green alternative solution to the problem of heavy metal pollution (Ali et al. 2013).
The accumulation of heavy metals in the shoots was described by Baker (1981), as a strategy to enable them to tolerate large amounts of these elements present in the root environment. Phytoremediation is the use of plants to remove pollutants from the environment or to render them harmless. The phytoremediation of toxic metals can be of great value due to the lack of affordable and effective alternative technologies. While organic molecules can be degraded by bioremediation, microbial toxic metals can be remedied only by collecting small amounts of metals dispersed into soil or water and thus removing them from the environment. This technology can provide an economically viable solution for the remediation of contaminated sites by heavy metals (Tangahu et al. 2011).
This technology, applied to the soil, removes, immobilizes, or renders harmless the contaminants present in the ecosystem (Tang et al. 2012). Moreover, the success of metal phytoremediation depends on the plant’s ability to tolerate and accumulate high metal concentrations, while obtaining a large biomass (Nascimento and Xing 2006; Davari et al. 2015). The plant’s ability to accumulate and tolerate high concentrations of heavy metals in the biomass is known as hyperaccumulation (Baker and Whiting 2002). These plants can accumulate concentrations of cobalt (Co), copper (Cu), chromium (Cr), lead (Pb), and nickel (Ni) in the tissue up to 0.1 % of dry mass, while zinc (Zn) or manganese (Mn) reaches up to 1 % and is useful in phytoremediation (Glick 2010; Jiménez et al. 2011; Kumari and Singh 2011).
Phytoextraction involves the removal of heavy metals from soil by hyperaccumulation, and phytostabilization immobilizes and/or detoxifies metals in soil, changing their state of oxidation (Andrade et al. 2007). Despite the various species of hyperaccumulators discovered and described in recent years, a major factor must be considered in applying this technology, which is the use of species adapted to specific environmental conditions of the area to be remedied (Bidar et al. 2007). However, the bioavailability of heavy metals in soil depends on the physical and chemical characteristics, especially the pH, the nature of the sorbent, the presence, and concentration of organic and inorganic ligands, including humic and fulvic acids, exudates, microbial metabolites, and nutrients (Violante et al. 2010). It is thus necessary to research both tolerant plant species and characteristics at the local level (Jiménez et al. 2011; Hao and Jiang 2015).
The chance of finding plants with a potential for heavy metal phytoaccumulation at a contaminated site is higher than at non-contaminated sites. From this perspective, studies worldwide have increased the search for plants with this capacity for specific contaminants in these contaminated areas.
This study aims to determine heavy metal concentrations in the biomass of species growing on a multi-metal-contaminated site of gold ore processing, to determine the metal translocation factor and bioconcentration factor of native species, to identify metal hyperaccumulators, and to provide insight for using native plants to remediate multi-metal-contaminated sites.
Material and methods
Site characterization
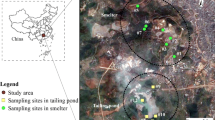
The soil, rhizospheric soil, and plants used in this study were collected at a known metal-contaminated site (30° 81′ 58″ S, 53° 92′ 05″ W) located near of the town of Lavras do Sul in the Southeast Region of Rio Grande do Sul State in Brazil (Fig. 1). The site area is 10,000 m2 and is surrounded by savanna vegetation with small ligneous trees and grasses that are predominant on the site, which is currently used as cattle pasture. The elevated metal concentrations at the depth of 0–20 cm on the site historically resulted from intensified gold ore processing from the 1960s to the 1980s. One hypothesis for contamination is that ore processed in the gold extraction mills had elevated levels of heavy metals (e.g., Galena, PbS), which resulted in contamination in the vicinity of the mills.
Soil and plant sample collection
The soils of the areas studied were classified as Entisol Orthent (SSS 2010). The soil samples were collected at a depth of 0–20 cm at previously determined points, in order to form a systematic “square mesh” distribution to characterize metal distribution on the site. The organic material at the surface had been removed before soil sample collections. Soil sample collections were based on eight points around a central point (simple samples) to form a composite sample, totalizing 12 composite soil samples. Plant samples were collected based on their coverage as well as plant health at each sampling point. Three plant samples were collected around sampling point number one and a total of 36 plant samples of seven species. Plant samples were separated into shoots and roots.
The rhizospheric soil samples were collected after stirring plant roots to remove the adhering soil, and a soil sample was collected outside the plant root zone. Soil samples were immediately stored in polystyrene boxes and maintained at a temperature of around 4 ± 2 °C.
Soil and plant sample preparation and chemical analysis
The rhizospheric soil samples were air-dried at room temperature and then sieved through a 2-mm stainless steel sieve. Soil pH was measured in water at a 1:2.5 soil/water ratio according to Tedesco et al. (1995). Water-soluble metal concentrations were found by a methodology described in Yoon et al. (2006) with adaptations. A volume of 25 ml of deionized distilled water was mixed with 2 g of soil and shaken for 12 h. The mixture was then centrifuged for 15 min at 3500 rpm. The supernatant was filtered by quantitative paper filter and then analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES) (PerkinElmer Optima 7000 DV, PerkinElmer Corp, Norwalk, CT). For heavy metal analysis, soil samples were digested according to the EPA 3050b method (USEPA 1998) and extracts analyzed by ICP-OES (PerkinElmer Optima 7000 DV, PerkinElmer Corp, Norwalk, CT).
A few selected characteristics of the soil samples collected from non-contaminated and contaminated sites are shown in Table 1.
The shoot samples were washed gently with deionized distilled water, and root samples were immersed in a solution of HCl 0.1 mol L−1 to remove metallic ions adhering to the root surface and then washed with deionized distilled water.
The shoot and root samples were placed in an oven with forced air at 65 ± 3 °C, where they remained for 72 h until constant weight was reached. The plant samples were ground using a Wiley Mill and then digested by nitro-perchloric acid solution (Tedesco et al. 1995). The extracts were analyzed by ICP-OES (PerkinElmer Optima 7000 DV, PerkinElmer Corp, Norwalk, CT).
Phytoextraction efficiency
The efficiency of phytoextraction can be quantified by calculating the bioconcentration factor (BCF) and translocation factor (TF). BCF indicates biota efficiency in accumulating a metal in its tissues from the surrounding medium (water) (Mackay 1982; Nowell et al. 1999). However, many authors (Yoon et al. 2006; Ladislas et al. 2012; Ali et al. 2013; Testiati et al. 2013) calculated BCF as the ratio between metal concentrations in root/metal concentration in soil outside the root zone. This study used a metal concentration from rhizospheric soil, considered to be the environment surrounding the roots, as described below:
where [metal]root is the concentration of the target metal in the harvested plant tissue and [metal]soil is the concentration of the same metal in the rhizospheric soil.
where [metal]shoot is the concentration of the metal in plant shoots and [metal]root is the concentration of the metal in plant roots (Yoon et al. 2006; Ali et al. 2013).
Statistical analysis
Data were subjected to analysis of variance (ANOVA), and Pearson correlation coefficients (r) were used to express the associations of quantitative variables. SISVAR software was used to analyze the data (Ferreira 2011).
Results and discussion
Chemical attributes of soils and metal concentrations
The soil pH in the area studied ranged from 5.7 to 6.4 (Table 1). According to Kabata-Pendias (2011), metal behavior affects soil conditions. Bioavailability may be easy or moderate according to soil conditions including pH. In acid soils (pH <3), several metals, such as Cd, Co, Ni, Cu, and Zn, are easily mobilized and available to plants, while in neutral or alkaline soils, metals are substantially less available. However, other metals are slightly less available to plants under the described soil conditions (Kabata-Pendias 2011).
The soil samples collected at the contaminated site presented a variable heavy metal concentration. The pseudo total concentration and water-soluble concentration of Zn, Cd, Pb, and As are presented in Table 1. The pseudo total Zn concentrations in the soil samples collected were variable, ranging from 35 at sampling point 11 to 553 mg kg−1 at sampling point 3. The permissible Zn concentrations for soil quality set out by the São Paulo Environmental Technology Company (CETESB) in Brazil are 60 mg kg−1 for quality reference, 300 mg kg−1 for prevention, and 450 mg kg−1 for agriculture activity intervention. According to the CETESB (2014) list of soil quality values, the concentrations of Zn are 60 mg kg−1 for soil quality reference and 86 mg kg−1 for prevention.
The soil samples presented a concentration of element Cd ranging from <0.2 (detection limit of method) at sampling points 8 and 11 to 2 mg kg−1 at sampling points 1 to 5. The permissible Cd concentrations for soil quality set out by the São Paulo Environmental Technology Company (CETESB) are <0.5 mg kg−1 for quality reference, 1.3 mg kg−1 for prevention, and 3 mg kg−1 for the intervention of agricultural activity. Therefore, the sampling points 6, 7, 8, 11, and 12 have a Cd concentration classified as safe without risks to human health. However, sampling points 1 to 5 have a Cd concentration classified between prevention and agricultural activity intervention (CETESB 2014).
The Pb concentration values ranged from 13 mg kg−1 at sampling point 11 to 947 mg kg−1 at sampling point 4. The permissible Pb concentration that can be considered for soil quality is 17 mg kg−1 (CETESB 2014). However, sampling points 1 to 5 contained elevated concentrations of Pb, considerably higher than the concentration estimated for residential intervention. Sampling points 1 to 5 and 10 presented a higher As concentration, ranging from 38 to 65 mg kg−1. The list of orientation values for substances in soil (CETESB 2014) considers a concentration of 35 to 55 mg kg−1, the upper and lower limits for agricultural intervention.
Total metal concentrations in the soil samples from the sampling points were highly correlated, with Pearson correlation coefficients ranging from r = 0.65 (p < 0.05, N = 10) to 0.97 (p < 0.01, N = 10), and water-soluble metal concentrations were correlated with pseudo-total metal concentrations, ranging from r = 0.48 (no significance, N = 10) to 0.94 (p < 0.01, N = 10). This result indicates that all heavy metals analyzed come from similar sources of contamination, and sampling points 1 to 5, 7, 9, and 10 were the most contaminated (Table 1).
Metal concentrations in plant shoots and roots
Heavy metal concentrations between different plant species and tissues cultivated or collected in uncontaminated soils varied greatly worldwide (Table 2). According to Cao and Chi (2001) and Gerber et al. (2002) and Hao and Jiang (2015) in general, the normal heavy metal concentrations of terrestrial plants growing in uncontaminated soils are in the range of 1–700 mg kg−1 for Mn, 0.2–0.8 mg kg−1 for Cd, 0.4–45.8 mg kg−1 for Cu, 0.1–41.7 mg kg−1 for Pb, and 1–160 mg kg−1 for Zn. Therefore, for these mine area plants, heavy metal contents greatly exceeded the upper limits of the normal range, except for Ba, demonstrating that the plants accumulated higher amounts of heavy metal including arsenic from substrate.
Heavy metal accumulation in plants depends on the plant species (Alloway et al. 1990; Yoon et al. 2006). Plant uptake of heavy metals from soil occurs either passively with the mass flow of water into the roots or through active transport crossing the plasma membrane of root epidermal cells (Yoon et al., 2006; Hao and Jiang 2015).
Cu is an essential element for plants. There is increasing evidence of the active absorption of Cu; however, passive absorption is likely to occur, especially in the toxic range of this metal in solutions. In root tissue, Cu is almost entirely in complexed forms; however, it is most likely that the metal enters root cells in dissociated forms (Kabata-Pendias 2011). Total Cu concentrations in the roots of collected plants ranged from 16.31 to 93.36 mg kg−1 with the maximum concentration found in Baccharis trimera. The Cu concentration ranged from 8.84 to 21.12 mg kg−1, the maximum accumulation being found in the shoots of H. brevipes.
Zn is also an essential element for plants. However, hyperaccumulation of Zn by plants is not very usual. It is very mobile during weathering processes, and its easily soluble compounds are readily precipitated by reactions with carbonates, or it is absorbed by minerals and organic compounds, especially in the presence of sulfur anions, hence minimizing uptake and transport from roots to the aerial parts of plants (Kabata-Pendias 2011; Hao and Jiang 2015). Total Zn concentration in the roots ranged from 188.4 to 1311.1 mg kg−1, this being the maximum concentration observed for H. brevipes. Moreover, the maximum Zn concentration in the shoots observed was higher in H. brevipes (Table 3). However, the roots of Eryngium horridum also contained high amounts of Zn. Hao and Jiang (2015), examining the heavy metal concentrations in dominant plants (shoot and root) in Mn mine area, observed Cu and Zn concentrations ranging 1.96–53.4 and 0.77–137.71 mg kg−1, respectively, lower than concentrations found in this study.
Total Cd concentrations in the roots ranged from 0.54 to 7.1 mg kg−1, the maximum occurring in H. brevipes. However, for shoots, the maximum concentration observed was in B. trimera, 2.1 mg kg−1 (Table 3). According to Kabata-Pendias (2011), soil pH is listed as the major soil factor controlling both total and relative uptake of Cd, as well as its concentration in growth media. Although soil characteristics other than the pH can also cause differences in the Cd absorption by roots, it may be stated that soluble forms of Cd in soil are always easily available to plants. Additionally, the higher total Ni concentration in the roots was 10.8 mg kg−1 (Cyperus eragrostis), and in the shoots, it was 2.76 mg kg−1 (S. brasiliensis) as shown in Table 3.
Total Pb concentration ranged from 22.5 to as high as 93.8 mg kg−1, the maximum being in the roots of S. brasiliensis. In addition, the roots of Dicranopteris nervosa and E. horridum also contained high amounts of Pb. The maximum concentration of Pb in the shoots was found in E. horridum (Table 3). In addition, the shoots of D. nervosa and B. trimera also contained high amounts of Pb (101.3 and 98.3 mg kg−1, respectively) compared to the contents presented in Table 2.
Bech et al. (2012), assessing spontaneous species from mine spoils in Peru, observed that Senecio sp. accumulated more than 4000 mg kg−1of Pb in its shoots from a contaminated site and 146 mg kg−1 of Pb at a reference site and in the roots ranging from 381 (reference site) to 451 mg kg−1 (contaminated site). However, many factors may be involved in these differences such as total Pb concentration in the sites, which ranged from 124 (reference) to 13,105 mg kg−1 (contaminated), higher than concentrations found at the studied contaminated sites (Table 1) (soil chemical characteristics, e.g., pH, CEC, adsorption capacity, climate, and others) (Keller 2006).
Total As concentrations in the plants ranged from 0.06 to 1.21 mg kg−1, the maximum being in the shoots of H. brevipes. Total Ba concentration in the roots ranged from 0.07 to 1.98 mg kg−1 and, in the shoots, from 0.05 to 0.62 mg kg−1 in E. horridum and H. brevipes, respectively.
Efficient phytoextraction requires plant species combining both high metal tolerance and elevated capacity for metal uptake and metal translocation to easily harvestable plant organs (e.g., shoots). Among plant species, there are large differences in the capacity to translocate and tolerate high concentrations of trace elements in the shoots. Most extreme behavior is displayed by metal hyperaccumulator plants (Bech et al. 2012).
None of the plant species showed metal concentrations that could be considered as hyperaccumulators based on the criteria: hyperaccumulators are plant species, which accumulate more than 100 mg kg−1 dry weight Cd or more than 1000 mg kg−1 dry weight Ni, Cu, and Pb or more than 10,000 mg kg−1 dry weight Zn and Mn in their shoots when grown on metal-rich soils (Baker and Brooks 1989) or, according to Van der Ent et al. (2013), the following concentration criteria for different metals and metalloids in dried foliage with plants growing in their natural habitats of 100 mg kg−1 for Cd, Se, and Ti; 300 mg kg−1 for Co, Cu, and Cr; 1000 mg kg−1 for Ni, Pb, and As; 3000 mg kg−1 for Zn; and 10,000 mg kg−1 for Mn.
The trend for heavy metal accumulation (mg kg−1dry weight) was Zn > Pb > Cu > Ni > Ba > As > Cd in the roots of D. nervosa and Pb > Zn > Cu > Ni > As > Cd > Ba in the shoots of D. nervosa and E. horridum. In the roots of E. horridum, it was Zn > Pb > Cu > Ni > Cd > As > Ba. In the roots of S. brasiliensis, it was Zn > Pb > Cu > Ni > Ba > Cd > As. In the shoots, it was Zn > Pb > Cu > Ni > Cd > Ba > As.
In the roots and shoots of Senecio leptolobus, it was Zn > Pb > Cu > Ni > Cd > Ba > As. In the roots of C. eragrostis, it was Zn > Pb > Cu > Ni > Ba > Cd >As, and in the shoots, it was Zn > Pb > Cu > Ni > Cd > As > Ba. In the roots of B. trimera, it was Zn > Cu > Pb > Ni > Cd > Ba > As, and in the shoots, it was Zn > Pb > Cu > Cd > Ni > Ba > As, and in the roots of H. brevipes, it was Zn > Pb > Cu > Cd >Ni > Ba > As, and in the shoots, it was Zn > Pb > Cu > Ni > As > Cd > Ba (Table 3).
Elements Zn and Pb were most absorbed by all species studied and Cd, As, and Ba the least absorbed. According to Mengel and Kirkby (2001) and Pandey (2012), Zn inhibits the Cd uptake due to its competitive behavior with Cd, because both metals are transported by a common carrier at the root plasma membrane, which has more affinity for Zn than Cd (Hart et al. 2005), explaining the low Cd concentrations in the plant tissue. However, it is known that the metal accumulation trend depends on some factors such as age and type of plant species, metal concentrations, pH, and season of sampling (Maiti and Jaiswal 2008; Pandey 2012).
Bioconcentration and translocation of metals in plants
Plant species with both bioconcentration factor (BCF) and translocation factor (TF) greater than one can potentially be used for heavy metal phytoextraction (Fitz and Wenzel 2002; Yoon et al. 2006). For Cu, B. trimera had the highest BCF (Table 4), though its TF was less than one. The TF of S. brasiliensis was the highest for Cu; however, the Cu concentration in the plant tissue was <300 mg kg−1, and it was not considered a Cu hyperaccumulator. The same behavior was observed for Zn. However, it was observed that S. leptolobus and B. trimera have BCF and TF greater than one, although the Zn concentration in rhizospheric soil is lower, which can explain the high factors (Table 4).
The Cd concentration in the rhizospheric soil was considered low, and plants did not present a hyperaccumulation feature (Table 3), although all of them, except D. nervosa and C. eragrostis, have BCF and TF greater than one. For element Ni, a higher BCF was observed only in E. horridum; it was higher than in other species studied (Table 4).
For element Pb, S. leptolobus and S. brasiliensis presented BCF and TF greater than one; therefore, they can potentially be used for metal phytoextraction. All plant species had TF greater than one for Pb, except H. brevipes. No BCF greater than one was observed for As, and only species D. nervosa and H. brevipes had TF greater than one. For element Ba, a higher BCF was observed in E. horridum (12.69), although the TF was lower than one. The species S. leptolobus and B. trimera had BCF greater than one and S. brasiliensis and H. brevipes the TF (Table 4).
According to Yoon et al. (2006), heavy metal-tolerant species with high BCF and low TF can be used for phytostabilization of a contaminated site, together with a plant cover. Phytostabilization uses plants to minimize the mobility and bioavailability of pollutants in the environment, either by immobilizing them or by preventing their migration (Tangahu et al. 2011). Species such as D. nervosa, E. horridum, S. leptolobus, and B. trimera could therefore be used for this purpose at a Cu-contaminated site. At a Zn-contaminated site, S. brasiliensis and H. brevipes could be used; for Cd, S. leptolobus and H. brevipes; and for Ba, S. leptolobus and B. trimera (Table 4). However, E. horridum showed the same characteristic for heavy metals Zn, Cd, Ni, and Ba; it may be considered a multi metal-tolerant plant and a good candidate for phytostabilization, preventing metals from being transferred to the food web.
Root exudates change the environmental characteristics around the root system, and plant species can immobilize heavy metals through absorption and accumulation by roots, adsorption onto roots, or precipitation within the rhizosphere, reducing metal bioavailability and mobility, because metal accumulated in the roots is considered relatively stable as far as release to the environment is concerned (Yoon et al. 2006). Moreover, root exudates change soil environmental (rhizosphere) stimulating plant–microorganism interactions, affecting the heavy metal uptake and translocation by microorganism bioremoval characteristics.
Conclusions
Among the plant species screened, none was identified as a metal hyperaccumulator. Heavy metal and metalloid levels in spontaneous plants greatly exceeded the upper limits of the normal range of terrestrial plants grown in uncontaminated soils, demonstrating that the plants accumulated higher heavy metal levels when grown in contaminated soils. S. leptolobus are most effective in taking up Zn and Pb; B. trimera are effective in taking up Cd and Senecio brasiliensis for Pb. These species may be used for phytoextraction at heavy metal-contaminated sites in each of the recovery steps. S. brasiliensis is most effective in translocating Cu, Cd, and Ba. For Zn and Pb translocation, B. trimera is most effective. D. nervosa and Hyptis brevipes are most effective for the As element. D nervosa for Cu; Eryngium horridum for Cu, Zn, Cd, Ni, and Ba; Senecio leptolobus for Zn and Pb; B. trimera for Cu, Zn, Cd, and Ba; and Hyptis brevipes for Zn and Cd have a BCF greater than one.
References
Andrade JCM, Tavares SRL, Mahler CF (2007) Phytoremediation: use of plants to improve environmental quality. Oficina de textos, São Paulo (in Portuguese)
Alloway BJ, Jackson AP, Morgan H (1990) The accumulation of cadmium by vegetables grown on soils contaminated from a variety of sources. Sci Total Environ 91:223–36
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Aliyu HG, Adamu HM (2014) The potential of maize as phytoremediation tool of heavy metals. Eur Sci J 10:30–37
Andrade MG, Melo VF, Gabardo J, Souza LCP, Reissmann CB (2009) Heavy metals in soils of a lead mining and metallurgy area. I—phytoextraction. Rev Bras Ci Solo 33:1879–1888 (in Portuguese)
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements: a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Baker AJM (1981) Accumulators and excluders—strategies in the response of plants to heavy metals. J Plant Nutri 3:643–654
Baker AJM, Whiting SN (2002) In search of the Holy Grail—a further step in understanding metal hyperaccumulation? New Phytol 155:1–7
Barceló J, Poschenrieder C (2003) Phytoremediation: principles and perspectives. Contrib Sci 2:333–344
Bech J, Duran P, Roca N, Poma W, Sánchez I, Roca-Pérez L, Boluda R, Barceló J, Poschenrieder C (2012) Accumulation of Pb and Zn in Bidens triplinervia and Senecio sp. spontaneous species from mine spoils in Peru and their potential use in phytoremediation. J Geochem Explor 123:109–113
Bidar G, Garcon G, Pruvot C, Dewaele D, Cazier F, Douay F, Shirali P (2007) Behavior of Trifolium repens and Lolium perenne growing in a heavy metal contaminated field: plant metal concentration and phytotoxicity. Environ Pollut 147:546–553
Cao JL, Chi BL (2001) Urban ecological corridor. Meteorological Press, Beijing
CETESB (2014) Guiding values report for soil and groundwater in São Paulo State. CETESB. http:// http://www.cetesb.sp.gov.br/userfiles/file/solo/valores-orientadores-nov-2014.pdf. Accessed 25 may 2015 (in Portuguese)
Coscione AR, Berton RS (2009) Barium extraction potential by mustard, sunflower and castor bean. Sci Agric 66:59–6
Davari M, Homaee M, Rahnemaie R (2015) An analytical deterministic model for simultaneous phytoremediation of Ni and Cd from contaminated soils. Environ Sci Pollut Res 22:4609–4620
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciênc Agrotec 35:1039–1042
Fitz WJ, Wenzel WW (2002) Arsenic transformation in the soil–rhizosphere–plant system, fundamentals and potential application of phytoremediation. J Biotechnol 99:259–78
Fumagalli P, Comolli R, Ferrè C, Ghiani A, Gentili R, Citterio S (2014) The rotation of white lupin (Lupinus albus L.) with metal-accumulating plant crops: a strategy to increase the benefits of soil phytoremediation. J Environ Manag 145:35–42
Gall JE, Boyd RS, Rajakaruna N (2015) Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess 187:201–222
Govindasamy C, Arulpriya M, Ruban P, Francisca LJ, Ilayaraja A (2011) Concentration of heavy metals in seagrasses tissue of the Palk Strait, Bay of Bengal. Int J Environ Sci 2:145–153
Gerber GB, Léonard A, Hantson PH (2002) Carcinogenicity, mutagenicity and teratogenicity of manganese compounds. Crit Rev Oncol Hematol 42:25–34
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:367–374
Grison CM, Mazel M, Sellini A, Escande V, Biton J, Grison C (2015) The leguminous species Anthyllis vulneraria as a Zn-hyperaccumulator and eco-Zn catalyst resources. Environ Sci Pollut Res 22:5667–5676
Hao Q, Jiang C (2015) Heavy metal concentrations in soils and plants in Rongxi Manganese Mine of Chongqing, Southwest of China. Acta Ecol Sin 35:46–51
Hart JJ, Welch RM, Norvell WA, Clarke JM, Kochian LV (2005) Zinc effects on cadmium accumulation and partitioning in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol 167:391–401
Jiménez MN, Bacchetta G, Casti M, Navarro FB, Lallena AM, Fernández-Ondono E (2011) Potential use in phytoremediation of three plant species growing on contaminated mine-tailing soils in Sardinia. Ecol Eng 37:392–398
Kabata-Pendias A (2011) Trace elements in soils and plants. Chemical Rubber Company Press, Boca Raton
Keller C (2006) Factors limiting efficiency of phytoextraction at multi-metal contaminated sites. In: Morel J, Echevarria G, Goncharova N (eds) Phytoremediation of metal-contaminated soils. Springer, Netherlands, pp 241–266
Kumari B, Singh SN (2011) Phytoremediation of metals from fly ash through bacterial augmentation. Ecotoxicology 2:166–176
Ladislas S, El-Mufleh A, Gerente C, Chazarenc F, Andres Y, Bechet B (2012) Potential of aquatic macrophytes as bioindicators of heavy metal pollution in urban storm water runoff. Water Air Soil Pollut 223:877–888
Ma L, Rao X, Lu P, Huang S, Chen X, Xu Z, Xie J (2015) Acid-tolerant plant species screened for rehabilitating acid mine drainage sites. J Soils Sediments 15:1104–1112
Mackay D (1982) Correlation of bioconcentration factors. Environ Sci Technol 16:274–278
Maiti SK, Jaiswal S (2008) Bioaccumulation and translocation of metals in the natural vegetation growing on fly ash lagoons: a field study from Santaldih thermal power plant, West Bengal India. Environ Monit Assess 136:355–370
Mengel K, Kirkby EA (2001) Principles of plant nutrition, 5th edn. Kluwer Academic Publishers, Dordrecht
Nascimento CWA, Xing B (2006) Phytoextraction: a review on enhanced metal availability and plant accumulation. Sci agric 63:299–311
Nowell LH, Capel PD, Dileanis PD (1999) Pesticides in stream sediment and aquatic biota: distribution, trends, and governing factors. Lewis Publishers, Boca Raton
Pandey VC (2012) Phytoremediation of heavy metals from fly ash pond by Azolla caroliniana. Ecotoxicol Environ Saf 82:8–12
Santos ACQ, Accioly AMA, Nascimento CWA, Santos NM, Melo EEC, Xavier BTL (2014) Competitive absorption of cadmium, zinc, and lead by Velvet Bean (Stizolobium aterrimum) and metal distribution among soil fractions. Commun Soil Sci Plant Anal 45:1499–1510
Satpathy D, Reddy MV, Dhal SP (2014) Risk assessment of heavy metals contamination in paddy soil, plants, and grains (Oryza sativa L.) at the east coast of India. Biomed Res Int 2014:1–11
Singh R, Singh DP, Kumar N, Bhargava SK, Barma SC (2010) Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area. J Environ Biol 31:421–430
Soil Survey Staff (2010) Soil taxonomy - a basic system of soil classification for making and interpreting soil survey, 11th edn. USDA, Washington
Tang YT, Deng THB, Wu QH, Wang SZ, Qiu RL, Wei ZB, Guo XF, Wu QT, Lei M, Chen TB, Echevarria G, Sterckeman T, Simonnot MO, Morel JL (2012) Designing cropping systems for metal-contaminated sites: a review. Pedosphere 22:470–488
Tangahu BV, Abdullah SRS, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng 21:1–31
Tedesco MJ, Gianello C, Bissani CA, Bohnen H, Volkweiss SJ (1995) Analysis of soil, plants and other materials, 2nd edn. UFRGS - Soil Department, Porto Alegre (in potuguese)
Testiati E, Parinet J, Massiani C, Laffont-Schwob I, Rabier J, Pfeifer H, Lenoble V, Masotti V, Prudent P (2013) Trace metal and metalloid contamination levels in soils and in two native plant species of a former industrial site: evaluation of the phytostabilization potential. J Hazar Mater 248–249:131–141
USEPA (1998) Method 3050B. http://www.epa.gov/wastes/hazard/testmethods/sw846/pdfs/3050b.pdf. Accessed 18 January 2015
Vamerali T, Bandiera M, Lucchini P, Mosca G (2015) Metal partitioning in plant–substrate–water compartments under EDDS-assisted phytoextraction of pyrite waste with Brassica carinata A. Braun. Environ Sci Pollut Res 22:2434–2446
Van Der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334
Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M (2010) Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutri 10:268–292
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464
Acknowledgments
This research received funding from the Brazilian National Research and Development Council (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Boechat, C.L., Pistóia, V.C., Gianelo, C. et al. Accumulation and translocation of heavy metal by spontaneous plants growing on multi-metal-contaminated site in the Southeast of Rio Grande do Sul state, Brazil. Environ Sci Pollut Res 23, 2371–2380 (2016). https://doi.org/10.1007/s11356-015-5342-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5342-5