Abstract
Irrigation with wastewater is a promising option to improve crop yields and to reduce pressure on freshwater sources. However, heavy metal concentrations in wastewater may cause health concerns. A greenhouse pot experiment was conducted in order to determine cadmium (Cd) and zinc (Zn) concentrations in sandy soil and plant tissues of tomato (Lycopersicon esculentum L.) and alfalfa (Medicago sativa L.). A 2 × 2 × 4 × 2 factorial treatment arrangement was utilized. Two water sources, fresh (FW) or treated wastewater (TWW), at two salinity levels (1 and 3 dS m−1) containing different levels of Cd and Zn were used. Samples were collected after a 90-day growth period. It was observed that the growth of both plants was depressed at the highest metal level (L3). Metal accumulation in plant parts increased with the increase of metal concentration and salinity in irrigation water. At low salinity, water source was the main factor which controlled metal accumulation, whereas, at high salinity, chloride appeared to be the principal factor controlling metal uptake regardless of water source. Metal translocation from roots to shoots increased in TWW-irrigated plants, even in the controls. Tomatoes accumulated Cd up to and above critical levels safe for human consumption, even though Cd concentration in irrigation water did not exceed the current recommended values. Therefore, food production in sandy soils may well pose a health hazard when irrigated with TWW containing heavy metals. Complexation with dissolved organic compounds (DOC) in TWW may be to be the principal factor responsible for increased metal uptake and transfer at low salinity, thereby increasing the risk of heavy metal contamination of food and forage crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While the availability of irrigation water in arid and semi-arid regions is the major limiting factor of agricultural production, the quality of the available water is also important. Due to water scarcity which is escalating in the Mediterranean countries (Aiello et al. 2007; Bakopoulou et al. 2011; Kouraa et al. 2002; Oron et al. 2001; Pedrero and Alarcón 2009), the use of alternative irrigation sources such as treated wastewater (TWW) has become a common practice. Alternative water sources contribute to alleviating the pressure on freshwater resources (Lado and Ben-Hur 2009). TWW contains nutrients and organic carbon that are beneficial to plant growth; nevertheless, there is an increased risk of soil salinization with TWW use (Feigin et al. 1991; Gharaibeh et al. 2007). In addition, TWW may also contain low concentrations of heavy metals which, after long-term application, may accumulate in the soil and crops posing environmental quality risks and health hazards to humans and animals (Datta et al. 2000; Nayek et al. 2010).
Metal levels in Jordanian treated wastewaters were reported to be lower than the Jordanian standards (JISM 2006; Ammary 2007). However, due to high water requirements, there is a potential increase in metal availability and uptake by crops which may exceed health or fodder standards. Therefore, there is a need to evaluate the effect of long-term irrigation with TWW on the accumulation of heavy metals in soil and their impact on plant uptake.
Salinity of soil and irrigation water can affect the transfer of heavy metals into the food chain. Chloride salinity is reported to reduce Cd sorption in soils by forming relatively strong soluble Cd complexes (Boekhold et al. 1993; O’Connor et al. 1984), thus increase Cd uptake in plants (McLaughlin et al. 1994; Weggler et al. 2004). Moreover, Chaney and Oliver (1996) reported that inorganic forms of Cd have higher phytoavailability than organic forms. Therefore, saline irrigation water containing elevated metal concentrations could increase the mobility of these metals and thus aggravate metal pollution problems. Furthermore, the solubility and bioavailability of metals may also be controlled by complexation reactions with DOM which would be likely to be present in elevated concentrations in TWW.
Cadmium (Cd) and zinc (Zn) are commonly present in wastewater and therefore in TWW-irrigated soils. Cadmium is highly mobile, non-essential, toxic metal and is readily absorbed by plant roots and translocated to above-ground parts. On the other hand, Zn is an essential trace element for plants and animals; however, it is phytotoxic when present at high concentrations (Kabata-Pendias and Mukherjee 2007; Sarwar et al. 2010). Das et al. (1997) and Cherif et al. (2011) reported that cadmium and Zn interactions can affect their transport and accumulation in edible plant parts. The main objective of this study was to investigate Cd and Zn uptake in tomato and alfalfa plants (representing the most consumed vegetable and fodder crop in Middle east) under the influence of two irrigation water sources: freshwater (FW) and TWW; two salinity levels corresponding to relative crop tolerance (low salinity (1 dS m−1) and high salinity (3 dS m−1)) amended with four Cd and Zn doses to simulate increased metal concentrations ranging from the recommended maximum concentrations of trace elements suggested by FAO (1992) to possible higher levels that could contaminate irrigation water.
Materials and methods
Collection and characterization of original soil and irrigation water
Soil material was collected from a sand-crushing facility near Karak City, Jordan (31° 22′ 28″ N, 35° 40′ 36″ E). Sandy soils are common in upland Jordanian agriculture, and their utilization for pot trials can be considered a worst-case scenario due to their low metal sorption capacity (Strawn et al. 2015). Soil physicochemical analysis was performed according to the standard methods described in Klute (1986) and Page et al. (1982).
Two irrigation water sources were used in this experiment: FW and TWW. FW was obtained from a groundwater well located at Jordan University of Science and Technology (JUST) campus, while TWW was obtained from Wadi Hassan wastewater treatment facility. All cations and metals were analyzed using inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Ciros, Spectro Analytical Instruments GmbH, Kleve, Germany), anions using ion chromatography (ProfIC, Metrohm AG, Herisau, Switzerland), and electrical conductivity (EC) and pH using EC and pH meter (InoLab 730, WTW GmbH, Weilheim, Germany). Chemical properties of both water sources are shown in Table 1.
Experimental treatment design
The multi-factorial pot experiment was conducted in the agricultural station greenhouse at JUST, Al-Ramtha, Irbid, Jordan (32° 28′ 44″ N, 35° 59′ 6″ E). A total of 32 treatments were tested, which resulted from a 2 × 2 × 4 × 2 factorial arrangement design. Factor A was the irrigation water source with two levels: FW and TWW. Factor B was the water salinity with two levels: 1 and 3 dS m−1. Factor C was Cd–Zn concentration with four levels: 0/0 mg L−1 (control (C)), 0.01/2 mg L−1 (L1), 0.16/32 mg L−1 (L2), and 2.56/512 mg L−1 (L3). Factor D was the plant species with two types: tomato and alfalfa. Each treatment had three replicates and, as a consequence, a total of 96 pots were utilized. The pots containing 2.5 kg of soil were arranged in a randomized complete block design. Two sub-blocks were assigned for each salinity level and water source. For alfalfa, 10–12 seeds were sown in each pot and thinned later to two plants, while tomato plants were grown from 5-cm seedlings for 3 months until maturity.
Salinity levels were chosen according to the guidelines of relative crop tolerance and yield potential adapted by Maas (1987). For both crops, full yield potential (no restriction on use) is obtained when irrigation water salinity does not exceed 1.7 and 1.3 dS m−1 for tomato and alfalfa, respectively. Increasing the salinity of irrigation water to 3.5 dS m−1 will result in a 25 % yield reduction of both plants.
For each water source, the two salinity levels (1 or 3 dS m−1) were obtained either by adding calcium chloride hexahydrate (CaCl2·6H2O; reagent grade, Sigma-Aldrich) or by diluting with FW to obtain the desired level.
Moreover, each water source (FW and TWW) was spiked with four levels of Cd/Zn concentrations: 0/0 mg L−1 (C), 0.01/2 mg L−1 (L1), 0.16/32 mg L−1 (L2), and 2.56/512 mg L−1 (L3). Metal concentration in L1 corresponds to the recommended maximum concentrations of trace elements in irrigation water suggested by FAO (1992). Preliminary experiments had shown that tomato yield was decreased only when Zn concentrations exceeded 250 mg L−1. In order to achieve this as an extreme treatment, L3 was adjusted to a Zn concentration of 512 mg L−1.
Irrigation was performed twice a week when soil surface was very dry. Prior to irrigation, water was spiked with the required volume of Cd–Zn solutions to obtain the desired level. Plants irrigated with FW were supplemented with 0.5 g L−1 NP (46:6) fertilizer (locally manufactured) to keep up with the increased demand for nutrients. The amount of applied water was determined from the weight loss of five representative pots from each crop. The temperature in the greenhouse was held at approximately 25 °C and 35 % relative humidity. The total amount of irrigation water supplied to tomato and alfalfa was 6.3 and 5.1 L pot−1, respectively.
Plant and soil analysis
Plants were harvested after 90 days and separated into fruits (for tomato), shoots, and roots. Tomato fruits were hand harvested at vine-ripe stage and oven-dried for 4 days at 70 °C. All other plant parts were oven-dried for 2 days at 70 °C. After drying, plant parts were finely ground to <0.25 mm using a rotating plant mill (ZM 100, Retsch, Titanium ring sieve) and then stored for metal analysis.
After roots were taken out, soils were air dried, sieved through a 2-mm sieve, and then oven-dried for 24 h at 105 °C. The oven-dried soil was ground to <0.25 mm using an agate ball mill (Fritsch pulverisette 7 premium) for 2 min at 25 Hz in zirconium oxide grinding jars.
Sample grinding was done separately and in ascending order of concentration to avoid cross-contamination. The finely ground materials (250 mg) were digested overnight by adding 10 mL of concentrated nitric acid. On the following day, the tubes were digested at 180 °C using microwave pressure method (Mars Xpress, CEM GmbH, Kamp-Lintfort, Germany). The digestion program involved four successive steps. Initially, the temperature was increased linearly from 25 to 90 °C over 4 min, followed by digestion at 90 °C for 2 min. After that, the temperature was increased linearly to 180 °C over 6 min, and in the last step, the temperature was held at 180 °C for 10 min. Samples were then filtered through acid-washed filter paper no. 640 (Sartorius AG, Göttingen, Germany) and transferred into 50-mL polyethylene bottles for metal analysis (Chander et al. 2008). Total Cd and Zn concentrations of soil and plant materials were analyzed using ICP-AES (Ciros, Spectro Analytical Instruments GmbH, Kleve, Germany). Each sample was measured twice, and the precision and accuracy of the inductively coupled plasma mass spectrometry (ICP-MS) measurements were proven by analyzing standard reference material SPS–SW2 (Spectrapure Standards AS) each in dilution 1:5 (v/v) and comparison to the certified values. Typical precision of duplicate measurement of ICP-MS was within 2 %.
Statistical analysis
The data presented in the tables and figures are arithmetic means of three replicates and expressed on an oven-dried basis for soil and plant samples. The normal distribution of the data sets was tested using the Shapiro-Wilk test. All data sets were log-transformed to fit a normal distribution. After log transformation, the data sets showed almost normal distribution. Analysis of variance (ANOVA) was carried out to test significant effects of treatments on the measured parameters using water source, salinity, and metal level as single factors. Further, the effect of interactions between the single factors on the measured parameters was tested. Within the treatments, the effects of the heavy metal level were identified by a Tukey post hoc test with p < 0.05. The effects of treatments were tested for plant species and plant parts in a separate ANOVA because of significant differences between single data sets. All statistical calculations were performed in IBM SPSS Statistics 19.
Results
Properties of original soil and irrigation water used in the experiment
Soil texture as determined by the pipette method was 98 % sand and <1 % clay, and soil pH and EC were 8.7 and 1.3 dS m−1, respectively. The cation exchange capacity (CEC) was low (4.5 cmolc kg−1), and soil metal concentrations in milligrams per kilogram were 0.2 (Cd), 2.5 (Zn), 1.15 (Cu), 2.71 (Pb), and 3.65 (Cr).
The freshwater had low salinity (0.6 dS m−1) and near-neutral pH of 7.4, whereas the treated wastewater had moderately saline value (1.5 dS m−1) and slightly alkaline pH (7.8). Metal concentrations in both water sources (Table 1) were below the safety guidelines for agricultural purposes (FAO 1992; EPA 2004). The maximum allowable Cd and Zn concentration for short- and long-term use in TWW effluents is 0.05 and 0.01 mg L−1 and 10 and 2 mg L−1, respectively (FAO 1992; EPA 2004).
Cadmium and Zn in soils after harvest
All treated soils receiving metal level <L3 accumulated less than 1 mg kg−1 Cd and below 200 mg kg−1 Zn, respectively (Table 2). Metal level, crop type, and their interaction significantly affected soil metal concentrations (p < 0.05), while the irrigation water source or salinity level had no significant effects on soil metal levels (Table 2).
Biomass yields
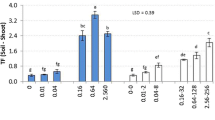
The effects of treatments on shoot dry weights of alfalfa and tomato at the end of the experiment are shown in Fig. 1. At both salinity levels, plants irrigated with FW produced significantly more biomass as compared to those irrigated with TWW. On the other hand, high salinity levels reduced the dry weights of tomato plants only when FW was used. The yield was mainly influenced by metal levels, where the highest level severely depressed the dry weights of both plants. Interestingly, the interactions between salinity and metal concentrations reduced alfalfa yields only with TWW irrigation, while tomato yields showed no significant response. The dry weights of tomato fruits ranged from 0.94 to 7.97 g and showed 35 % yield reduction with highest heavy metal level compared to other treatments.
Effects of water source (FW and TWW), salinity (dS m−1), and metal level of the irrigation water on the shoot dry weights (g per pot) of alfalfa and tomato at the end of the experiment. Capital letters show significant differences between salinity level, and little letters show significant differences between heavy metal level (p < 0.05) within the respective salinity level. Error bars show standard error with n = 3
Metal concentration in plants
The control treatments showed that Zn uptake in both plant shoots was significantly higher in TWW-irrigated plants than in FW-irrigated treatments (Fig. 2a). This was most pronounced for tomato, where Zn concentrations were higher than in alfalfa, and roughly doubled in TWW treatments. Salinity had no effect on alfalfa shoot Zn concentrations and had inconsistent effects on tomato shoot. Furthermore, Zn levels in FW-irrigated plants were significantly higher at higher salinity, while the opposite was the case in TWW-irrigated plants.
Mean concentration of Zn in alfalfa and tomato roots (a) and shoots (b) in control soils. Capital letters show significant differences between plants, and little stars show significant differences (p < 0.05) between water quality within the respective plant over both salinity levels. Error bars show standard error with n = 3
Zn in roots showed a similar distribution among the treatments and plants (Fig. 2b). In alfalfa, Zn concentrations in roots and shoots were similar, with slightly higher values under FW irrigation. For tomato, Zn concentration in roots and shoots was similar in FW-irrigated plants, while shoot/root ratios were much higher in TWW-irrigated plants.
As shown in Tables 3 and 4, metal shoot concentrations in both crops increased significantly with increasing metal levels with both irrigation water sources. However, the increase was not proportional to metal concentration in irrigation water. Cd and Zn levels were higher at the higher salinity level with both water sources compared to those irrigated with lower salinity level. As salinity increased from 1 to 3 dS m−1, Cd levels in L1 treatment increased significantly with both water sources.
For alfalfa, Cd and Zn in shoots were influenced by all factors significantly. However, the concentrations in roots were slightly influenced by metal levels in irrigation water (Table 3). Tomato plants showed generally higher Cd and Zn concentrations than alfalfa plants. The shoot Cd metal concentration was influenced by all main factors significantly, while shoot Zn concentration was affected by metal level. The roots showed stronger response to the main factors for both metals.
Moreover, the relationship between soil and shoot metal concentration were often linear (Figs. 3 and 4) but showed clear effects of irrigation water source and salinity. At the lower salinity level, metal uptake was higher in TWW than in FW-irrigated crops (except for Zn in tomato shoots). At the higher salinity level, plant metal uptake relative to soil concentration was generally much higher than at lower salinity but independent of water source.
Metal concentration in alfalfa and tomato roots
Similar to shoot concentration, metals in roots increased with metal level in the irrigation water (Tables 3 and 4). In contrast to the shoot values, the relative increases were less pronounced.
For the illustration of water quality effects on metal root concentrations, the mean concentration of Cd and Zn in the roots of both crops treated with metal level 1 is shown in Fig. 5. Clearly, at low salinity, TWW compared to FW greatly enhanced Zn and Cd uptake of both crops. At higher salinity, metal root concentrations in TWW and FW were similar in both plants but higher than at the lower salinity level. In addition, metal root concentrations increased considerably with increasing salinity in FW compared to that in TWW. The same trend was observed with the other metal levels. In all treatments, Cd and Zn accumulation in tomato roots was, on average, twice that of alfalfa roots. For alfalfa roots, Cd and Zn concentrations were mainly controlled by metal level (Table 4).
Mean concentration of Cd (a) and Zn (b) in roots of both crops grown in soils irrigated with freshwater (FW) and treated wastewater (TWW) of low or high salinity and spiked with metal level 1. Capital letters show significant differences between salinity level, and little stars show significant differences (p < 0.05) between water quality within each plant group. Error bars show standard error with n = 3
Metal concentration in tomato fruits
Metal concentrations in the tomato fruits also increased with metal level in irrigation water (Table 5), but differences were much lower than in shoots or roots. Tomato fruits from treatments with elevated metal level (L2) and fruits from the TWW 3 dS m−1 also contained Cd concentrations of 0.8 to 29 mg kg−1 dry weight (DW). Zinc levels in tomato fruits increased at elevated salinity, i.e., from 30.8 to 44.5 mg kg−1 in FW treatments and from 41.0 to 49.5 mg kg−1 in TWW, respectively. The ANOVA showed that metal level was the most important factor controlling metal concentrations in tomatoes (Table 5).
Discussion
Soil metal concentrations
Soil metal concentrations were clearly elevated in response to metal additions in irrigation water. Except for soils receiving the highest metal dose, metal levels in all other soils fall within FAO/WHO (1999) limits for unpolluted soils (0.1–1.0 mg Cd kg−1 and 200 mg Zn kg−1) and European maximum permissible limit (MPL) for Cd (5 mg kg−1) and Zn (300 mg kg−1) (FAO/WHO 2001; Kabata-Pendias and Mukherjee 2007). Apparently, metal uptake/translocation by plant parts at lower metal levels occurred at higher magnitude, whereas the translocation mechanism could be broken down at the highest metal level which would result in metal buildup exceeding the adsorptive capacity of sandy soils (Strawn et al. 2015).
Metal and salinity effects on biomass yields
The observed depressions of alfalfa and tomato shoot biomass at the highest metal level can largely be attributed to Zn which is well known to cause plant growth reductions (Chaney 1993). Phytotoxicity of Cd has only been observed above 10 mg kg−1 soil Cd concentrations which is consistent with the findings reported by Mohammad et al. (2009). The more pronounced yield reductions in tomato than in alfalfa at the higher salinity level in FW correspond to the higher salt tolerance of alfalfa (FAO 1992; Maas and Grattan 1999). However, the much lower shoot yields of both crops, especially tomato under TWW irrigation, may be due to a lower nutrient availability in TWW compared to the fertilizer-supplemented FW.
Metal uptake by plants
Metal concentrations in plant parts increased with metal dose. However, at lower dosages, the uptake did not increase proportionally to metal concentrations in the irrigation water, while at high dosage, the relative increase often overrides that of metals supplied with the irrigation water. This could be related to the presence of free sorption sites and ligands at the low metal loading which reduces metal availability. At high metal loading, these sites and ligands are saturated and this is commonly observed in sorption isotherms (Degryse et al. 2006). Moreover, at low metal loading, roots have effective protection mechanisms that reduce metal uptake (Marschner 1995). This may be supported by the presence of certain cations, such as Fe or Mg (Marschner 1995). At high metal concentrations, these protective mechanisms can break down due to direct toxic effects or due to insufficient concentrations of competing cations (e.g., Fe or Mg). In addition, due to the severe growth depressions at the highest metal dosage (L3), metals are more concentrated in the lower biomass.
At high salinity, metal uptake by both plants was higher and independent of irrigation water source. The most likely mechanism for enhanced metal uptake is the formation of metal chloro-complexes, which reduce sorption and enhance plant availability. Chlorides in saline irrigation waters were reported to increase Cd availability and uptake by forming soluble and highly phytoavailable species (MeCl+ and MeCl2 o) (McLaughlin et al. 1994; Khoshgoftar et al. 2004; Usman et al. 2005). Smolders et al. (1998) and Smolders and McLaughlin (1996) showed that increased Cd uptake was mainly due to increased Cd diffusion to plant roots by chloro-complexation of free Cd2+ ions. Furthermore, mobilization of metals like Cd and Zn in saline soils can also be due to divalent cations (Ca and Mg) competing for sorption sites and ligands (Paalman et al. 1994).
There is much evidence in the literature that DOM decreases metal sorption onto TWW-irrigated soils. The decrease is attributed to the formation of soluble and insoluble organic complexes (Hesterberg 1998). However, the decrease in metal sorption in the presence of DOM is likely to increase their mobility (Gove et al. 2001; Ashworth and Alloway 2008) yet does not necessarily increase the bioavailability (Bolan et al. 2003). Interestingly, the root–shoot transfer (represented by the root/shoot ratio) of Cd and Zn was also enhanced under TWW irrigation, indicating higher mobility of these organic complexes within the plants. Therefore, future work should focus on possible mechanisms that increase metal availability in TWW soils.
Food and fodder safety aspects
Tomato fruits accumulated Cd above the safe levels for human consumption, even when irrigated with water containing low levels of metals within the recommended limits of irrigation water (L1 = 0.01 mg L−1) but at elevated salinity. Therefore, food production on such sandy soils with low sorption capacity may well pose a health hazard even if irrigation water contains metal concentrations within the permissible limits. This result was unexpected since Cd and Zn uptake from alkaline soils is generally not considered a problem due to the low solubility of these metals at elevated pH (Chaney 2010). Cadmium concentrations are considered unsafe for human consumption if they exceed 0.2 mg kg−1 FW or 1.3 mg kg−1 DW limit (WHO 1993). Moreover, Ewers (1991) and Begerow et al. (2008) reported stricter limits (0.1 mg kg−1 FW or 0.7 mg kg−1 DW) as the MPL for tomato fruits. It is interesting to note that there is no European guideline present on MPL for Zn in food products. However, in China, 20 mg Zn kg−1 FW is MPL for food which is equivalent to 135–170 mg Zn kg−1 DW (MOH 1991).
Tomato shoots in the control and L1 contained Cd levels below the permissible limits, while almost all L2 and L3 treatments exceeded the limits for fodder crops, which may be relevant since greens are often fed to goats or cows, thus posing a risk to animals when this material is a major part of their diet. In the treatments with metal additions, Cd concentrations in alfalfa shoots were well below the FAO/WHO (2001) permissible limits for fodder crops in L1 and L2 (Cd < 1.0 mg kg−1 FW, equivalent to <6.7–8.3 mg kg−1 DW at 85–88 % water content). In the L3 treatments, these values were exceeded, but due to the severe yield reductions, this is of no practical concern. For Zn, no regulatory limits exist; however, the observed Zn concentrations of 200–500 mg kg−1 are of no concern in animal feed (Ewers 1991; Begerow et al. 2008).
Conclusions
Our results showed that irrigation with TWW and elevated salinity can increase metal uptake in tomato and alfalfa plants. Since TWW is generally more saline and richer with DOM than well or surface waters, the mobility and bioavailability of metals by forming soluble complexes may be enhanced. Interestingly, this was observed to also affect the transfer from roots to shoots. The results of Cd accumulation in tomatoes show that food safety may be compromised even if irrigation water Cd concentrations do not exceed current recommended values if irrigation water had high salinity levels. Furthermore, as heavy metals accumulate in soils, problems with Cd transfer into edible plant parts will most likely aggravate with time, as observed with the effect of higher metal dosage in this study.
Further research on the identification of organic complexes responsible for enhanced metal uptake in TWW and their practical implications is needed. This includes identification of organic complexes responsible for elevated metal uptake, metal levels that induce phytotoxicity, antagonistic and competitive effects from salts, increased metal risk with TWW use even at low concentrations, possible reduction of uptake by the presence of other metals, and reduced risk of Zn deficiency in calcareous soils with TWW irrigation.
References
Aiello R, Cirelli GL, Consoli S (2007) Effects of reclaimed wastewater irrigation on soil and tomato fruits: a case study in Sicily (Italy). Agr Water Manage 93:65–72
Ammary BY (2007) Wastewater reuse in Jordan: present status and future plans. Desalination 211:164–176
Ashworth DJ, Alloway BJ (2008) Influence of dissolved organic matter on the solubility of heavy metals in sewage-sludge-amended soils. Commun Soil Sci Plant Anal 39:538–550
Bakopoulou S, Emmanouil C, Kungolos A (2011) Assessment of wastewater effluent quality in Thessaly region, Greece, for determining its irrigation reuse potential. Ecotox Environ Safe 74:188–194
Begerow J, Crößmann G, Ewers U, Finck M (2008) Standards and regulations regarding metals and their compounds in environmental materials, drinking water, food, feeding-stuff, consumer products, and other materials: elements and their compounds in the environment. Wiley-VCH Verlag GmbH, 1498–1524.
Boekhold A, Equine A, Temminghoff JM, Van der Zee SEATM (1993) Influence of electrolyte composition and pH on cadmium sorption by an acid sandy soil. J Soil Sci 44:85–96
Bolan NS, Adriano DC, Mani S, Khan A (2003) Adsorption, complexation, and phytoavailability of copper as influenced by organic manure. Environ Toxicol Chem 22:450–456
Chander K, Hartmann G, Joergensen RG, Khan KS, Lamersdorf N (2008) Comparison of three methods for measuring heavy metals in soils contaminated by different sources. Arch Agron Soil Sci 54:413–422
Chaney RL (1993) Zinc phytotoxicity. In: Robson AD (ed) Zinc in soils and plants. Springer, Netherlands, pp 135–150
Chaney RL (2010) Cadmium and zinc. In: Hooda PS (ed) Trace elements in soils. Wiley, Chippenham, pp 409–439
Chaney RL, Oliver DP (1996) Sources, potential adverse effects and remediation of agricultural soil contaminants. In: Naidu R, Kookana RS, Oliver DP, Rogers S, McLaughlin MJ (eds) Contaminants and the soil environment in the Australasia-Pacific region. Springer, Netherlands, pp 323–359
Cherif J, Mediouni C, Ammar WB, Jemal F (2011) Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solanum lycopersicum). J Environ Sci 23(5):837–844
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98:29–36
Datta SP, Biswas DR, Saharan N, Ghosh SK, Rattan RK (2000) Effect of long-term application of sewage effluents on organic carbon, bioavailable phosphorus, potassium and heavy metals status of soils and content of heavy metals in crops grown thereon. J Indian Soc Soil Sci 48:836–839
Degryse F, Smolders E, Parker DR (2006) Partitioning of metals (Cd, Co, Cu, Ni, Pb, Zn) in soils: concepts, methodologies, prediction and applications—a review. Europ J Soil Sci 60:590–612
EPA (2004) Guidelines for water reuse. EPA/625/R-04/108, Washington, 450 pp.
Ewers U (1991) Standards, guidelines and legislative regulations concerning metals and their compounds. In: Merian E (ed) Metals and their compounds in the environment: occurrence, analysis and biological relevance. Weinheim, VCH, pp 458–468
FAO (1992) Wastewater treatment and use in agriculture. Irrigation and Drainage Paper, # 47, M.B. Pescod, Rome, 125 pp
FAO/WHO (2001) Codex Alimentarius Commission Food Additives and Contaminants. Joint FAO/WHO Food Standards Program, ALINORM 01/12A:1–289
Feigin A, Ravina I, Shalhevet J (1991) Irrigation with treated sewage effluent: management for environmental protection. Springer, Berlin, 224pp
Gharaibeh MA, Eltaif NI, Al-Abdullah B (2007) Impact of field application of treated wastewater on hydraulic properties of vertisols. Water Air Soil Poll 184:347–353
Gove L, Cooke CM, Nicholson FA, Beck AJ (2001) Movement of water and heavy metals (Zn, Cu, Pb and Ni) through sand and sandy loam amended with biosolids under steady-state hydrological conditions. Bioresource Technol 78:171–179
Hesterberg D (1998) Biogeochemical cycles and processes leading to changes in mobility of chemicals in soils. Agric Ecosyst Environ 67:121–133
JISM (2006) Reclaimed domestic wastewater standard. No. 893/2006, Technical Regulation, Jordanian Institution for Standards and Metrology, Jordan.
Joint FAO/WHO Committee on Food Additives (1999) Joint FAO/WHO Expert Committee Report on Food Additives. WHO Technical Report Series, 922 (Rome, Italy)
Kabata-Pendias A, Mukherjee AB (2007) Trace elements of group 12 (previously group IIb): trace elements from soil to human. Springer Berlin , Heidelberg, pp 283–319
Khoshgoftar AH, Shariatmadari H, Karimian N, Kalbasi M, van der Zee SEATM, Parker DR (2004) Salinity and zinc application effects on phytoavailability of cadmium and zinc. Soil Sci Soc Amer J 6:1885–1889
Kouraa A, Fethi F, Fahde A, Lahlou A, Ouazzani N (2002) Reuse of urban wastewater treated by a combined stabilisation pond system in Benslimane (Morocco). Urban Water 4:373–378
Klute A (1986) Methods of soil analysis, part 1: Physical and mineralogical methods (2nd edition). Agronomy Monograph 9, Madison: WI, ASA and SSSA, pp 1188
Lado M, Ben-Hur M (2009) Treated domestic sewage irrigation effects on soil hydraulic properties in arid and semiarid zones: a review. Soil Till Res 106:152–163
Maas EV (1987) Salt tolerance of plants. In: Christie BR (ed) CRC handbook of plant science in agriculture. CRC Press, Boca Raton, pp 57–75
Maas EV, Grattan SR (1999) Crop yields as affected by salinity. In: Skaggs RW, Schilfgaarde, JV (eds) Agricultural drainage. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, pp 55–108
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London, 889pp
McLaughlin MJ, Tiller KG, Beech T, Smart MK (1994) Increased soil salinity causes elevated cadmium concentrations in field-grown potato tubers. J Environ Qual 23:1013–1018
MOH - Ministry of Health of China (1991) Sanitary standard of zinc level for vegetables, GB13106-91.
Mohammad A, Seema MA (2009) The influence of single and multiple soil contamination of cadmium with lead and zinc on growth, chlorophyll contents, uptake and translocation of cadmium in tomato plants. Arch Agron Soil Sci 55(4):407–413
Nayek S, Gupta S, Saha RN (2010) Metal accumulation and its effects in relation to biochemical response of vegetables irrigated with metal contaminated water and wastewater. J Hazard Mater 178:588–595
O’Connor GA, O’Connor C, Cline GR (1984) Sorption of cadmium by calcareous soils: influence of solution composition. Soil Sci Soc Am J 48:1244–1247
Oron G, Armon R, Mandelbaum R, Manor Y, Campos C, Gillerman L, Saigot M, Gerba C, Klein I, Enriquez C (2001) Secondary wastewater disposal for crop irrigation with minimal risks. Water Sci Technol 43:139–146
Paalman MAA, van der Weijden CH, Loch JPG (1994) Sorption of cadmium on suspended matter under estuarine conditions: competition and complexation with major seawater ions. Water Air Soil Pollut 73:49–60
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis, Part 2: Chemical and microbiological properties (2nd edn). Agronomy Monograph 9, Madison: WI, ASA and SSSA, pp 1184
Pedrero F, Alarcón JJ (2009) Effects of treated wastewater irrigation on lemon trees. Desalination 246:631–639
Sarwar N, Saifullah Malhi SS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agr 90:925–937
Smolders E, McLaughlin MJ (1996) Chloride increases cadmium uptake in Swiss chard in a resin-buffered nutrient solution. Soil Sci Soc Am J 60:1443–1447
Smolders E, Lambergts RM, McLaughlin MJ, Tiller KG (1998) Effect of soil solution chloride on cadmium availability to Swiss chard. J Environ Qual 27:426–31
Strawn D, Bohn H, O’Connor G (2015) Soil chemistry, 4th edn. Wiley-Blackwell, UK, 392 pp
Usman ARA, Kuzyakov Y, Stahr K (2005) Effect of immobilizing substances and salinity on heavy metals availability to wheat grown on sewage sludge contaminated soil. Soil Sediment Contam 14:329–344
Weggler K, McLaughlin MJ, Graham RD (2004) Effect of chloride in soil solution on the plant availability of biosolid-borne cadmium. J Environ Qual 33:496–504
WHO (1993) Evaluation of certain food additives and contaminants. 41rd Report of the Joint FAO/WHO Expert Committee on Food Additives, Technical Report Series. WHO: Geneva
Acknowledgments
The authors would like to acknowledge the partial fund from the deanship of research at the Jordan University of Science and Technology and the German Research Foundation (DFG) for financial support of the research visit. The authors acknowledge the support from the Department of Soil Science/Soil Ecology, Institute of Geography, Ruhr-University Bochum, Germany for using lab facilities.
Compliance with ethical standards
The manuscript has not been submitted to more than one journal for simultaneous consideration. The manuscript has not been published previously (partly or in full). This is a single study which is not split up into several parts and has not been submitted to various journals. No data have been fabricated or manipulated (including images) to support our conclusions. No data, text, or theories by others are presented; this is solely single research done by the authors. Consent to submit has been received explicitly from all co-authors before the work was submitted. Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results. No changes of authorship or in the order of authors will be changed after acceptance of a manuscript. If requested, authors are prepared to send relevant documentation or data in order to verify the validity of the results. The authors declare that there is no misconduct that has been established.
Conflict of interest
The authors declare that they have no competing interests
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Gharaibeh, M.A., Marschner, B. & Heinze, S. Metal uptake of tomato and alfalfa plants as affected by water source, salinity, and Cd and Zn levels under greenhouse conditions. Environ Sci Pollut Res 22, 18894–18905 (2015). https://doi.org/10.1007/s11356-015-5077-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5077-3