Abstract
Ambient levels of formaldehyde (HCHO) were measured in the tropical urban mega city of Kolkata from July 2012 to April 2014, based on USEPA Compendium Method TO-11A using 2,4-dinitrophenylhydrazine (2,4-DNPH). The samples were analyzed by isocratic reverse-phase high-performance liquid chromatography (HPLC) with an ultraviolet detector at a wavelength of 360 nm. High values of HCHO were recorded at our site. The highest and average HCHO mixing ratio measured for the entire study period was 803 and 217 ppbv respectively. Seasonal wind regimes have been found to influence the seasonal pattern of HCHO mixing ratio at this site resulting in relatively higher mixing ratio of HCHO during the pre-monsoon periods as compared to the others. Apart from these, sampling-based measurements of CH4 and continuous measurements of ozone (O3) and CO were also performed with the objective to study the interrelationship of HCHO with these species. The results suggest the presence of highly complex chemistry among them.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Formaldehyde (HCHO) is the most abundant aldehyde present in the atmosphere. It is a highly reactive component present in both urban (Feng et al. 2005; Grosjean 1982; Pang and Mu 2006; Possanzini et al. 2002; Ren et al. 2003; Viskari et al. 2000) and rural areas (Christensen et al. 2000; Granby et al. 1997; Solberg et al. 2001). Natural sources of formaldehyde include combustion, biodegradation, photo-dissociation of organic matter and oxidation of naturally occurring hydrocarbons like methane, isoprene and terpenes (Goldan et al. 2000; Miller et al. 2008; Singh et al. 2000). In the methane oxidation cycle, HCHO is produced as an important atmospheric intermediate. In presence of intense sunlight, HCHO may also be produced by the photochemical oxidation of non-methane hydrocarbons and higher aldehydes (Friedfeld et al. 2002; Wuebbles and Hayhoe 2000). Compared to natural sources, anthropogenic sources contribute a major portion to the total HCHO levels of the atmosphere. Of all the anthropogenic sources, automobile emissions make a significant contribution in the urban areas (Alzueta and Glarborg 2003; Mitchell and Olsen 2000; Wuebbles and Hayhoe 2000). There are various other primary sources, such as production of petrochemicals, manufacturing of plastics, paints, varnishes, processing of coal, pressed wood products containing urea-formaldehyde resins, carpenting, cosmetics, nail hardeners, cigarette smoke and sewage treatment plants (Dasgupta et al. 2005; Karar and Gupta 2006; Environmental Protection Agency 2003; World Health Organization 1999) which contribute towards the total HCHO mixing ratio of the atmosphere. Once produced, HCHO is broken down into some free radicals, H2 and CO in presence of sunlight (refer to R6a and R6b in Table 2). Thus in remote troposphere, photolysis is the dominant process for the loss of HCHO during the daytime. In polluted urban areas, HCHO is equivalently destroyed through reactions with OH and HO2. Night time loss of HCHO is mainly by reaction with nitrate radical (refer R7b in Table 2), though the rate of reaction is much slower, as compared to the daytime loss processes (Wuebbles and Hayhoe 2000). HCHO plays a major role in chemistry of the free troposphere by acting as a precursor for ozone as well as HO2 (Finlayson-Pitts and Pitts 1997; Pang et al. 2009). Thus, measurement and quantification of ambient HCHO is necessary for correlating the same with the ozone levels near the surface (Ahammed et al. 2006; Duan et al. 2008). The lifetime of HCHO in the troposphere is of the order of few hours due to several removal pathways following both chemical and physical mechanisms (De More et al. 1992). Thus, the observed daytime mixing ratio mainly depends on its production from precursors and direct emission from nearby sources (De Smedt et al. 2008; Li et al. 2010). HCHO being a highly toxic compound, its presence in the air in high quantity (~100 ppmv or above) can be fatal to living beings. Short-term exposure (3–4 h) to about 300 ppmv of HCHO is known to cause irritation of the eyes, nose and mucous membranes of the upper respiratory tract. Long-term exposure (few days) is associated with skin irritation and respiratory symptoms (U.S. Environmental Protection Agency 1988; U.S. Environmental Protection Agency 1999a). The main objective of this paper is to report the measurement of ambient HCHO in Kolkata, along the Indo-Gangetic Plain (IGP) in the eastern part of India. Kolkata is a densely populated metropolitan city with many small-scale industries spread across the city and large scale industries nearby. The measured values are correlated to other trace species such as ozone, CO and methane. In addition to this, a comparative study between measured surface HCHO and its corresponding columnar counterpart from surrounding areas is also performed to find out any regional contribution of HCHO from various sources other than local chemistry.
Materials and methods
Experimental site
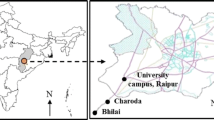
Ambient formaldehyde is measured in an urban location within greater Kolkata (22°33′N, 88°30′E; 6 m above mean sea level [amsl]), India. Kolkata is a metropolitan city situated on the deltaic plain of river Ganga. It is one of the densely populated urban areas in the world (Population: 4.5 million; http://www.censusindia.gov.in/2011-prov-results/prov_data_products_wb.html). The city is highly polluted (Brewer and Belzer 2001; Gupta et al. 2007). Such high levels of pollution is contributed by automobile emissions due to heavy traffic (Mallik et al. 2014) and numerous small-scale industries (agriculture, textiles, pharmaceuticals, mineral processing, food processing, heavy engineering, electronics, etc.) which are present all over the city (Ghose et al. 2004). According to Köppen Climate Classification, Kolkata experiences a tropical wet and dry climate. Summer is hot and humid (RH: ~69 %) and winter is pleasant (RH: ~66 %). Summer rainfall or monsoon is prevalent in this part with a total annual rainfall of 1,600 mm (Ghosh et al. 2013). Moderately blowing winds are experienced in the city with an average speed of 7 km/h throughout the year (Gupta et al. 2007).
The experiments were carried out in the outskirts of the city in Jadavpur University, Salt Lake (hereafter referred to as JUSL) campus, from July 2012 to April 2014. The JUSL campus is situated in a residential locality. A municipal solid waste landfill (Dhapa) is located approximately 2 km to the south and the airport about 10 km to the north-east of the experimental site (Ghosh et al. 2015). The geographical location of the experimental site is given in Fig. 1.
Sampling methodology
Sampling and analysis of HCHO
Carbonyl samples were collected from ambient air using 2,4-dinitrophenylhydrazine (DNPH)-adsorbent cartridges following the USEPA Compendium Method TO-11A (US-EPA 1999). The sampling system consists of a sampling pump (Model: Escort ELF, Make: Zefon International), a DNPH-adsorbent cartridge (Lp DNPH S10L, Make: Supelco) and a KI ozone scrubber (Lp DNPH Ozone Scrubber, Make: Supelco). The Escort ELF pump was calibrated after every 200 h of use. At first, the inlet filters of the pump were checked for dust loading. In case of overloading of dust, the filters were changed. The pump was set at a flow rate of 2.5 L/min and checked for any leaks by temporarily blocking the pump inlet. If the value displayed was <0.02 L/min, it passed the leak proof test. Thereafter, it was connected to a primary calibration standard (Model: Digical Primary Calibrator; Make: Zefon International). The pump was calibrated for different flow rates (0.8, 0.9, 1.0, 1.1, 1.2 L/min) with an accuracy of ±1.5 %.
In case of HCHO, the collection efficiency of a cartridge is greater than 0.95 for sampling rates up to 2.0 L/min (Environmental Protection Agency 1999b). Previous works involving DNPH impingers (attached downstream of DNPH-coated cartridges) have shown no effect of humidity on the collection efficiency of the cartridges for RH > 28 % (Grosjean 1991). The duration of experiment ranged from 0800 to 1900 hours of the day. Each sample was collected for a time duration of 1 h at a steady flow rate of 1.0 L/min. Thus the total amount of sample collected in each instance was ~60 L. Once sampling was over, the cartridges were sealed to prevent any leakage and infiltration of air before the analysis. These were then stored in a cool dry place under refrigerated condition both before and after sampling. All the samples were shipped in this condition from the experimental site (JUSL) to the Lab, which is situated about 12 km away from JUSL. Within 24 h of sampling, the samples were eluted with 5 mL of high-performance liquid chromatography (HPLC)-grade acetonitrile and analyzed by isocratic reverse-phase liquid chromatography with ultraviolet detector (Model: Waters 2489; Make: Waters) at a wavelength of 360 nm. In this method, the carbonyl in the ambient air reacts with 2,4-DNPH in the cartridge to form a hydrazone, which is eluted with acetonitrile. On analysis using HPLC (Make: Waters), the DNPH derivatives of formaldehyde and other carbonyls are separated. The HPLC was periodically calibrated using a standard HCHO-DNPH solution at different mixing ratios. The calibration curve of the HCHO-DNPH peak areas at various known mixing ratios is given in Fig. 2. Formaldehyde, in the sample, was identified and quantified by comparing the retention times and peak heights or peak areas to those of the standard solutions. For quantitative evaluation of formaldehyde, a blank cartridge and solvent blank were likewise analyzed for determining the amount of any formaldehyde present in these. The mass of average blank cartridge per cartridge was found to be 62,231 ng whereas solvent was found to be free of any amount of formaldehyde. The actual quantity of formaldehyde present in the air sample was then computed from the difference in values between the cartridge mixing ratio and the sum of blank cartridge and solvent blank mixing ratio.
Sampling methodology of O3 and CO
Ozone and CO were continuously monitored using online ozone analyzer (Model 49i; Make: Thermo Fisher Scientific, USA; Principle: UV photometry) and online CO analyzer (Model 48i; Make: Thermo Fisher Scientific; Principle: gas filter correlation technique), respectively, installed in the JUSL Campus laboratory. The lower detectable limit of the ozone and CO analyzers are 1.0 and 40 ppbv, respectively, and the data are stored as 15-min average.
Both the analyzers were periodically calibrated for zero and span. Zero calibration was carried out using zero air cylinders from British Oxygen Corporation, India. For span calibration of the ozone analyzer, the multi-point calibrator was used to calibrate the instrument at six different span values (200, 150, 100, 75, 50 and 20 ppbv). In case of CO, the span calibration was done using CO calibration gas cylinder (Make: Inox Air Products, India) having a tank concentration of 5.08 ppmv with balance nitrogen. Calibration was performed at every 100 ppbv interval at lower mixing ratios from 200 ppbv to 1.0 ppmv, and at every 1.0 ppmv interval thereafter up to 5 ppmv. A multi-gas calibrator (Model: MCG146i; Make: Thermo Fischer Scientific) was used for controlling the gas flow and to obtain the desired mixing ratios to carry out multi-point calibrations.
Sampling and analysis of methane
For estimation of methane, an oil-free air compressor (Make: Metal Bellow, USA; Model: MB-158-E,) was used to collect air samples in pre-evacuated glass sampling bottles (Make: Vensil Glass Works Pvt. Ltd., India) of 900 mL volume capacity. Air samples were collected at 3-h intervals over the same time period as HCHO. The bottles were then shipped to the laboratory in PRL, Ahmedabad for analysis. Gas chromatographic method was used for analysis and all the analyses were performed within a month of their sampling (Mallik et al. 2014).
Satellite data
The NASA Giovanni Portal (disc.sci.gsfc.nasa.gov/giovanni) gives access to several global datasets for the environment. Columnar HCHO data were collected from Ozone Monitoring Instrument (OMI) on board the Aura satellite. The Netherlands’s Agency for Aerospace Programs (NIVR) and the Finnish Meteorological Institute (FMI) together have built a nadir-viewing near-UV/Visible CCD spectrometer called the OMI which is placed on board of NASA’s Earth Observing System’s (EOS) Aura satellite and has a data record since August 9, 2004. Aura was launched with an objective to study the chemistry and dynamics of different atmospheric constituents. The satellite provides a total latitudinal coverage from 82°N to 82°S. The atmospheric constituents measured by OMI are both from natural (forest fire, volcanic and biological activity) and anthropogenic sources (biomass burning, combustion). It is a wide angle, non-scanning instrument designed for the measurement of solar back scattered irradiance. It has a 115° field of view in across track dimension. OMI covers a spectral region of 264–504 nm and has a spectral resolution in between 0.42 and 0.63 nm. The detector used in this instrument is a two-dimensional CCD spectrometer having 780 × 576 pixels (spectral × spatial). Columnar HCHO is collected from OMI L2G. This study uses the monthly averaged columnar HCHO data from OMI L2G with a spatial resolution of 0.25° × 0.25° (latitude × longitude) and a temporal resolution of approximately 1 day. This data was analyzed over a grid, spanning 2° × 2° in the region having Kolkata at the centre.
Tropospheric chemistry involving HCHO
In remote areas or in the regions near wetlands or landfill, CH4 is the major source of HCHO. Partial oxidation of methane (directly), to produce HCHO, is difficult due to much higher C-H bond energy in methane than in HCHO. Catalytic conversion of CH4, to produce HCHO has a success rate of <4 % (Zhang et al. 2012) till date. However, in presence of NOx or H2O2, the oxidation is highly favoured. NOx plays the role of a promoter in the reaction, thus reducing the high transition barrier i.e. the initiation temperature of methane oxidation (Tabata et al. 2002). The reactions involved in the formation and destruction of HCHO are given in Table 1. Under low nitrogen oxide condition, CH4 oxidation proceeds through the formation of methyl peroxide (CH3O2). It has a long half-life against photolysis and so it reacts with hydroxyl radical to produce HCHO. However, this phenomenon is mainly observed in rural areas (Wuebbles and Hayhoe 2000). In urban polluted environment, the reactions of methyl peroxide with NO and HO2 are the most important ones (R2, R3). Formaldehyde is a photochemically active molecule. So once formed, it easily undergoes photo-dissociation in the wavelength range of 325–360 nm (R6a and R6b). The branching ratio of R6a and R6b is given by the ratio of the two photolytic rate constants: j6a/j6b. HCHO also reacts with OH to produce both CO and HO2 (R7, R8). Therefore, HCHO acts as a major source of HO2 in the free troposphere. Hydrogen peroxy radical has a very significant role in the chemistry of tropospheric ozone. Along with other peroxy radicals, they are involved in the formation of the primary precursor NO2 for tropospheric ozone formation (R9, R13).
Results and discussion
Frequency distribution of HCHO
The frequency distribution of measured HCHO from July 2012 till April 2014 is tabulated in Table 2. The frequency distribution represents the range of HCHO at every 100 ppbv interval. The relative frequency of occurrence shows a maximum occurrence of 33.70 % in between 0 and 100 ppbv. The percentage gradually decreases with an increase in mixing ratio range and reduces to a value of ~1 % at mixing ratios greater than 600 ppbv. Further, a breakup of distribution of upto 100 ppbv is also provided in the Table 2. The relative frequency of occurrence of HCHO remains comparable in the ranges: 11–25 ppbv (29.03 %), 26–50 ppbv (25.81 %) and 51–75 ppbv (22.58 %) with the maximum occurring in the range of 11–25 ppbv. An important meteorological parameter—ventilation coefficient (VC)—is also computed in order to understand such high values of HCHO at the site. Ventilation coefficient is used to determine the air quality and pollution potential of a region (Iyer and Raj 2013). Mathematically, it is expressed as a product of mixed layer height and mean wind speed within this layer. Thus, higher values of VC indicate greater dispersion of pollutants whereas lower value is indicative of atmospheric stagnation and poor air quality. Figure 3 represents the variation of HCHO with ventilation coefficient. It is expected that lower VC should be associated with higher HCHO values and vice versa. But in this case, no such relationship is observed. This factor again points towards the highly complex chemistry governing the HCHO mixing ratio in Kolkata. The presence of occasional high values of HCHO may be explained on the basis of the methane oxidation cycle. The experimental site (JUSL) is located within 2 km north of the Municipal Solid Waste (MSW) Landfill (DHAPA) (refer Fig. 1). High amounts of methane are released from Dhapa on a regular basis. It is highly likely that this methane undergoes oxidation to produce high mixing ratios of HCHO (refer Table 1), which disperse convectively along the wind very easily. The wind rose plots for different seasons are given in Fig. 4 which represents the contribution of surface winds at our study site.
Diurnal variations of HCHO, ozone, CO and CH4
Figure 5 represents the box-whisker plots showing the hourly averaged diurnal variation of HCHO, ozone, CO and CH4. Since samples were mostly taken during daytime, the time duration here represents 12-h daytime (0800–2000 hours) values only. The hourly average variation of all the species are shown along with the corresponding maximum and minimum mixing ratios of the species, respectively. The maximum, minimum and average atmospheric mixing ratios of HCHO were 803, 3 and 217 ppbv, respectively, for the entire period of study. Compared to the average mixing ratio range of HCHO, measured at different parts of the world (Table 3), observed HCHO values are found much higher in Kolkata. The general trend for nearly 2-year span shows more or less a smooth and uniform trend for HCHO throughout the day. However, mixing ratios were found to be lower in the morning (0800–0900 hours) which steadily increased till noon. The increase in HCHO mixing ratio may be accounted from significant amounts of HCHO that is contributed from traffic emissions during morning rush hours. From noon onwards, the mixing ratio of HCHO starts decreasing till 1600 hours. This is clearly due to the photochemical dissociation (R6). HCHO itself being a photochemically active molecule, easily dissociates to corresponding radicals which further take part in other cycles of atmospheric chemistry. This accounts for the gradual decrease in the mixing ratio in the late afternoon. In the evening (1600–2000 hours), HCHO mixing ratio starts increasing again and reaches highest around 1900 hours. The escalation in the mixing ratios of HCHO during this time is a cumulative effect of traffic emissions as well as production from methane oxidation. Existence of early evening capped inversion cannot be also ruled out.
Over the same time period, the diurnal patterns of ozone, CO and CH4 are also plotted. Ozone, being produced photochemically, shows its characteristic trend; it shows a minimum during morning and evening and peaks at noon. The highest and lowest measured mixing ratios were 64 and 2 ppbv, respectively. In the atmosphere, the principal sources of CO are various combustion processes such as biomass burning and automobile emissions. Substantial amounts of CO are also formed by the natural oxidation of methane and isoprene. The diurnal trend shows morning and evening highs with maximum and minimum mixing ratios of 5,182 and 387 ppbv, respectively. The morning and evening highs are predominantly due to the presence of automobile emissions during office hours. CH4 is a very important precursor of formaldehyde. The average CH4 mixing ratio at our site was 2,124 ppbv; with a maximum and minimum values of 5,899 and 1,459 ppbv, respectively (Mallik et al. 2014). JUSL is situated very close to a vast MSW Landfill (DHAPA) site, which lies exactly to the south of the site within a distance of a few kilo metres (~2 km). Thus oxidation of CH4 is a potential source of HCHO at our site. Besides, there is a huge source of CH4 in the vicinity, the east Kolkata wetland, which contributes in a way towards the local methane budget.
Seasonal variation of surface HCHO
The seasonal variations of HCHO are plotted in Fig. 6. According to the guidelines of Indian Meteorological Department, a year is divided into four different seasons: winter (1st of January to 28th of February), pre-monsoon (1st of March to 7th of June), monsoon (8th of June to 10th of October) and post-monsoon (11th of October to 31st of December). Our study period overlaps with the Monsoon (2012), Post-Monsoon (2012), Winter (2013, 2014) and Pre-Monsoon (2013, 2014) periods of the region. Due to some technical problems, sufficient data were not present during Monsoon 2013 and Post-Monsoon 2013, so these seasons were not considered for the study. Of the six seasons studied, the highest average values appear during both the Pre-Monsoon periods, with Pre-Monsoon 2014 (361 ppbv) exceeding the Pre-Monsoon 2013 results (306 ppbv). Kolkata experiences warm to hot days in the Pre-Monsoon periods as this region falls in the Tropical wet-dry region according to Koppen Classification. The presence of such high levels is due to both primary emission and secondary production as discussed in details in the section “Tropospheric Chemistry involving HCHO.” Various relevant reactions involved in these processes are given in Table 1. In Monsoon 2012 (161 ppbv) and Post-Monsoon 2012 (184 ppbv), intermediate mixing ratios were observed. Figure 7a, b represent polar plots of HCHO and CH4 mixing ratios along with wind directions. On Monsoon 2012 and Post-Monsoon 2014, the mixing ratio of HCHO and CH4 come from the same regions (S). High mixing ratio of HCHO at JUSL may be explained on this basis that from the Dhapa region high quantities of methane are released which undergo oxidation to produce this HCHO. Winter 2013 is an exception, where unusually low mixing ratios of HCHO were measured. If we observe the two plots of methane and HCHO, it is evident that the source contribution of HCHO mixing ratio is different from the methane source region of Dhapa. For the rest of the seasons studied for HCHO, more or less the source may be identified as Dhapa. Thus among all the six different seasons studied, it may be summarized that high HCHO mixing ratio measured at JUSL is mainly contributed from methane emission from Dhapa which is situated so near the experimental site.
Regional source contribution: variation of column HCHO from satellite data
The study of column HCHO over Kolkata and its surrounding regions (parts of IGP and the Gangetic Delta plains) during the six seasons using OMI data from NASA Giovanni Portal (disc.sci.gsfc.nasa.gov/giovanni) reveals distinct monthly and seasonal variability of column HCHO in this region.
In Fig. 8, the variation of monthly average columnar HCHO is represented along with the monthly max and min values, as obtained from OMI. The columnar HCHO values range from 0 to 30 × 1015 molecule cm−2. The monthly average columnar mixing ratios were higher during the monsoon periods (Jun–Sep). The mixing ratio decreases gradually as post-monsoon approaches and during the winter the amount is a minimum.
Seasonal variation of the columnar HCHO was also observed. Figure 9 provides with a comparison of seasonal average of column HCHO from OMI and surface HCHO measured at JUSL for various seasons superimposed on them. Of the five seasons studied, the highest seasonal average appears on Monsoon 2012 (25.25 × 1015 molecule cm−2). In the other five seasons, the average seasonal values remain below 20 × 1015 molecule cm−2. In order to find out any contribution of columnar HCHO mixing ratio to the near surface values, the seasonal average values of surface HCHO has been overlaid on the columnar values. The trends in the changes of these values show no direct correlation among them. Thus it may be stated that columnar values are not impacted by surface emissions only and major role of chemistry/photochemistry could be inferred to account for the seasonal differences in the values of near surface HCHO mixing ratios.
Primary and secondary sources of HCHO: Chemistry
Ambient level of HCHO may be considered as an aggregate of both primary emissions and secondary formation. Since the time of sampling for our experiments is mainly from 0800 to 2000 hours, it is most likely that both primary and secondary HCHO contribute towards the net amount of HCHO measured at our site. It is difficult to segregate between primary emissions and secondary formation of ambient HCHO through chemical quantification. Given the fact that HCHO is a short lived species during the daytime and is involved in active chemistry along with the other trace gases, contribution of the local chemistry towards the actual mixing ratio of HCHO cannot be totally neglected. The inter relationships among various trace gases with HCHO have been found out in order to find out any possible correlation amongst them. The chemistry involving all these species are already discussed earlier and the reactions are given in detail in Table 1. HCHO acts as a precursor for both CO and ozone in ambient air. Hence, their correlation is observed taking HCHO as the independent variable. In case of methane, the case is reversed as HCHO is produced as an important intermediate in the methane oxidation cycle. In Fig. 10, we present the variation of HCHO with methane at three different times: 0800–1300, 1300–2000 and 2000–0500 hours (night). During daytime (Fig. 9a, b), there is an exponential decay in the mixing ratio of HCHO with increase in methane. The exponential regression gives a better result from 1300 to 2000 hours (R 2 = 0.77) as compared to 0800–1300 hours (R 2 = 0.71). However at night, this trend is not followed. On regression, a very poor correlation among these two is found. A polynomial fit (order 3) gives the R 2 value as 0.31. The methane oxidation process via the free radical mechanism is photochemistry dependant. At night due to absence of sunlight, this oxidation process to produce HCHO cannot take place. This explains the poor correlation between HCHO and CH4 at night.
Summary and conclusions
An attempt has been made to study the behavioral characteristics of ambient levels of HCHO and other gases such as CO, CH4 and ozone in and around JUSL, within the tropical urban mega city of Kolkata. Successful experiments were performed for the measurement of HCHO based on USEPA Compendium Method TO-11A using 2,4-dinitrophenylhydrazine (2,4-DNPH). Very high values of HCHO were measured. The duration of study was mainly focused on six seasons—Monsoon (2012), Post-Monsoon (2012), Winter (2013, 2014) and Pre-Monsoon (2013, 2014). Seasonal values of HCHO showed high mixing ratios during both the pre-monsoon periods due to the influence of seasonal wind patterns. Satellite data from OMI also support the scenario. Besides these, the study shows very poor correlation among various species indicating a highly complex chemistry between them. Nevertheless, characterization of this HCHO cycle in a metropolitan city may be possible with the measurements of more number of chemical species which are involved in the chemical cycle. Alongside, the emission scenarios over this region may be immensely helpful to come to an overall conclusion for the actual amount of HCHO present in the air.
References
Ahammed YN, Reddy RR, Gopal KR, Narasimhulu K, Basha DB, Reddy L, Rao TVR (2006) Seasonal variation of the surface ozone and its precursor gases during 2001–2003, measured at Anantapur (14.62 N), a semi-arid site in India. Atmos Res 80:151–164
Alzueta MU, Glarborg P (2003) Formation and destruction of CH2O in the exhaust system of a gas engine. Environ Sci Technol 37:4512–4516
Brewer R, Belzer W (2001) Assessment of metal concentrations in atmospheric particles from Burnaby Lake, British Columbia, Canada. Atmospheric Environ 35:5223–5233
Carroll MA, Bertman SB, Shepson PB (2001) Overview of the program for research on oxidants: photochemistry, emissions, and transport (PROPHET) summer 1998 measurements intensive. J Geophysical Res: Atmospheres 106:24275–24288
Christensen CS, Skov H, Nielsen T, Lohse C (2000) Temporal variation of carbonyl compound concentrations at a semi-rural site in Denmark. Atmos Environ 34:287–296
Dasgupta PK, Li J, Zhang G, Luke WT, McClenny WA, Stutz J, Fried A (2005) Summertime ambient formaldehyde in five US metropolitan areas: Nashville, Atlanta, Houston, Philadelphia, and Tampa. Environ Sci Technol 39:4767–4783
De More WB et al (1992) Chemical kinetics and photochemical data for the use of stratospheric modeling: evaluation Number 10. JPL Publication 92–20, Jet Propulsion Labs, Pasadena
De Smedt I, Müller JF, Stavrakou T, van der A R, Eskes H, Van Roozendael M (2008) Twelve years of global observations of formaldehyde in the troposphere using GOME and SCIAMACHY sensors Atmospheric Chem Physics 8
Duan J, Tan J, Yang L, Wu S, Hao J (2008) Concentration, sources and ozone formation potential of volatile organic compounds (VOCs) during ozone episode in Beijing. Atmos Res 88:25–35
U.S. Environmental Protection Agency (1999b) In: “Compendium method TO 11A, Determination of formaldehyde in ambient air using absorbent cartridge followed by high performance liquid chromatography (HPLC) (Active Sampling Methodology), United States Environmental Agency
U.S. Environmental Protection Agency (2003) In: “Formaldehyde: hazard summary”
Feng Y et al (2005) Ambient levels of carbonyl compounds and their sources in Guangzhou, China. Atmospheric Environ 39:1789–1800
Finlayson-Pitts BJ, Pitts JN (1997) Tropospheric air pollution: ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science 276:1045–1051
Friedfeld S, Fraser M, Ensor K, Tribble S, Rehle D, Leleux D, Tittel F (2002) Statistical analysis of primary and secondary atmospheric formaldehyde. Atmos Environ 36:4767–4775
Ghose MK, Paul R, Banerjee SK (2004) Assessment of the impacts of vehicular emissions on urban air quality and its management in Indian context: the case of Kolkata (Calcutta). Environ Sci Pol 7:345–351
Ghosh D, Lal S, Sarkar U (2013) High nocturnal ozone levels at a surface site in Kolkata, India: trade-off between meteorology and specific nocturnal chemistry. Urban Climate 5:82–103
Ghosh D, Midya SK, Sarkar U, Mukherjee T (2015) Variability of surface ozone with cloud coverage over Kolkata, India J Earth Syst Sci (accepted for publication)
Goldan PD et al (2000) Airborne measurements of isoprene, CO, and anthropogenic hydrocarbons and their implications. J Geophysical Res: Atmospheres 105:9091–9105
Granby K, Christensen CS, Lohse C (1997) Urban and semi-rural observations of carboxylic acids and carbonyls. Atmos Environ 31:1403–1415
Grosjean D (1982) Formaldehyde and other carbonyls in Los Angeles ambient air. Environ Sci Technol 16:254–262
Grosjean D (1991) Ambient levels of formaldehyde, acetaldehyde and formic acid in southern California: results of a one-year baseline study. Environ Sci Technol 25:710–715
Grosjean E, Williams EL, Grosjean D (1993) Ambient levels of formaldehyde and acetaldehyde in Atlanta. Georgia Air Waste 43:469–474
Grutter M, Flores E, Andraca-Ayala G, Baez A (2005) Formaldehyde levels in downtown Mexico City during 2003. Atmos Environ 39:1027–1034
Gupta AK, Karar K, Srivastava A (2007) Chemical mass balance source apportionment of PM10 and TSP in residential and industrial sites of an urban region of Kolkata, India. J Hazardous Materials 142:279–287
Iyer US, Raj PE (2013) Ventilation coefficient trends in the recent decades over four major Indian metropolitan cities. J Earth Syst Sci 122:537–549
Karar K, Gupta AK (2006) Seasonal variations and chemical characterization of ambient PM < sub > 10</sub > at residential and industrial sites of an urban region of Kolkata (Calcutta), India. Atmospheric Res 81:36–53
Khare P, Satsangi GS, Kumar N, Maharaj Kumari K, Srivastava SS (1997) HCHO, HCOOH and CH3COOH in air and rain water at a rural tropical site in North Central India. Atmos Environ 31:3867–3875
Li Y, Shao M, Lu S, Chang C-C, Dasgupta PK (2010) Variations and sources of ambient formaldehyde for the 2008 Beijing Olympic games. Atmos Environ 44:2632–2639
Mallik C, Ghosh D, Ghosh D, Sarkar U, Lal S, Venkataramani S (2014) Variability of SO2, CO, and light hydrocarbons over a megacity in Eastern India: effects of emissions and transport Environ Sci Poll Res: 1–15
Miller SM et al. (2008) Sources of carbon monoxide and formaldehyde in North America determined from high-resolution atmospheric data Atmospheric Chemistry & Physics Discussions 8
Mitchell CE, Olsen DB (2000) Formaldehyde formation in large bore natural gas engines part 1: formation mechanisms. J Eng Gas Turbines Power 122:603–610
Pang X, Mu Y (2006) Seasonal and diurnal variations of carbonyl compounds in Beijing ambient air. Atmos Environ 40:6313–6320
Pang X, Mu Y, Lee X, Zhang Y, Xu Z (2009) Influences of characteristic meteorological conditions on atmospheric carbonyls in Beijing, China. Atmos Res 93:913–919
Possanzini M, Palo VD, Cecinato A (2002) Sources and photodecomposition of formaldehyde and acetaldehyde in Rome ambient air. Atmos Environ 36:3195–3201
Ren X et al (2003) OH and HO2 Chemistry in the urban atmosphere of New York City. Atmos Environ 37:3639–3651
Singh H et al (2000) Distribution and fate of selected oxygenated organic species in the troposphere and lower stratosphere over the Atlantic. J Geophysical Res: Atmospheres 105:3795–3805
Slemr J, Junkermann W, Volz-Thomas A (1996) Temporal variations in formaldehyde, acetaldehyde and acetone and budget of formaldehyde at a rural site in southern Germany. Atmos Environ 30:3667–3676
Solberg S, Dye C, Walker S-E, Simpson D (2001) Long-term measurements and model calculations of formaldehyde at rural European monitoring sites. Atmos Environ 35:195–207
Tabata K, Teng Y, Takemoto T, Suzuki E, Banares MA, Pena MA, Fierro JLG (2002) Activation of methane by oxygen and nitrogen oxides. Catalysis Rev 44:1–58
Trapp D, De Serves C (1995) Intercomparison of formaldehyde measurements in the tropical atmosphere. Atmos Environ 29:3239–3243
U.S. Environmental Protection Agency (1988) In: “Health and environmental effects profile for formaldehyde. EPA/600/x-85/362. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development, Cincinnati
U.S. Environmental Protection Agency (1999) In: “Integrated risk information system (IRIS) on formaldehyde. National Center for Environmental Assessment, Office of Research and Development, Washington
US-EPA (1999) In: Compendium method TO 11A, Determination of formaldehyde in ambient air using absorbent cartridge followed by high performance liquid chromatography (HPLC) (Active Sampling Methodology), United States Environmental Agency
Viskari E-L, Vartiainen M, Pasanen P (2000) Seasonal and diurnal variation in formaldehyde and acetaldehyde concentrations along a highway in Eastern Finland. Atmos Environ 34:917–923
World Health Organization (1999) In: “International Program on Chemical Safety, Environ Health Criteria 89: formaldehyde
Wuebbles DJ, Hayhoe K (2000) Atmospheric methane: trends and impacts. In: Non-CO2 greenhouse gases: scientific understanding, control and implementation. Springer, pp 1–44
Xiaoyan W, Huixiang W, Shaoli W (2010) Ambient formaldehyde and its contributing factor to ozone and OH radical in a rural area. Atmos Environ 44:2074–2078
Zhang J, Burklé-Vitzthum V, Marquaire P (2012) NO2-promoted oxidation of methane to formaldehyde at very short residence time. Part I: experimental results. Chem Eng J 197:123–134
Acknowledgments
This research work is done with financial support from Indian Space Research Organisation’s ISRO-AT-CTM programme. The authors are also grateful to CSIR for funding the research fellowship of Ms. Debreka Ghosh. The authors would also like to thank the Goddard Earth Sciences Data and Information Services Center (GES DISC) as the analyses and visualizations used in this paper were produced with the Giovanni online data system, developed and maintained by the NASA GES DISC.
Compliance with ethical standards
ᅟ
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
No experiments were performed involving Human Participants and/or Animals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerhard Lammel
Rights and permissions
About this article
Cite this article
Ghosh, D., Sarkar, U. & De, S. Analysis of ambient formaldehyde in the eastern region of India along Indo-Gangetic Plain. Environ Sci Pollut Res 22, 18718–18730 (2015). https://doi.org/10.1007/s11356-015-5029-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5029-y