Abstract
In this work, we report the adaptation of bacteria to stress conditions that induce instability of their cultural, morphological, and enzymatic characters, on which the identification of pathogenic bacteria is based. These can raise serious issues during the characterization of bacteria. The timely detection of pathogens is also a subject of great importance. For this reason, our objective is oriented towards developing an immunosensing system for rapid detection and quantification of Staphylococcus aureus. Polyclonal anti-S. aureus are immobilized onto modified gold electrode by self-assembled molecular monolayer (SAM) method. The electrochemical performances of the developed immunosensor were evaluated by impedance spectroscopy through the monitoring of the charge transfer resistance at the modified solid/liquid interface using ferri-/ferrocyanide as redox probe. The developed immunosensor was applied to detect stressed and resuscitate bacteria. As a result, a stable and reproducible immunosensor with sensitivity of 15 kΩ/decade and a detection limit of 10 CFU/mL was obtained for the S. aureus concentrations ranging from 101 to 107 CFU/mL. A low deviation in the immunosensor response (±10 %) was signed when it is exposed to stressed and not stressed bacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus is a major human pathogen responsible for a broad range of infection and chronic diseases. S. aureus expresses multiple virulence factors such as adhesions and toxins. In addition, it expresses specific surface-associated proteins such as the polysaccharide intercellular adhesion (PIA) that constitutes a keystone in biofilm formation (Ferreira et al. 2013) and allows the organisms to interact specifically with extracellular matrix proteins of the host cell, such as fibronectin and fibrinogen (Blickwede et al. 2005).
As it is well known, bacteria adopt several strategies to adapt rapidly to environmental changes (Dash et al. 2013). One of the most frequently observed behaviors in the nutrient starvation response of bacteria is the size reduction, cell morphology conversion from rod to coccoid shape, cell surface alteration, and evolution to viable but non-cultivable state (Bakhrouf et al. 2008). The characteristics of the suspending medium such as pH, osmolarity, and temperature are considered to be important factors in altering the physicochemical properties of a bacterial surface (Hamadi et al. 2004) and bacterial cultivability (Bakhrouf et al. 2008). In such conditions, the conventional methods for bacterial detection, based on bacterial culture and colony counting, can be a hurdle in the characterization and identification of stressed bacteria (Khandekar et al. 2013). However, biosensors are currently imposed as powerful sensitive and specific tools for the real-time detection of such stressed bacteria. Several successful biosensors for microbial agent were developed (Baccar et al. 2010; Mejri et al. 2010; Varshney and Li 2007; Byrne et al. 2009).
In the present study, an immunosensor was developed based on self-assembled molecular monolayer (SAM) strategy. Specific anti-S. aureus antibodies were immobilized on 16-mercaptohexanoic acid modified gold electrode. Voltammetry and impedance spectroscopy methods were used to characterize the developed biosystem. The latter was applied to identify stressed and non-stressed S. aureus bacteria. The reference strain of S. aureus (ATCC 25923) was incubated for 5 years in sea water and was resuscitated in tryptic soy broth (TSB, Difco). The biochemical profile was achieved using API staph system as recommended by manufacturer’s recommendation. The morphological alteration of resuscitate cells was performed using atomic force microscopy.
Materials and methods
Reagents and antibodies
Polyclonal antibodies (developed in rabbit) against S. aureus were provided by BIOtech RDP (Sfax, Tunisia). 16-Mercaptohexadecanoic acid, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), sodium nitrite, phosphate buffered saline (PBS), potassium ferrocyanide [K4Fe(CN)6], potassium ferricyanide [K3Fe(CN)6], hydrogen peroxide (30 %), ethanolamine (25 %), and bovine serum albumin (BSA) were purchased from Sigma-Aldrich.
Bacterial strains and growth conditions
S. aureus ATCC 25923 was used in this study. For the experiments, cells were grown at 37 °C in tryptic soy broth (TSB, Difco) for 24 h. Natural seawater (100 mL) was sampled from the coast of Monastir (salinity 4 %, pH 8.0), filtered through membranes (pore size, 0.22 μm; Millipore Corp., Bedford, MA), and autoclaved at 121 °C for 20 min. Then, S. aureus cells were washed three times by centrifugation (13,000 rpm for 10 min) with autoclaved seawater (Ellafi et al. 2009). The microcosms (100 mL) were inoculated with these suspensions (approximately 108 CFU/mL) and then incubated in a static state at room temperature.
Resuscitation of VBNC cells
Starved cells of S. aureus (ATCC 25923), incubated during 5 years in natural sea water microcosm at a static state at room temperature, were resuscitated by addition of tryptic soy broth, and cultivability was performed in tryptic soy agar (TSA, Pronadisa, Spain) according to the previous described method (Ben Kahla-Nakbi et al. 2006; Bakhrouf et al. 2008). The biochemical and the enzymatic profiles of resuscitate S. aureus cells were characterized using API staph system (bio-Merieux, France) and API-ZYM (bio-Merieux, France), respectively.
Determination of morphological changes by AFM
In order to visualize any morphological changes, starved cells were examined by AFM (Nanoscope IIIA, Digital Instrument, VEECO). In brief, the cells were collected, washed three times with phosphate-buffered saline (PBS), and centrifuged. The final pellet was resuspended in PBS, placed on a round microscope cover slide, and dried in air according to the method described previously (Braga and Ricci 1998; Bakhrouf et al. 2008).
PCR confirmation of S. aureus strain
The extraction of the chromosomal DNA was carried out using a Wizard Genomic Purification Kit (Promega, Lyon, France) according to the manufacturer’s recommendation.
PCR were performed in a total volume 25 μL containing 50 ng of extracted DNA, 5 μL green Go Taq buffer (5×), 0.25 μL dNTPs (10 mM), 0.5 μL MgCl2 (50 mM), 1 μL of each primer (25 pM), and 1 U of GO Taq DNA polymerase (Promega, USA). The used primers were specific to Sa442 gene (5′-CGTAATGAGATTTCAGTAGATAATACAACA-3′ and 5′-AATCTTTGTCGGTACACGATATTCTTCACG-3′) (Murdoch et al. 2004; Bekir et al. 2011). The PCR was performed using the following thermal cycles: an initial denaturation step at 94 °C for 5 min followed by 35 cycles (94 °C for 90 s, 57 °C for 30 s, and 72 °C for 90 s), followed by a final extension step at 72 °C for 10 min. PCR products were analyzed on 1.5 % gel and stained with ethidium bromide (0.5 mg/mL). Gel was visualized using ultraviolet transilluminator.
Electrochemical instrumentation
Electrochemical measurements were performed using a potentiostat galvanostat instrument (Voltalab 40). The electrochemical measurements were performed using three-electrode cell (volume of 5 mL) containing the modified gold electrode (geometrical surface of 0.07 cm2) as working electrode, a saturated calomel electrode (SCE) as reference electrode, and a platinum plate as counter electrode. All electrochemical measurements were carried out in a Faraday cage at room temperature to avoid external interference which can perturbate the impedance response.
Two-electrochemical methods and modeling software were used in this work as follows:
-
(i)
Cyclic voltammetry was performed at a scan rate of 100 mV/s in electrolyte support PBS solution (8 mM) containing 5 mM ferro-/ferricyanide and 100 mM of NaCl.
-
(ii)
Faradaic electrochemical impedance spectroscopy (EIS) was used by applying a small sinusoidal signal (amplitude 10 mV; frequency range 100 mHz to 100 kHz) to the system at open circuit potential in 5 mM ferro-/ferricyanide PBS solution (8 mM).
-
(iii)
The Zview/Zplot modeling software compatible with Windows (provided by Scribner Associate Inc, Southern Pines, NC, USA) was used for fitting the Faradaic impedance spectra.
Elaboration of the immunosensor
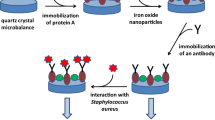
The cleaning and activation of the electrode surface represent a key step to developing a successful electrochemical sensor. In our work, gold electrodes were ultrasonically cleaned in acetone and then immersed for 5 min in a piranha mixture (HCl/H2O2: 3/1). After such step, the gold electrodes were rinsed thoroughly with ultrapure water and then rinsed with absolute ethanol and dried in a flow of nitrogen. After cleaning, gold electrodes were immersed into 10 mM of 16-mercaptohexanoic acid (thiol)/ethanol solution for 12 h. The adsorbed SAM thiol layer was activated by incubation in a mixture of 0.1 M EDC and 0.1 M NHS for 1 h at room temperature. After rinsing with PBS, the activated modified electrodes were incubated for 1 h in a 0.3 mg/mL solution of specific anti-S. aureus antibody. The terminal amine groups on the antibody enabled covalent bonding to occur through the activated carboxylic functions of the adsorbed thiol layer. Finally, after rinsing with PBS, the electrodes were incubated for 20 min in ethanolamine to deactivate the remaining acidic functionalities. The different steps of the immunosensor elaboration are presented in Fig. 1. The electrochemical performances of the developed bioarchitecture were characterized by cyclic voltammetry and impedance spectroscopy techniques. The immunosensor response towards different concentrations of the S. aureus bacteria before and after resuscitation in sea water microcosms was investigated by impedance spectroscopy. The modified electrode was incubated in different aliquots corresponding to different bacterial concentrations in CFU/mL.
Results and discussion
Resuscitation of VBNC cells
The results developed in this study showed that S. aureus cells were cultivable on TSA after 24 h of incubation in TSB; later, they had been incubated during 5 years in sea water microcosm. We have noted that the biochemical profile of the 24-h-resuscitated cells was completely inactive on API staph system. The stressed bacteria recuperate their initial characters after 8 months of resuscitation in TSB (Table 1).
These results showed that Gram-positive bacteria as S. aureus are able to adapt and survive for a long period under extremely stressing conditions. Viable but non-culturable (VBNC) cells of S. aureus were resuscitated after 24 h of incubation in TSB. It has been clearly demonstrated that many bacteria can enter the VBNC state when faced with these conditions (Besnard et al. 2002; Bakhrouf et al. 2008). Several researches indicate that VBNC state is an adaptive strategy developed by the microorganisms to face stressing conditions. In this state, bacteria are still viable and show metabolic activity and respiration but cannot be shown as colony-forming units by the conventional plate counts and hence remain hidden (Colwell 1996). Indeed, the cells can find their initial state when the good conditions are restored (Huq and Colwell 1995).
It was reported that strains of Salmonella bovismorbificans incubated during 13 years in seawater microcosms were cultivable after 24 h of incubation in nutrient broth (Bakhrouf et al. 2008).
The survival of S. aureus in seawater microcosm for a long period confirmed an adapted profile of this bacterium under starvation conditions, findings that are in agreement with those noted for Vibrio cholerae 01 (Colwell 1996), Vibrio vulnificus (Oliver and Bockian 1995), enteropathogenic Escherichia coli (Pommepuy et al. 1996), Shigella dysenteriae (Rahman et al. 1994), Vibrio alginolyticus (Ben Kahla-Nakbi et al. 2006), and Salmonella (Bakhrouf et al. 2008). In contrast, resuscitation has been found to be impossible for other bacteria such as Vibrio parahaemolyticus (Jiang and Chai 1996) and Vibrio splendidus (Armada et al. 2003).
The biochemical modifications observed in the starved strain are probably due to the oligotrophy of the medium. S. aureus recuperated its initial biochemical profiles after 8 months of incubation in TSB. According to a previous study, cells in the VBNC state could be metabolically reactivated (Villarino et al. 2000). Furthermore, it has been demonstrated that S. bovismorbificans incubated during 13 years in seawater microcosms recuperated most of their biochemical characters after 6 months of resuscitation (Bakhrouf et al. 2008).
Morphological variations of stressed strain
Alterations in cell morphology due to starvation stress in sea water were examined by AFM (Fig. 2). The control S. aureus cells have a diameter varying from 0.7 to 1 μm (a). Whereas, the cells obtained after 8 months of resuscitation have a coccoid shape with a size of less than 0.4 μm (b). As described previously, several bacteria such as Salmonella can survive for a long period under stressing environmental conditions owing to gradual changes in cellular physiology and morphology (Morita 1982).
The reduction of the bacteria size during the stress is a strategy of survival to minimize the needs for the cell in nutriments (Jiang and Chai 1996). This reduction of cell size is the result of the cytoplasmic contraction and the volume of the bacterial periplasm reduction (Huisman et al. 1996).
Molecular confirmation of resuscitated strain
After 24 h of resuscitation, S. aureus becomes non-identifiable by the API staph system. For this reason, we have used the technique of PCR to identify the stressed strains. After amplification of Sa442 gene by PCR, we confirmed the identity of the investigated Staphylococcus strains (Fig. 3). The present study shows that Salmonella is a ubiquitous germ as it can evolve towards a VBNC state with biochemical and morphological modifications to face stressing environmental conditions. It can be resuscitated, and then it can recuperate its initial characters when the good conditions are estabilished even if the stress period is too long.
Electrochemical recognition of stressed pathogenic S. aureus using EIS immunosensor
Electrochemical characterization of modified gold electrode
The self-assembled monolayers formed onto the gold electrode surface and the immobilization of antibodies were investigated by cyclic voltammetry and impedance spectroscopy in the presence of ferro-/ferricyanide as a redox probe. Figure 4 shows the voltammogram of bare and modified gold electrode. As a result, the current through the modified working electrode is effectively decreased after the deposit monolayers. This phenomenon can be attributed to the decrease of the electron transfer rate through the modified SAMs multilayers.
Nyquist plots of impedance spectra obtained before and after the different functionalization steps show a noticeable increase of the charge transfer resistance R CT (Fig. 5) after SAM modification and a further increase after immobilization of anti-S. aureus antibody. The Nyquist plots are fitted with the electrical equivalent circuit presented in Fig. 6. It was shown that the electrochemical modified solid/liquid interface can be depicted by an electrical circuit containing the following:
-
A solution resistance (R 0).
-
A charge transfer resistance of the redox reaction at the biofunctionalized electrode (R CT).
-
A constant phase element (CPE) that is related to the capacitance of the biofunctionalized Au electrode/electrolyte interface. CPE reflects the non-ideality of the double layer at the biofunctionalized gold electrode/electrolyte interface due to the roughness and porosity of the biofilm.
-
A Warburg element (Z w)-specific electrochemical element of diffusion.
Detection of bacteria cells using electrochemical impedance spectroscopy
The electrochemical response of the modified electrode incubated in different aliquots corresponding to different bacterial concentrations (UFC/mL) was performed by impedance spectroscopy. Figure 7 shows the impedance spectra obtained for different concentration of S. aureus (ATCC 25923) bacteria before (A) and after (B) resuscitation in the concentration range between 101 and 107 UFC/mL. We can see an increase of the charge transfer resistance when the concentration of bacteria increases which is due to the specific antibodies-bacteria interaction. The same behavior was observed for S. aureus bacteria before and after resuscitation increases after consecutive incubations.
A linear relationship between the charge transfer resistance and decimal logarithmic value of S. aureus concentrations was found in the concentration range from 101 to 107 CFU/mL for stressed and resuscitated bacteria (Fig. 8). A low deviation in the electrochemical immunosensor response of 10 % was obtained for tested stressed and resuscitated bacteria. A sensitivity of 15 kΩ/decade (±10 %) was defined in linear concentration range from 101 to 107 CFU/mL. A low detection limit of 10 CFU/mL was observed for different modified electrodes.
Our results showed that there is low difference in sensitivity of detection between stressed and non-stressed bacteria in terms of detection percentage (Fig. 8). This behavior can be attributed to the conservation of surface antigen integrity in stressed S. aureus. According to Bekir et al. (2011), stress induces the reduction of the cell size, the increase in cell number, the lost of small granules and inclusion bodies, the lost of the distinct three-layered integrity of the outer membrane, peptidoglycan, and the inner membrane to retain only remnants of those structures, the compression of the nuclear region into the cell center surrounded by a denser cytoplasm, and the formation of extended or convoluted structures from the cell wall which are pulled away from the cell membrane. In previous work, it was shown that stress phenomenon can lead to the alteration of the S. bovismorbificans envelope (Bakhrouf et al. 2008). These results suggest two possibilities: either the exopolysaccharides on the outer membrane of the cells might be untangled and released or the peptidoglycan of the cytoplasmic membrane might be perturbed (Slavik et al. 1995).
Conclusion
In this work, a highly sensitive immunosensor was developed for stressed and resuscitated pathogenic S. aureus bacteria recognition. The specific immobilized antibodies and bacteria interaction was characterized by voltammetry and impedance spectroscopy. The bacteria morphology after starvation stress in sea water was examined by AFM observation. The developed immunosensor was used to recognize stressed and resuscitation bacteria using impedance spectroscopy measurements. As a result, a high and linear response with a low detection limit was observed in dynamic concentration range from 101 to 107 CFU/mL. The same sensitivity (15 kΩ/decade) was observed for stressed and non-stressed bacteria with low difference less than 10 %. After some optimization of the biodetection procedure, we assume that the developed immunosensor can be used in bacteriology laboratory for routine analysis. The reproducible results obtained from successful measurements demonstrated the potentiality of the bioanalytical system. Finally, the developed bioarchitecture system can be used to detect pathogenic S. aureus bacteria in environmental and food disease even though in stress condition.
References
Armada SP, Farto R, Perez MJ, Nieto TP (2003) Effect of temperature, salinity and nutrient content on the survival responses of Vibrio splendidus biotype I. Microbiology 149:369–375
Baccar H, Mejri MB, Hafaiedh I et al (2010) Magnetic nanoparticles immobilization and functionalization for biosensor applications. Talanta 82:810–814
Bakhrouf A, Ben Abdallah F, Lagha R (2008) Morphological changes of starved Salmonella enterica serovar Agona cells in soil after resuscitation. Ann Microbiol 58:521–552
Bekir K, Ben Abdallah F, Ellafi A, Bakhrouf A (2011) Adherence assays and slime production of Staphylococcus aureus strains after their incubation in seawater microcosms. Ann Microbiol 61:819–823
Ben Kahla-Nakbi A, Chaieb K, Besbes A et al (2006) Virulence and enterobacterial repetitive intergenic consensus PCR of Vibrio alginolyticus strains isolated from Tunisian cultured gilthead sea bream and sea bass outbreaks. Vet Microbiol 117:321–327
Besnard V, Federighi M, Declerq E, Jugiau F, Cappelier JM (2002) Environmental and physico-chemical factors induce VBNC state in Listeria monocytogenes. Vet Res 33:359–370
Blickwede M, Goethe R, Wolz C et al (2005) Molecular basis of florfenicol-induced increase in adherence of Staphylococcus aureus strain Newman. J Antimicrob Chemother 56:315–323
Braga PC, Ricci D (1998) Atomic force microscopy: application to investigation of Escherichia coli morphology before and after exposure to cefodizime. Antimicrob Agents Chemother 42:18–22
Byrne B, Stack E, Gilmartin N, Kennedy R (2009) Antibody-based sensors: principles, problems and potential for detection of pathogens and associated toxins. Sensors 9:4407–4445
Colwell RR (1996) Global climate and infectious disease: the cholera paradigm. Science 274:2025–2031
Dash HR, Mangwani N, Chakraborty J, Kumari S, Das S (2013) Marine bacteria: potential candidates for enhanced bioremediation. Appl Microbiol Biotechnol 97:561–571
Ellafi A, Denden I, Ben Abdallah F, Souissi I, Bakhrouf A (2009) Survival and adhesion ability of Shigella spp. strains after their incubation in seawater microcosms. World J Microbiol Biotechnol 25(1161):1168
Ferreira AS, Silva IN, Oliveira VH, Becker JD, Givskov M, Ryan RP, Fernandes F, Moreira LM (2013) Comparative transcriptomic analysis of the Burkholderia cepacia tyrosine kinase bceF mutant reveals a role in tolerance to stress, biofilm formation, and virulence. Appl Environ Microbiol 79:3009–3020
Hamadi NK, Swaminathan S, Chen XD (2004) Adsorption of paraquat dichloride from aqueous solution by activated carbon derived from used tires. J Hazard Mater 112:133–141
Huisman GW, Siegele DA, Zambrano MM et al (1996) Morphological and physiological changes during stationary phase in Escherichia coli and Salmonella. In: Neidhardt FC, Curtiss R, Gross CA, Ingraham JL, Lin EEC (eds) Cellular and molecular biology. American Society for Microbiology, Washington, DC, pp 1672–1682
Huq A, Colwell RR (1995) A microbiological paradox: viable but nonculturable bacteria with special reference to Vibrio cholerae. J Food Prot 59:96–101
Jiang X, Chai TJ (1996) Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable, nonculturable cells. Appl Environ Microbiol 62:1300–1305
Khandekar N, Willcox MD, Shih S, Simmons P, Vehige J, Garrett Q (2013) Decrease in hyperosmotic stress–induced corneal epithelial cell apoptosis by L-carnitine. Mol Vis 19:1945–1956
Mejri MB, Baccar H, Baldrich E, Del Campo FJ, Helali S, Ktari T, Simonian A, Aouni M, Abdelghani A (2010) Impedance biosensing using phages for bacteria detection: generation of dual signals as the clue for in-chip assay confirmation. Biosens Bioelectron 26:1261–1267
Morita RY (1982) Starvation-induced survival of heterotrophs in the marine environment. Adv microb Ecol 6:171–198
Murdoch FE, Sammons RL, Chapple IL (2004) Isolation and characterization of subgingival staphylococci from periodontitis patients and controls. Oral Dis 10:155–162
Oliver JD, Bockian R (1995) In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol 61:2620–2623
Pommepuy M, Butin M, Derrien A, Gourmelon M, Colwell RR, Cormier M (1996) Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol 62:4621–4626
Rahman I, Shahamat M, Kirchman PA, Russek-Cohen E, Colwell RR (1994) Methionine uptake and cytopathogenicity of viable but nonculturable Shigella dysenteriae type 1. Appl Environ Microbiol 60:3573–3578
Slavik MF, Kim WJ, Walker JT (1995) Reduction of Salmonella and Campylobacter on chicken carcasses by changing scalding temperature. J Food Prot 58:689–691
Varshney M, Li Y (2007) Interdigitated array microelectrode based impedance biosensor coupled with magnetic nanoparticle-antibody conjugates for detection of Escherichia coli O157:H7 in food samples. Biosens Bioelectron 22:2408–2424
Villarino A, Bouvet OM, Regnault B et al (2000) Exploring the frontier between life and death in Escherichia coli: evaluation of different viability markers in live and heat- or UV-killed cells. Res Microbiol 151:755–768
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bekir, K., Barhoumi, H., Braiek, M. et al. Electrochemical impedance immunosensor for rapid detection of stressed pathogenic Staphylococcus aureus bacteria. Environ Sci Pollut Res 22, 15796–15803 (2015). https://doi.org/10.1007/s11356-015-4761-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4761-7