Abstract
The bioaccumulation of artificial Cs-137 and natural radionuclides Th-234, Ra-226, and K-40 by Basidiomycetes of several species is studied and evaluated in relation to their substratum soils. For this reason, 32 fungal samples, representing 30 species of Basidiomycetes, were collected along with their substratum soil samples, from six selected sampling areas in Greece. The fungal fruit bodies and the soil samples were properly treated and the activity concentrations of the studied radionuclides were measured by gamma spectroscopy. The measured radioactivity levels ranged as follows: Cs-137 from <0.1 to 87.2 ± 0.4 Bq kg−1 fresh weight (F.W.), Th-234 from <0.5 ± 0.9 to 28.3 ± 25.5 Bq kg−1 F.W., Ra-226 from <0.3 to 1.0 ± 0.5 Bq kg−1 F.W., and K-40 from 56.4 ± 3.0 to 759.0 ± 28.3 Bq kg−1 F.W. The analysis of the results supported that the bioaccumulation of the studied natural radionuclides and Cs-137 is dependent on the species and the functional group of the fungi. Fungi were found to accumulate Th-234 and not U-238. What is more, potential bioindicators for each radionuclide among the 32 species studied could be suggested for each habitat, based on their estimated concentration ratios (CRs). The calculation of the CRs’ mean values for each radionuclide revealed a rank in decreasing order for all the species studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are several studies in the scientific literature that deal with the bioaccumulation of radionuclides (both natural and artificial), as well as with conventional pollutants, concluding that fungi can be considered as a tool for assessing the early response to contamination and the dispersion and behavior of natural elements—including radionuclides—in the ecosystem (Baeza et al. 2004; Castro et al. 2012; Duff and Ramsey 2008; Fraiture et al. 1990; Rakić et al. 2014). The factors that may influence radionuclide uptake by fungi have been reported by several authors. The most significant factors are related with the ecology and geology of their habitats and include environmental parameters, such as type of climate, rainfall patterns, type of soil and humus, soil pH and texture, type of forest canopy, and soil stable Cs and K concentrations; and biological traits such as depth and life span of the mycelium, fungal species, functional group of fungi with regards to their mode of nutrition, and type of basidiome. (Baeza et al. 2004; Bazala et al. 2008; Fraiture 1992; Gillet and Crout 2000; Heinrich 1993; Kuwahara et al. 2005; Nimis et al. 1992; Rakić et al. 2014; Yoshida et al.,1994). In conclusion, fungi may be used as bioindicators for assessing the radioactive contamination of a territory and the changes in the biological availability of radionuclides (Dighton et al. 2008; Fraiture et al. 1990).
Regarding the behavior of fungi toward radionuclide accumulation from terrestrial ecosystems in Greece, there is limited published research in the international literature. On the contrary, there are a lot of studies with respect to fungal Ecology and Systematics in Greek ecosystems (Delivorias et al. 2010; Gonou-Zagou and Delivorias 2011; Tsopelas et al. 2009). The present study is the result of a joint research initiative of the mycological research group of the University of Athens and the Environmental Radioactivity Laboratory (ERL) of the National Center for Scientific Research “Demokritos” (NCSR “D”). Consequently, several studies have been conducted, and the results have been presented in national and international conferences (Gonou-Zagou et al. 2006; Kioupi et al. 2011; Kioupi et al. 2004; Kioupi 2004; Mantzourani Α 2002; Mantzourani Α, Florou Ε, Kapsanaki-Gotsi Ε, Gonou-Zagou Ζ 2001; Mylogiannaki Ν 2004). Τhe main findings of the former studies suggested that specific fungal species can be used as a tool for assessing the radiological status of a terrestrial ecosystem.
The primary research task of the present study was to investigate the accumulation of Cs-137, which resulted mainly from the deposition of the radioactive fallout of the Chernobyl accident on Greek soils, and the natural Th-234, Ra-226, and K-40 by fungi from various habitats in Greece. Furthermore, the respective activity concentrations in their substrate soils were measured and analyzed in order to estimate the CRs for the radionuclides considered and compare them with results from the international literature. The obtained data were treated and interpreted by use of appropriate statistics. Furthermore, the radiological role of the various species of Basidiomycetes and the dependence of the activity concentrations on the sampling area, family, and functional group of fungi in terms of nutrition were also evaluated. Finally, an effort was also made to suggest some species as bioindicators for the studied radionuclides in terms of their ability to show a remarkable response to the pollutants introduced to their habitat.
Materials and methods
Sampling and treatment of fungal fruit bodies and soils
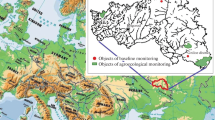
Thirty-two composite samples of fungal fruit bodies (basidiomata), representing 30 species of Basidiomycetes, were collected from six sampling areas of 5–20 m2 in central and northern Greece, between September 2002 and November 2002 (Fig. 1 and Table 1). In almost all the cases, the substrate of the fungi was soil, except for Mycena seynii, which was found on fallen Pinus halepensis cones and Armillaria mellea which was found growing on buried wood (Table 1).
The sampling weight for the fungi was about 1–2 kg fresh weight (F.W.), consisting of samples and their replicates. This was considered enough to obtain the adequate mass to complete the polyurethane measurement pot (called white cup, d = 70 mm, h = 20 mm, V = 76 cm3), taking into account that the percentage ashing factor varies from 1.2 to 6.1 % (Table 1).
Fungal fruit bodies were collected from natural (Agrafa, Diakopi, Dirfys) and semi-natural environments (Florina, Ymittos, Aigaleo) directly. The sampling areas were mainly natural forest areas dominated by the Greek fir (Abies cephalonica; Agrafa, Diakopi, Dirfys), exurban forest areas dominated by the Mediterranean pine (P. halepensis; Ymittos, Aigaleo), and grasslands (Florina).
Care was taken in order for the fungal fruit body to remain intact during sampling. The fungi collected, in terms of systematics, belong to the class Basidiomycetes and represent 16 families. They were identified up to species level, with the exception of six samples (Table 1). In terms of their nutrition mode, which affects the biological accumulation of radionuclides, 17 of the species collected were mycorrhizal, 13 were saprotrophic, and only 1 was characterized as parasitic to trees (Table 1).
Twelve soil samples were collected from the same sampling areas as the fungi (Fig. 1 and Table 1) between September 2002 and June 2003. The soil texture of each sampling area ranges from fine (35 % < clay < 60 %) in Diakopi to medium (18 % < clay < 35 % and ≥15 % sand, or 18 % < clay and 15 % < sand < 65 %) in Agrafa, Dirfys, Ymittos, and Aigaleo to coarse (18 % < clay and >65 % sand) in Florina (Soil Texture and European Commission 2001). Soil samples (0–10-cm depth) were collected using a spade under the fruit body in a 10 cm × 10 cm square. For each sampling area, composite soil samples were collected, consisting of samples and their replicates. In areas where more than one composite soil samples were collected (Diakopi and Ymittos, Table 2), the calculation of radionuclide activity concentrations was performed by using the mean value.
After sampling, the fungal specimens, were immediately transferred to the laboratory, they were cleaned from soil and other impurities first by hand and then washed with tap water and finally rinsed with bidistilled water in order to be perfectly free from residuals. After that, they were dried and finally F. W. was appropriately obtained. Then, they were ashed at 360 °C (>90 % recovery of radionuclides according to ERL treatment protocols, IAEA TRS No 118 of 1970 and IAEA TRS No 295 of 1989) until constant weight which was measured and the percentage ratio (ashing factor) was estimated (Table 1). Finally, they were homogenized up to fine powder and enclosed in white cups for immediate gamma measurement. The soil samples were initially sieved (0.6 mm in diameter) to collect the fine fraction and after that dried at 105 °C until constant dry weight (D.W.), which was obtained. Then, they were enclosed in white cups for immediate gamma measurement, according to the ERL physical treatment protocols and the TRS No 295 of IAEA (1989).
Measurements
The activity concentrations of the artificial Cs-137 and natural radionuclides Th-234, Ra-226, and K-40 for both the fungal and the soil samples were measured for 70,000 s in a high-resolution gamma spectrometry system of the ERL of the NCSR “D,” with a high-purity Ge detector of 20 % relative efficiency and computerized multichannel analyzer of 4000 channels in a total spectrum area of 2000 keV. ORTEC software was used for the analyses of the obtained spectra. The relative statistical error (1σ) did not exceed 18 %. The efficiency was determined by a Ra-226 standard source of 240 Bq. The calibration correction was made by a Co-60 point standard source of 3.7 × 104 Bq, in the 1173.2- and 1332.5-keV peaks. Additional calibration in the range below 150 keV was made by use of U-238 standard and the IAEA reference material RGU-1, as well. Quality control was certified through the participation of ERL in the ALMERA network of IAEA and especially through its participation in the interlaboratory proficiency tests (PTs) and intercomparison exercises such as First ALMERA PT in soil of 2001 (IAEA 2005) and EC-JRC-IRMM Interlaboratory Comparison on natural radioactivity and Cs-137 in soil run IAEA-375 (Strachnov et al. 1996). It must be noted that interlaboratory tests and intercomparison exercises always include a quality control sample aiming at a constant check of the detector's energy and efficiency calibration.
The minimum detectable activities for each radionuclide were as follows: for Cs-137 0.01 Bq/sample, for K-40 0.14 Bq/sample, for U-238/Th-234 0.22 Bq/sample, and for Ra-226 0.02/per sample. The activity concentrations for the fungi were calculated on the basis of F.W. (Bq kg−1) taking into account the ashing factor of each fungal sample.
Uranium-238 activity concentrations were calculated viaTh-234 peaks, namely, the one at 63.3 keV and the double peak at 92.4 and 92.8 keV. In the soil, U-238 is in secular equilibrium with its daughter radionuclide Th-234 in most of the cases. A second measurement of U-238/Th-234 activity concentrations in the soil samples took place about 1 month after the first one to reveal if the radionuclide follows the decay of U-238 (t 1/2 = 4468 billion years) or Th-234 (t 1/2 = 24 days). The second measurement was similar to the first one and, thus, the radionuclide follows the decay of U-238. In basidiomata, which are short-lived, Th-234 is not in secular equilibrium with its parent radionuclide U-238. In order to determine if fungi accumulate U-238 or Th-234, a second measurement was carried out about 1 month after the first one. The U-238/Th-234 activity concentrations in the second measurement were found below the lower limit of detection (LLD), which was 0.5 Bq kg−1 (Tables 2 and 4). Consequently, the radionuclide present in the fungal samples follows the decay of Th-234 and, thus, Th-234 is accumulated by fungi and not U-238.
Radium-226 activity concentrations were measured about a month after sealing the measurement pots, so that secular equilibrium was established between Ra-226 and its daughter radionuclide Rn-222. Consequently, calculations were made via the two peaks of Pb-214 at 295.2 and 351.9 keV and via the peak of Bi-214 at 609.3 keV. The LLD for Ra-226 was 0.3 Bq kg−1 (Table 4).
Potassium-40 activity concentrations were calculated via its only peak at 1460.8 keV, Cs-137 activity concentrations via its only peak at 661.7 keV, and Cs-134 activity concentrations via its two peaks at 604.7 and 795.8 keV. In cases where more than two peaks were used, calculation of the mean value was performed. The respective LLD for K-40 was 0.2 Bq kg−1 F.W. and for Cs-137 was 0.1 Bq kg−1 F.W. (Tables 2 and 4).
Concentration ratios and statistical treatment
The concentration ratios (CRs) for the artificial Cs-137 and natural Th-234, Ra-226, and K-40 (Table 6) were calculated on the basis of the IAEA TRS No 479 (2014) CRs as follows:
- AC :
-
radionuclide activity concentration
The statistical treatment of the results was processed using PASW Statistics 18 software. This is a software package for statistical analysis, data management, and data documentation (Statistics 2009). More specifically, the features of the program described below were used:
-
1.
The Shapiro–Wilks W test to check if the activity concentrations in both the fungal and the soil samples followed normal distribution. This test was appropriate for our samples because it is used for a number of samples (N) from 3 to 2000. Results for this test are provided in Tables 3 and 5.
Table 3 Descriptive values and Shapiro–Wilks W Test results for Cs-137, U-238, Ra-226, and K-40 activity concentrations (Bq kg−1 F.W.) in the 12 soil samples -
2.
The Kruskal–Wallis H test (one-way analysis of variance by ranks) is a non-parametric method for testing whether the samples originate from the same distribution. Results for this test are provided in Table 7.
-
3.
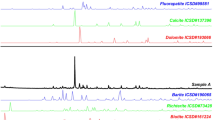
The cluster analysis is a common technique used for grouping a set of data into clusters based on their similarity and distance. Results for this test are provided in Fig. 2.
For the statistical analyses of activity concentrations of the radionuclides in the fungal species, the values below the LLD were omitted, whereas 10 % of the distribution for Cs-137, 5 % of Th-234, and 3 % of K-40 was winsorized (Table 5). This was done in order to give less weight to values in the tails of the distribution (outliers). Very low activity concentrations (close to the LLD) and very high ones were changed to the next largest or the next smallest score, respectively, in order to obtain more robust mean values. The level of significance (p value) in all the analyses was set at 0.05.
Results and discussion
Activity concentrations in the substratum soils
The activity concentrations for the artificial radionuclide Cs-137 in the soil samples ranged from 44.8 ± 1.6 Bq kg−1 in Dirfys to 571.7 ± 4.6 Bq kg−1 D.W. in Diakopi (Fig. 1 and Table 2). Cs-137 was found in the range of the LLD of 0.1 Bq kg−1 D.W. only in one soil sample from Ymittos (Fig. 1 and Table 2). This was a very disturbed soil sample collected in the wider area of the University of Athens campus. From the bulk of 12 soil samples measured for Cs-134, only 3 of them, collected in Diakopi and Florina (Fig. 1), were found to be >0.1 Bq kg−1 D.W. (LLD). The observed values for Cs-134 ranged from 2.3 ± 0.6 to 4.9 ± 1.4 Bq kg−1 D.W. (Table 2).
Compared with results from North Lebanon Province that ranged from 2 to 113 Bq kg−1 (Samad et al. 2013) and from western Serbia that ranged from 2.6 to 55.3 Bq kg−1 (Dugalic et al. 2010), the present values for Cs-137 are higher. They are, however, comparable with values from Turkey that ranged from 27 to 775 Bq kg−1 (Celik et al. 2009).
Caesium-137 concentrations in Greek soils were relatively high as the Chernobyl fallout had a significant impact on the Greek territory (Kritidis and Florou 2001). Activity concentrations for Cs-137 in the soil samples followed the deposition pattern of total Cs in Greece as it is reported in the map of total cesium in Greece (Greece: Total Caesium 1996), i.e., higher concentrations were found in Western Macedonia (Florina), Thessalia (Agrafa), and parts of Sterea Hellas (Diakopi), which were mostly contaminated after the Chernobyl accident due to heavy rainfalls. On the other hand, lower concentrations were found in Athens (Ymittos and Aigaleo) and parts of Evia (Dirfys), where less Cs was deposited after the Chernobyl accident. Consequently, the deposition seems to be the main parameter affecting the soil concentrations. This can also be noted from the almost similar concentrations in Dirfys undisturbed and Ymittos disturbed soil samples (Table 2).
In terms of the natural radionuclides determined in this study, U-238 concentrations ranged from 23.5 ± 12.9 Bq kg−1 D.W. in Diakopi to 132.5 ± 17.3 Bq kg−1 D.W. in Dirfys (Fig. 1 and Table 2). Three soil samples from Diakopi, Ymittos, and Aigaleo presented values <0.5 Bq kg−1 D.W. (LLD, Table 2). Data from North Lebanon province showed activity concentrations for U-238 from 4 to 37 Bq kg−1 D.W. (Samad et al. 2013), from Thailand from 13.9 to 76.8 Bq kg−1 D.W. (Santawamaitre et al. 2014), and from western Serbia from 12 ± 9 to 90 ± 30 Bq kg−1 D.W. (Dugalic et al. 2010). It is clear that the data presented in this study seem to be to the upper part of the observed spectrum worldwide.
Radium-226 concentrations ranged from 18.1 ± 2.2 Bq kg−1 D.W. in Diakopi to 57.2 ± 1.7 Bq kg−1 D.W. in Agrafa (Fig. 1 and Table 2). In the scientific literature, results for Ra-226 ranged from 28.3 ± 0.5 to 81.0 ± 1.7 Bq kg−1 in India (Singh et al. 2009), 12 to 120 Bq kg−1 in Turkey (Celik et al. 2009), and 7.8 ± 0.7 to 45.6 ± 2.7 Bq kg−1 in Western Serbia (Dugalic et al. 2010), which are almost similar to the present values.
Potassium-40 activity concentrations ranged from 401.2 ± 25.5 Bq kg−1 D.W. in Aigaleo to 851.5 ± 23.0 Bq kg−1 D.W. in Dirfys (Fig. 1 and Table 2). For K-40, recent data from other studies presented radioactivity levels from 363.4 ± 4.9 to 1002 ± 11.2 Bq kg−1 in India (Santawamaitre et al. 2014) and 204 to 1295 Bq kg−1 in Turkey (Celik et al. 2009), which are higher than the ones reported in this study, and 121 ± 15 to 458 ± 27 Bq kg−1 in western Serbia (Dugalic et al. 2010), which are lower, accordingly.
The activity concentrations for each radionuclide in the soil samples were checked for normality using the Shapiro–Wilks W test (Table 3). For the radionuclides Ra-226, K-40, and Cs-137, the hypothesis that they are normally distributed cannot be rejected based on their p values (Table 3). This was not the case for U-238 (p value = 0.001, Table 3).
Activity concentrations in fungi
General comments
Activity concentrations for all the radionuclides assessed in this study are shown on Table 4. The distributions of activity concentrations for all the radionuclides were checked for normality using the Shapiro–Wilks W Test after winsorizing the values in the cases of Cs-137 (10 %), K-40 (3 %), and Th-234 (5 %). The results in Table 5 showed that Cs-137, Th-234, and K-40 activity concentrations did not follow normal distribution (p values were 0.000, 0.001, and 0.021, respectively), but Ra-226 (p value = 0.182) seemed to follow normal distribution.
It has been suggested that the bioaccumulation of radionuclides in fungi depends on the specific characteristics of each fungal species (Duff and Ramsey 2008). To assess that, cluster analysis was performed in our data for all the radionuclides in order to organize them into clusters (groups), based on their similarity. First, a two-step cluster analysis was carried out in order to determine the number of clusters that best fits our data. Good quality of cohesion and separation was observed for the two-cluster solution (C1 and C2). After that, hierarchical cluster analysis was applied which revealed that the larger cluster (C1) included 75 % (24) of the fungal species that showed lower activity concentrations and the smaller cluster (C2) included 25 % (8) of the fungal species that showed higher activity concentrations (Fig. 2). The eight species (cluster C2) that showed higher activity concentrations for all the radionuclides were Ramaria formosa, Sarcodon martioflavus, Hydnellum concrescens, Clavariadelphus truncatus, Macrolepiota mastoidea, Melanoleuca cognata, Russula delica, and Lactarius salmonicolor (Fig. 2 and Table 1). More specifically, the cluster C2 seemed to include three groups of potential bioaccumulators (Fig. 2). The first group with the “higher” values consisted of S. martioflavus, the second group with “medium” values consisted of Ramaria formosa and H. concrescens, and the third group with the “lower” values consisted of Russula delica, Lactarius salmonicolor, C. truncatus, Melanoleuca cognate, and Macrolepiota mastoidea.
From the eight species of cluster C2, six are mycorrhizal and two saprotrophic (Table 1). Two of these eight species belong to the family Bankeraceae (S. martioflavus and H. concrescens; Table 1) and two to the family Russulaceae (Russula delica and Lactarius salmonicolor; Table 1). Four of the species were collected in Diakopi (H. concrescens, C. truncatus, Macrolepiota mastoidea, and Lactarius salmonicolor; Table 1), while three were collected in Agrafa (Ramaria formosa, S. martioflavus, and Russula delica; Table 1). In the scientific literature (Duff and Ramsey 2008; Gillet and Crout 2000), four of these species’ genera (Lactarius, Russula, Sarcodon, and Macrolepiota) were reported as bioaccumulators whose behavior (activity concentrations and CRs) varied widely (from low to very high depending on species) toward the presence of Cs-137.
Mycena seynni and Armillaria mellea were the two fungal species that did not grow on soil but on fallen P. halepensis cones and buried wood respectively. Their measured radioactivity concentrations probably occurred via the transfer of radionuclides through the plant material on which they grow and obtain nutrients.
Cs-137
The values for Cs-137 ranged from 0.21 ± 0.02 to 87.2 ± 0.4 Bq kg−1 F.W. (Table 4). Five fungal species’ activity concentrations (16 % of the samples) from different sampling sites, functional groups, and families were measured in the range of the LLD of 0.1 Bq kg−1 F.W. Those species were Armillaria mellea, Agaricus xanthodermus, Clitocybe sp.2, Lepista nuda, and Boletus sp. (Table 4). The present values seem to be higher than those of previous studies from Greece that ranged from 0.11 to 17.80 Bq kg−1 F.W. (Mantzourani Α, Florou Ε, Kapsanaki-Gotsi Ε, Gonou-Zagou Ζ 2001). However, when taking into consideration the ashing factor, the concentrations of Cs-137 (10.0–4035.2 Βq kg−1 D.W.) were also higher in comparison with data from other areas worldwide, e.g., Mexico, ranging from 0.2 to 91 Bq kg−1 D.W. (Gaso et al. 2000); Brazil, ranging from 1.45 to 10.6 Bq kg−1 D.W. (Castro et al. 2012); Spain, ranging from <LLD to 47 ± 7 Bq kg−1 D.W. (Baeza et al. 2004); and Turkey, ranging from 9.81 to 401 Bq kg−1 D.W. (Karadeniz and Yaprak 2007).
The lowest value for Cs-137 was found in the mycorrhizal Lycoperdon perlatum collected in Diakopi, while the highest value was found in the mycorrhizal Ramaria formosa collected in Agrafa (Fig. 1 and Table 4). It is important to mention that the Cs-137 activity concentration in the soil of Agrafa was not the highest detected (Table 2). Other species presenting high Cs-137 activity concentrations were the mycorrhizal S. martioflavus (42.5 ± 2.8 Bq kg−1) and H. concrescens (30.7 ± 0.5 Bq kg−1) collected in Agrafa and Diakopi respectively (Fig. 1 and Table 4). These findings suggest species-specific accumulation.
As for the CRs for Cs-137, they ranged from 0.0005 for Lycoperdon perlatum to 0.2780 for Ramaria formosa (Table 6). As Ramaria formosa showed both the highest activity concentration (Table 4) and the highest CR for Cs-137 (Table 6), it seems to bioaccumulate Cs-137 and it could be used as bioindicator for the radiological assessment of Cs-137 in this habitat. The CRs for Cs-137 previously reported for Greece by Mantzourani Α, Florou Ε, Kapsanaki-Gotsi Ε, Gonou-Zagou Ζ (2001) ranged from 0.001 to 0.95 and were found to be higher than the present ones. In terms of CR values reported in the international literature, the CRs presented in this study were also found to be lower than, e.g., the CRs reported from Germany that were 0.001–5.4 (Kammerer et al. 1994) and from Norway that were 0.02–8.2 (Amundsen et al. 1996).
When taking into account the D.W. of the fungi, the present CRs are found higher than the CRs from Turkey that ranged from 0.19 to 3.15 (Karadeniz and Yaprak 2007), but are significantly lower when compared with data from Great Britain that showed a spectrum from 0.0125 to 57.1 (Barnett et al. 1999). Generally, it is difficult to compare and contrast CRs for Cs-137 because various authors employ different approaches in determining the CRs (Gillet and Crout 2000). For calculating the CRs in this study, we used homogenized soils taken from the upper layer because it is useful for practical assessment after an accident (IAEA TRS No 472 of 2010 and IAEA TRS No 479 of 2014).
The effect of functional group, family, and sampling site on the activity concentrations of Cs-137 in fungi was assessed using the Kruskal Wallis test (Table 7). The 32 species were categorized in three groups (saprotrophic, mycorrhizal, and parasitic, Table 1). Then, the Kruskal–Wallis H test was applied, considering the nominal parameter for each species as follows: 1 for saprotrophic, 2 for mycorrhizal, and 3 for parasitic. The same test was applied for family and sampling site, with 16 nominal parameters representing the 16 fungal families and 6 nominal parameters corresponding to the six sampling sites. The results (Table 7) showed that the effect was statistically significant only in the case of the functional group for Cs-137 (p value = 0.038); thus, functional group plays a role in Cs-137 accumulation by fungi. As a result, mycorrhizal fungi seemed to show higher Cs-137 concentrations than saprotrophic and parasitic ones and saprotrophic fungi showed higher Cs-137 activity concentrations than parasitic ones (Table 1 and 4). A relation for Cs-137 concentrations as mycorrhizal > saprotrophic ≥ parasitic is supported by other works (Baeza et al. 2004; Mietelski et al. 2010; Steiner et al. 2002). This relation seems to also apply in the case of CRs for Cs-137, when comparing mean CR values, i.e., for mycorrhizal fungi CRmean = 0.045, for saprotrophic CRmean = 0.013, and for parasitic CRmean = 0.0019 (Table 7). This is probably due to the fact that the uptake of Cs is related to the uptake of K, particularly in fungi (Bystrzejewska-Piotrowska and Bazala 2008), as these two radionuclides are analogues (Myttenaere et al. 1993). Potassium-40 is taken up together with stable K because it is essential for fungal metabolism. Mycorrhizal fungi, due to their symbiotic relationship with the roots of plants, absorb higher quantities of stable K/K-40 and Cs-137 for their metabolic needs compared with the saprotrophic ones (Brunner et al. 1996; Rosen et al. 2005). Parasitic fungi absorb these radionuclides directly from their host plants.
Th-234
Fungi accumulate Th-234 and not its parent radionuclide U-238. This is supported by the fact that the values for U-238/Th-234 determined by the second gamma measurements, conducted about a month after the original ones, were <LLD (Table 4).
The activity concentrations for this radionuclide ranged from 1.5 ± 0.9 to 28.3 ± 25.5 Bq kg−1 F.W. (Table 4). The percentage of samples found in the range of the LLD of 0.5 Bq kg−1 F.W. was almost 44 % (14 samples; Table 4). The CRs were from 0.0434 for Boletus sp. in Florina to 0.9625 for S. martioflavus in Agrafa (Table 6). Previous data from Greece (Mantzourani Α, Florou Ε, Kapsanaki-Gotsi Ε, Gonou-Zagou Ζ 2001) reported Th-234 concentrations between 0.11 and 9.85 Bq kg−1 F.W. and CRs between 0.005 and 0.1742, which are lower than the present results. Thorium-234 activity concentrations in herbal plants from Poland ranged from 0.173 to 1.941 Bq kg−1 D.W. (Solecki et al. 2003) and are lower than the ones reported for fungi.
The lowest activity concentration was found in the mycorrhizal Ramaria obtusissima in Diakopi, while the highest was found in the mycorrhizal S. martioflavus in Agrafa (Table 4). It is important to mention that the highest value for Th-234 in fungi did not correspond to the highest value in the substratum soil. This suggests species-specific accumulation. As S. martioflavus showed both the highest activity concentration (Table 4) and the highest CR for Th-234 of 0.9625 (Table 6), it could be considered as bioindicator for Th-234 in this habitat.
The applied Kruskal–Wallis H test results (Table 7) in order to assess the effect of functional group (p value = 0.236), family (p value = 0.317), and sampling site (p value = 0.522) on the accumulation of this radionuclide by fungi indicated that Th-234 uptake is possibly independent of all three factors.
Ra-226
The spectrum of Ra-226 activity concentrations was between 0.3 ± 0.1 and 1.0 ± 0.5 Bq kg−1 F.W. (Table 4). The percentage of samples found in the range of the LLD of 0.3 Bq kg−1 F.W. was almost 78 % (25 samples, Table 4). The concentrations for Ra-226 previously assessed in Greece were from 0.11 to 4.51 Bq kg−1 F.W. (Mantzourani Α, Florou Ε, Kapsanaki-Gotsi Ε, Gonou-Zagou Ζ 2001), which are comparable with the present values. Very few published scientific papers report Ra-226 activity concentrations. One study from Serbia (Rakić et al. 2014) presented values from 3 to 39 Bq kg−1 D.W. which are comparable with the present ones in terms of D.W. (4.1 to 43 Bq kg−1). Results from Spain reported slightly higher activity concentrations for Ra-226 in 35 fungal species and were from <LLD to 87 Bq kg−1 D.W. (Baeza et al. 2004). The same applies for results from Brazil that presented Ra-226 values from 14 to 66 Bq kg−1 D.W. (Castro et al. 2012).
The lowest activity concentration for Ra-226 was detected in the mycorrhizal Lactarius salmonicolor, while the highest in the mycorrhizal H. concrescens (Table 4), both collected in Diakopi. The CRs for Ra-226 ranged from 0.0070 for Russula delica and Ramaria formosa (Agrafa) to 0.0350 for H. concrescens (Diakopi) (Table 6). Previous reports from Greece showed CRs from 0.0001 to 0.0961 (Mantzourani Α, Florou Ε, Kapsanaki-Gotsi Ε, Gonou-Zagou Ζ 2001) which are comparable with ours. As H. concrescens showed both the highest activity concentration and the highest CR for Ra-226, it could be considered as bioindicator for Ra-226 in this habitat.
The applied Kruskal–Wallis H test results (Table 7) in order to assess the effect of functional group (p value = 0.718), family (p value = 0.826), and sampling site (p value = 0.667) on the accumulation of this radionuclide by fungi indicated that Th-234 uptake is possibly independent of all three factors.
K-40
The values for K-40 ranged from 56.4 ± 3.0 to 759.0 ± 28.3 Bq kg−1 F.W. (Table 4). These values in terms of D.W. (213.8–10,502.0 Bq kg−1) are significantly higher when compared with results from Brazil that reported values from 462 to 1735 Bq kg−1 D.W. (Castro et al. 2012), from Spain that ranged from 493 ± 27 to 2670 ± 24 Bq kg−1 D.W. (Baeza et al. 2004), and from Serbia that ranged from 29 to 3020 Bq kg−1 (Rakić et al. 2014).
The lowest value for K-40 activity concentrations was found in the saprotrophic Macrolepiota mastoidea collected in Diakopi, while the highest in the mycorrhizal S. martioflavus collected in Agrafa (Fig. 1 and Table 4). The activity concentration of the soil sample from Agrafa was not the highest detected (Table 2). This suggests species-specific accumulation.
The CRs for K-40 were higher than those for the other radionuclides reported and ranged from 0.0786 for Armillaria mellea to 1.4960 for S. martioflavus (Table 6). As S. martioflavus showed the highest activity concentration and the highest CR for K-40, it could be considered as a bioindicator for K-40 in this habitat. Results from Turkey showed higher CRs for K-40 than the present study, and the reported values were in the range of 0.67–5.58 (Karadeniz and Yaprak 2011).
The applied Kruskal–Wallis H test results (Table 7) in order to assess the effect of functional group (p value = 0.738) and sampling site (p value = 0.237) on the accumulation of this radionuclide by fungi indicated that K-40 uptake is possibly independent of these two factors. Furthermore, the activity concentrations for K-40 also appeared to be fairly constant among the different fungal families without statistically significant differences as Kruskal–Wallis H test showed (p value = 0.462; Table 7). Similar constancy has been reported by other authors (Battiston et al. 1989; Mantzourani Α, Florou Ε, Kapsanaki-Gotsi Ε, Gonou-Zagou Ζ 2001; Yoshida and Muramatsu 1994).
Conclusions
In terms of the artificial Cs-137, (a) Ramaria formosa collected in Agrafa presented the highest value (87.2 ± 0.4 Bq kg−1 F.W.), even though its substratum soil did not present the respective highest one; (b) subsequently, Ramaria formosa also presented the highest CR (0.2780), with the lowest one observed in Lycoperdon perlatum (0.0005).
Concerning the natural radionuclides, a selective bioaccumulation of Th-234 was observed, instead of its parent U-238 in fungi. Among the 18 species considered, S. martioflavus presented the highest value of activity concentration (28.3 ± 25.5 Bq kg−1 F.W.) and CR (0.9625) for Th-234. The lowest CR value was found in Boletus sp. (0.0434). For Ra-226, H. concrescens presented a CR over an order of magnitude higher than that of the other species. For K-40, the highest CR was found in S. martioflavus (1.4960).
As general conclusions, it can be noted that (a) mycorrhizal fungi were better indicators for the Cs-137 impact assessment than saprotrophic and parasitic ones, and (b) a relation as CRsΚ-40 > CRsTh-234 > CRsCs-137 > CRsRa-226 was observed for all the radionuclides reported, whereas in terms of species-specific bioaccumulation, a rank of eight fungal species as S. martioflavus > Ramaria formosa and H. concrescens > C. truncatus, Macrolepiota mastoidea, Melanoleuca cognata, Russula delica and Lactarius salmonicolor resulted from the cluster analysis.
References
Amundsen I, Gulden G, Strand P (1996) Accumulation and long term behaviour of Cs-137 in Norwegian fungi. Sci Total Environ 184:163–171
Baeza A, Guillén J, Mietelski JW (2004) Uptake of alpha and beta emitters by mushrooms collected and cultured in Spain. J Radioanal Nucl Chem 261:375–380
Barnett CL, Beresford NA, Self PL, Howard BJ, Frankland JC, Fulker MJ, Dodd BA, Marriott JVR (1999) Cs-137 activity concentrations in the fruit-bodies of macrofungi in Great Britain and an assessment of dietary intake habits. Sci Total Environ 231:67–83
Battiston GA, Degetto S, Gerbasi R, Sbrignadello G (1989) Radioactivity in mushrooms in northeast Italy following the Chernobyl accident. J Environ Radioact 9:53–60
Bazala MA, Golda K, Bystrzejewska-Piotrowska G (2008) Transport of Cs-137 in mycelium and its translocation to fruitbodies of a saprophytic macromycete. J Environ Radioact 99:1200–1202
Brunner I, Frey B, Riesen TK (1996) Influence of ectomycorrhization andcesium / potassium ratio on uptake and localization of cesium in Norway spruce seedlings. Tree Physiol 16:705–711
Bystrzejewska-Piotrowska G, Bazala MA (2008) A study of mechanisms responsible for incorporation of cesium and Cs-137 into fruitbodies of king oyster mushroom (Pleurotus eryngii). J Environ Radioact 99:1185–1191
Castro LP, Maihara VA, Silva PSC, Figueira RCL (2012) Artificial and natural radioactivity in edible mushrooms from Sao Paulo Brazil. J Environ Radioact 113:150–154
Celik N, Cevik U, Celik A, Koz B (2009) Natural and artificial radioactivity measurements in eastern black Sea region of turkey. J Hazard Mater 162:146–153
Delivorias P, Dimitriadis I, Gonou-Zagou Ζ, Kapsanaki-Gotsi E (2010) Some interesting species of Hymenoscyphus from Greece. Mycol Balc 7:87–92
Dighton J, Tugay T, Zhdanova N (2008) Fungi and ionizing radiation from radionuclides. FEMS Microbiol Lett 281:109–120
Duff MC, Ramsey ML (2008) Accumulation of Cs-137 by mushrooms in the environment: a literature review. J Environ Radioact 99:912–932
Dugalic G, Krstic D, Jelic M, Nikezic D, Milenkovic B, Pucarevic M, Zeremski-Skoric T (2010) Heavy metals, organics and radioactivity in soil of western Serbia. J Hazard Mater 177:697–702
Fraiture A (1992) Introduction to the radioecology of forest ecosystems and survey of radioactive contamination in food products from forests. Report for the Commission of the European Communities, Directorate-General environment, civil protection and nuclear safety, Belgium, pp. 116
Fraiture A, Guillitte O, Lambinon J (1990) Interest of fungi as bioindicators of the radiocontamination in forest ecosystems. In: Desmet G, Nassimbeni P, Belli M (eds) Proc. CEC workshop on the transfer of radionuclides in natural and semi-natural environments. Elsevier Applied Science, London, pp 477–484
Gaso MI, Segovia N, Morton O, Cervantes ML, Godinez L, Peña P, Acosta E (2000) 137Cs and relationships with major and trace elements in edible mushrooms from México. Sci Total Environ 262(1-2):73–89
Gillet AG, Crout NMJ (2000) A review of 137 Cs transfer to fungi and consequences for modeling environmental transfer. J Environ Radioact 48(1):95–121
Gonou-Zagou Z, Delivorias P (2011) The genus resupinatus in Greece. Nova Hedwig 92:513–522
Gonou-Zagou Z, Kapsanaki-Gotsi E, Florou E, Kioupi V (2006) Cs-137 concentrations in Basidiomycetes collected in forest ecosystems of Greece. In: Velchev V, Peev D (eds) Book of abstracts IV Balkan botanical congress on plant, fungal and habitats diversity investigation and conservation. Bulgarian Academy of Sciences, Sofia, Bulgaria, p 248
Heinrich G (1993) Distribution of Cs-137 in the different parts of mushrooms. J Environ Radioact 18:229–245
IAEA (1989) Measurement of radionuclides in food and in the environment, Technical report series No 295. IAEA, Vienna
IAEA (2005) Report on the proficiency test of the analytical laboratories for the measurement of environmental radioactivity (ALMERA) Network. IAEA/AL/152, Sebeisdorf, Austria
IAEA (2010) Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater environments, Technical report series No 472. IAEA, Vienna
IAEA (2014) Handbook of parameter values for the prediction of radionuclide transfer to wildlife, Technical report series No 479. IAEA, Vienna
IAES (1970) Reference methods for marine radioactivity studies, Technical report series No. 118, IAEA, Vienna
Kammerer L, Hiersche L, Wirth E (1994) Uptake of Cs-137 by different species of mushrooms. J Environ Radioact 23:135–150
Karadeniz O, Yaprak G (2007) Dynamic equilibrium of Cs-137 with stable cesium within the soil - mushroom system in Turkish pine forest. Environ Pollut 148:316–324
Karadeniz, Yaprak (2011) Soil-to-mushroom transfer of 137Cs, 40K, alkali–alkaline earth element and heavy metal in forest sites of Izmir, Turkey. J Radioanal Nucl Chem 288:261–270
Kioupi V (2004) Bioaccumulation of artificial and natural gamma-emitting radionuclides in macromycetes of Greece. Undergraduate dissertation. National and Kapodistrian University of Athens, Faculty of Biology, Department of Ecology and Systematics, Athens. (In Greek)
Kioupi V, Florou Ε, Gonou-Zagou Ζ, Kapsanaki-Gotsi Ε (2004) Bioaccumulation of 137Cs in the fruitbodies of basidiomycetes of Greece, in: Margaritis, L.C., (Ed.) Proc. 26th Scientific Conference of the Hellenic Society for Biological Sciences, Volos, Greece, pp. 136-137
Kioupi V, Florou E, Gonou-Zagou Z, Delivorias P, Kapsanaki–Gotsi E (2011) Bioaccumulation of the artificial radionuclide 137Cs in Basidiomycota in Greece. In: Diamandis S, Topalidou E (eds) Book of abstracts, XVI congress of european mycologists. Halkidiki, Greece, pp 99–100
Kritidis P, Florou H (2001) Radiological impact in Greece of the Chernobyl accident- a 10-y retrospective synopsis. Health Phys 80(5):440–446
Kuwahara C, Fukumoto A, Ohsone A, Furuya N, Shibata H, Sugiyama H, Kato F (2005) Accumulation of Cs-137 in wild mushrooms collected from a Japanese forest and cesium uptake by microorganisms isolated from the mushroom-growing soils. Sci Total Environ 345:165–173
Mantzourani Α (2002) Bioaccumulation of gamma–emitting radionuclides in Macromycetes of Greece. Undergraduate dissertation. National and Kapodistrian University of Athens, Faculty of Biology, Department of Ecology and Systematics, Athens. (In Greek)
Mantzourani Α, Florou Ε, Kapsanaki-Gotsi Ε, Gonou-Zagou Ζ (2001) Macromycetes as bioindicators of radioactive soil pollution, in: F., E. (Ed.) Proc. 1st national conference on environmental radioactivity, NCSR “Demokritos”, Athens, Greece, 23-24 November 2001, pp. 171-181
Mietelski JW, Dubchak S, Blazej S, Anielska T, Turnau K (2010) 137Cs and 40K in fruiting bodies of different fungal species collected in a single forest in Southern Poland. J Environ Radioact 101:706–711
Mylogiannaki Ν (2004) Bioaccumulation of gamma-emitting radionuclides in Macromycetes of Greece. Undergraduate dissertation. National and Kapodistrian University of Athens, Faculty of Biology, Department of Ecology and Systematics, Athens. (In Greek)
Myttenaere C, Schell WR, Thiry Y, Sombre L, Ronneau C, van der Stegen de Schriek J (1993) Modelling of Cs-137 cycling in forests: recent developments and research needed. Sci Total Environ 136:77–91
Nimis Pl, Melin J, Guillette O, Wirth E, Palo T, Bergman R, Wickman G (1992) Cycling of cesium and strontium in natural ecosystems
Rakić M, Karaman M, Forkapić S, Hansman J, Kebert M, Bikit K, Mrdja D (2014) Radionuclides in some edible and medicinal macrofungal species from Tara Mountain, Serbia. Environ Sci Pollut Res 21:11283–11292
Rosen K, Weiliang Z, Martensson A (2005) Arbuscular mycorrhizal fungi mediated uptake of 137Cs in leek and ryegrass. Sci Total Environ 338:283–290
Samad OE, Baydoun R, Nsouli B, Darwish T (2013) Determination of natural and artificial radioactivity in soil at North Lebanon province. J Environ Radioact 125:36–39
Santawamaitre T, Malain D, Al-Sulaiti HA, Bradley DA, Matthews MC, Regan PH (2014) Determination of 238U, 232Th and 40K activity concentrations in riverbank soil along the Chao Phraya river basin in Thailand. J Environ Radioact 138:80–86
Singh J, Singh H, Singh S, Bajwa BS, Sonkawade RG (2009) Comparative study of natural radioactivity levels in soil samples from the upper siwaliks and Punjab, India using gamma-ray spectrometry. J Environ Radioact 100:94–98
Solecki J, Reszka M, Chibowski S (2003) 90Sr and 137Cs radioisotopes and heavy metal concentrations in pharmaceutical herbal plants from the Lublin (Poland) region. J Radioanal Nucl Chem 257(2):261–265
Steiner M, Linkov I, Yoshida S (2002) The role of fungi in the transfer and cycling of radionuclides in forest ecosystems. J Environ Radioact 58:217–241
Strachnov V, LaRosa J, Dekner R, Zeisler R, Fajgelj A (1996) Report on the interocomparison run IAEA-375: radionuclides in soil. IAEA/AL/075, IAEA, Vienna, Austria
Tsopelas P, Slippers B, Gonou-Zagou Z, Wingfield MJ (2009) First report of Diplodia corticola in Greece on kermes oak (Quercus coccifera). Plant Pathol 59(4):805
Yoshida S, Muramatsu Y, Ogawa M (1994) Cs-137 concentrations in mushrooms collected in Japan. J Environ Radioact 22:141–154
Yoshida S, Muramatsu Y (1994) Accumulation of Cs-137 in basidiomycetes collected from Japanese forests. Sci Total Environ 157:197–205
Web references
Greece: Total Caesium Deposition (1996) Available at: ipta.demokritos.gr/erl/info_ces.html (Accessed: 27 November 2014)
PASW Statistics (2009) Available at: www.spss.com.hk/statistics/ (Accessed: 27 November 2014)
Soil Texture Map, European Commission (2001) Available at: http://eusoils.jrc.ec.europa.eu/ (Accessed: 29 March 2015)
Acknowledgments
The authors wish to thank Dr. Panos Delivorias (research associate at the Mycology Laboratory of the University of Athens) for his invaluable help in the sampling and identification of the fungal samples and the personnel of the Environmental Radioactivity Laboratory for sharing with us their experience, materials, and expertise on the gamma spectrometry system measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kioupi, V., Florou, H., Kapsanaki-Gotsi, E. et al. Bioaccumulation of the artificial Cs-137 and the natural radionuclides Th-234, Ra-226, and K-40 in the fruit bodies of Basidiomycetes in Greece. Environ Sci Pollut Res 23, 613–624 (2016). https://doi.org/10.1007/s11356-015-5298-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5298-5