Abstract
A low crystalline Fe-oxyhydroxy sulfate (FeOS) was used to immobilize arsenic (As) in soils in this study. The effects of FeOS amount, treatment time and soil moisture on As immobilization were investigated. The results showed that water-soluble and NaHCO3-extractable As were immobilized by 53.4–99.8 and 13.8–73.3 % respectively, with 1–10 % of FeOS addition. The highest immobilization of water-soluble (98.5 %) and NaHCO3-extractable arsenic (47.2 %) was achieved under condition of 4 % of FeOS and 80 % of soil moisture. Further, more amounts of FeOS addition resulted in less time requirement for As immobilization. Sequential chemical extraction experiment revealed that easily mobile arsenic phase was transferred to less mobile phase. The FeOS-bonded As may play a significant role in arsenic immobilization. Under leaching with simulated acid rain at 60 times pore volumes, accumulation amount of As release from untreated soil and soil amended with FeOS were 98.4 and 1.2 mg, respectively, which correspond to 7.69 and 0.09 % of total As amounts in soil. The result showed that the low crystalline FeOS can be used as a suitable additive for arsenic immobilization in soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Serious arsenic contamination of soil and groundwater in many countries has raised environmental concern (Ng et al. 2003; Zhu et al. 2008). In addition to geochemical weathering processes, mining, beneficiation, and smelting activities (Carbonell-Barrachina et al. 2004), irrigation with As-contaminated wastewater, and widespread use of arsenic-containing products can also result in soil contamination. In mining and smelting areas, soil contamination of arsenic is particularly serious and arsenic content in contaminated soil can reach to several thousand milligrams per kilogram. Acute exposure to high levels of arsenic can cause nausea, vomiting, bloody urine, anuria, colicky abdominal pain, even death in the most severe cases. Even at low concentration, long-term exposure to arsenic can lead to diabetes, hypertension, neurological complications, reproductive disorders, respiratory disease, and cardiovascular disease (Jomova et al. 2011). Inorganic arsenicals have been classified as group 1 of human carcinogen. Therefore, it is necessary to find an effective way to remediate As contamination in soils.
Several technologies have been proposed for the remediation of As-contaminated soils, including soil replacement, chemical immobilization, electrokinetics, soil flushing, vitrification, and phytoremediation. Chemical immobilization of As in the contaminated soils has been paid attentions by many researchers due to its low cost and less disruptive to soil system. Many chemicals have been used to treat As-contaminated soils, including iron compounds, aluminum oxides, manganese oxides, and clay minerals (Kumpiene et al. 2008). Due to its adsorption properties, iron-containing materials have been extensively investigated in the immobilization of As (Komárek et al. 2013). Zero-valent iron (Ascher et al. 2009; Kumpiene et al. 2012) and ferrous sulfate (Kim et al. 2003; Moore et al. 2000; Xenidis et al. 2010) have demonstrated its great ability to reduce the mobility of As. However, the application of zero-valent iron more than 5 % by weight can lead to soil structure problems, such as changes in porosity and aggregate (Mench et al. 1999). Ferrous sulfate can cause the acidification of soil and re-release of the immobilized As. Besides that, several Fe oxide minerals were also used for As immobilization, including goethite (α-FeOOH), lepidocrocite (g-FeOOH) and ferrihydrite (5Fe2O3·9H2O or Fe5HO8·4H2O) (García-Sanchez et al. 2002; Hartley et al. 2004; Nielsen et al. 2011). It was reported that water-soluble As reduced 55–100 % by using synthetic FeOOH (1–5 %) and limonite (1–10 %) (García-Sanchez et al. 2002). Recently, ferric hydroxide (Ko et al. 2012) and nano-sized iron particles (Kim et al. 2012) were also employed to treat As-contaminated soil. The synthesis of nano-sized materials gains significant attention due to their high reactivity to capture As in the contaminated soil. However, the magnetic aggregates of nano-sized iron particles can result in the clogging of soil pores, which limited its availability. Consequently, it is necessary to develop new iron-containing immobilizers to avoid soil acidification and reduce foreign substances addition into the As-contaminated soil.
A poorly crystalline Fe-oxyhydroxy sulfate mineral was found in acidic iron and sulfate-rich environment. This mineral is commonly shown as a brownish yellow material and called schwertmannite (Fe8O8(OH)8-2x (SO4) x ; 1 < X < 1.75) (Blgham et al. 1990). Schwertmannite has high-specific surface area and containing a large number of hydroxyl, sulfate and other groups, which can be used to precipitation and adsorption of toxic elements (Jönsson et al. 2005; Lee and Kim 2008). Many studies have shown that these minerals have a good effect on arsenate adsorption in aqueous. However, little is known concerning the treatment of arsenate contaminated soil by this mineral. The main objectives of this study are to: (1) investigate the potential application of poorly crystalline Fe-oxyhydroxy sulfate mineral in treatment As-contaminated soils, (2) optimize the treatment parameters, and (3) examine the release of As from treated and original As-contaminated soil.
Materials and methods
Materials
Analytical grade hydrogen peroxide (H2O2) and ferrous sulfate heptahydrate (FeSO4·7H2O) were used to prepare FeOS. All solutions were prepared with ultrapure water (18.25 MΩ·cm).
Soil sample
The soil sample was collected from a realgar mine area located in Hunan province, China (111°02′E, 29°39′N). The soil was Calcaric Cambisol. The soil sample was air-dried at room temperature and sieved to remove the coarse fraction (>2.0 mm), whereas the fine fraction (less than 2.0 mm) was homogenized and stored prior to experiment. The soil properties are listed in Table 1.
Preparation and characterization of FeOS
The poorly crystalline Fe-oxyhydroxy sulfate was prepared following the procedures described by Paikaray et al. (Paikaray et al. 2011). The synthesized product was ground into fine powders for the measurements. The crystal structure of FeOS was characterized by an X-ray diffraction (XRD, D/max 2550 VB+X). The morphology of FeOS was observed using a scanning electron microscope (SEM, Nova NanoSEM 230) and a transmission electron microscopy (TEM, Tecnai G2). A Fourier transform infrared spectra of FeOS was recorded using a FTIR spectrometer (NICOLET IS10). The sample was scanned under 400 to 4,000 cm−1 at 4-cm−1 resolution.
Arsenic immobilization in soil
To examine the effects of FeOS addition for As immobilization in soils, a series of batch experiments were carried out. For determining the effect of FeOS dosage on As immobilization, 10 g of the soil sample was placed in a 150-ml conical flask, and mixed with different dosages of FeOS according to FeOS-to-soil mass percentage of 1, 2, 4, 5, 6, 8, and 10 %. Deionized water was added in the mixture based on a soil/water ratio of 1:1 (w/v) followed by immobilization for 7 days. Then, the mixtures were withdrawn to analyze the concentrations of water-soluble and NaHCO3-extractable As in soils.
In order to investigate the influence of reaction time on As immobilization, soil samples were spiked with 1, 2, 4, 5, 6, and 8 % (w/w) of FeOS, respectively, added with deionized water at soil/water ratio of 1:1 (w/v), and then kept under room temperature. At 7, 14, 21, 28, 40, and 60 days, the mixtures were withdrawn to analyze the concentrations of water-soluble and NaHCO3-extractable As.
To investigate the effect of soil moisture on immobilization of As, 10 g soil and 0.4 g FeOS were thoroughly mixed, and then added deionized water to adjust the soil moisture content to 30, 40, 50, 60, 70, 80, 100, and 120 % of water-holding capacity. The field capacity of the soil in this study was 40 % (w/w). After 7 days, the mixtures were withdrawn to analyze the concentrations of water-soluble and NaHCO3-extractable As in soils.
All experiments were conducted in triplicate.
Sequential chemical extraction experiment
A sequential extraction procedure was carried out to study the changes of arsenic species distribution in soils after immobilization (Wenzel et al. 2001). Briefly, 1 g soil was placed in a 50-ml polypropylene centrifuge tube and followed by a four-step sequential chemical extraction procedure as described in Table 2. Residual in the final digestion step was conducted with 10 ml aqua regia.
Soil column leaching experiment with simulated acid rain
To investigate the performance of the soil treated by FeOS in the long term, soil samples were artificially leached with simulated acid rain. The pH of acid rain ranges from 3.43 to 4.8 at the sampling area. Therefore, synthetic acid rain of pH 4.0 was prepared with 4:1 (v/v) H2SO4–HNO3 solution mixture. For leaching experiment, 400 g of soil were placed in a Plexiglas column (locally fabricated) with an interior diameter 5 cm and 30 cm in height. The length of the soil columns in Plexiglas tubes were approximately 17 cm with a typical pore volume of 214 cm3. Simulated acid rain was pumped by a peristaltic pump to percolate through the packed soil columns at a flow rate of 0.8–1.1 ml/min. Blank column containing only contaminated soil was used for control. The leachates were collected for determining As concentration. As concentrations and accumulated As amount were presented at the relative pore volume number (V/V 0), where V 0 is the pore volume of soil column and V was the volumes of simulated acid rain.
Chemical analysis
The water-soluble and NaHCO3-extractable As were extracted by deionized water and 0.5 mol/l sodium bicarbonate solution at soil/water ratio of 1:10 (w/v) for 2 h. For determining total As, 0.5 g soil was digested with 10 ml aqua regia in a boiling water bath for 2 h. The concentration of water-soluble, NaHCO3-extractable, and total As in solution were determined with an atomic fluorescence spectrometry (AFS-8x). Soil pH was measured by using soil/water 1:5 and a pH meter. The content of organic matter was analyzed by Walkley-black titration. The cation exchange capacity of soils was determined by the ammonium acetate method. The determinations of total N and total P were Kjeldahl and molybdenum blue method, respectively.
Results and discussion
Characterization of FeOS
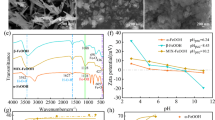
The X-ray diffraction pattern showed that FeOS had a poorly crystalline structure because there is no significantly sharp peak observed (Fig. 1). Based on the peaks detected and their respective intensities, it can be concluded that the powder was FeOS mineral.
The FeOS particles were small spheroids with a mean diameter of 500 nm. The scanning electron microscope (SEM) image showed that the FeOS was characterized by heterogeneous spheroidal size with uneven surface, in which radially arranging acicular on surface to form a “pincushion” morphology (Fig. 2). Based on transmission electron microscopy (TEM) pattern, the “fluff” on the particle surface with about 25-nm size can be clearly seen.
As shown in the FTIR spectra of FeOS, the sharp peak at 1,630.80 cm−1 in the curve could be attributed to the adsorbed water within the sample (Fig. 3). Three dashed lines in the infrared spectra indicated the typical absorption bands of SO4 2− at 1,123.62 cm−1 (ν3(SO4)), 980.85 cm−1 (ν1(SO4)), 614.20 cm−1 (ν4(SO4)), and three significant absorption bands of OH deformations were observed at 699.61 cm−1, 838.08 cm−1, and 3,286.04 cm−1 according to the previous literatures (Boily et al. 2010; Paikaray et al. 2011; Regenspurg et al. 2004).
The point of zero charge pH (pHpzc) of FeOS was 5.1 via measuring its zeta potential at various pH values (Fig. 4). This result was similar with the result obtained by Liao et al. (2011). Therefore, the surface of the FeOS was negatively charged at pH > 5.1.
Effect of FeOS on soil pH
As shown in Fig. 5, with increasing of FeOS from 1 to 10 %, the soil pH decreased from about 7.5 to 6.5. It was reported that soil pH decreased from the initial value of 7.8 down to 6.4 when ferrous sulfate was added into soil at a rate of 1.5 % (w/w) (Xenidis et al. 2010). Although FeOS application slightly decreased soil pH, this effect was less than that of ferrous sulfate amendment. The results showed that the pH of FeOS-amended soil maintained a relatively high value even at 10 % of FeOS addition. This can be explained that FeOS has a high point of zero charge pH (pHpzc).
Effect of FeOS/soil mass ratio on As immobilization
Figure 6 shows the immobilization efficiency of water-soluble and NaHCO3-extractable As in soils at different mass ratios of FeOS/soil (w/w). It can be seen that the immobilization efficiency of water-soluble and NaHCO3-extractable As increased with increasing FeOS amount. The immobilization percentages of water-soluble and NaHCO3-extractable arsenic increased from 53.4 to 99.8 % and from 13.8 to 73.3 %, respectively, when mass ratio of FeOS/soil increased from 1 to 10 %. When FeOS addition exceeded more than 4 %, the immobilization percentage of water-soluble As was over 95 %. Regarding NaHCO3-extractable As, it immobilization percentage reached up to 50.5 % when the addition of FeOS was over 4 %. Thereafter, As immobilization percentage tended to be stable and reached up to 67 % with 6 % of FeOS addition. In this study, it was found that water-soluble As was subjected to immobilization in soils than NaHCO3-extractable form. This phenomenon could be explained by that water-soluble arsenic was free form in soils.
Due to pH of the contaminated soil was higher than 5.1, the FeOS were negatively charged, which was not conducive to arsenate (arsenite) adsorption. However, according to its good efficiency of As immobilization, the adsorption of arsenate or arsenite occurred in the surface of the FeOS was not electrostatic adsorption since As immobilization was effective. It has been reported that the mechanisms of arsenic removal by Fe-oxyhydroxy mineral may include two adsorption processes, which were surface complexation with iron hydroxyl groups on the mineral surface and exchange interaction with sulfate complexes present in the mineral structure (Antelo et al. 2012; Burton et al. 2009; Guo et al. 2007). The immobilization efficiency of water-soluble and NaHCO3-extractable depended on Fe addition amount in soils. When iron reached 6 %, the efficiency of As immobilization remained unchanged. These results were consistent with several previous research results (Moore et al. 2000; Subacz et al. 2007).
Effect of reaction time on As immobilization
The effects of reaction time on the immobilization percentage of water-soluble and NaHCO3-extractable As are shown in Fig. 7. When the dose of FeOS exceeded 4 %, no significant change of water-extractable As immobilization efficiency was observed in the period of 7 to 60 days (Fig. 1a). However, with 1 % of FeOS addition, the immobilization percentage of water-soluble As gradually increased from 27.2 to 45.4 %, and eventually maintained stable after immobilized for 40 days. Similar trend was observed at 2 % FeOS addition except for the maximum immobilization percentage of water-soluble As (80.4 %) after 28 days. Further, the time of reaction equilibrium for As immobilization at 1 % of FeOS addition was longer than that at 2 % of FeOS addition.
The immobilization efficiencies of NaHCO3-extractable As in soils at 4, 6, and 8 % of FeOS addition were 52.6, 56, and 72.5 %, respectively, after 21 days. Thereafter, little change of NaHCO3-extractable As immobilization was observed. However, with 1 and 2 % of FeOS addition, the immobilization efficiency of NaHCO3-extractable As steadily increased until 40 days. It was observed that the time for water-soluble As immobilization was shorter than that for NaHCO3-extractable As immobilization under the same FeOS dosage. Meanwhile, more FeOS addition caused less time for both water-soluble and NaHCO3-extractable As immobilization. This phenomenon could be ascribed to that an increase in the amount of the FeOS increased the contact sites between As and FeOS.
Effect of soil moisture on As immobilization
Soil moisture can promote complete mixture between FeOS and soil and provide reaction site for metals bonding onto FeOS. In practical application, excessive water will lead to waste of water resources, as well as bring pernicious elements leaching into non-contaminated soil or ground water area, causing secondary pollution of the environment.
In order to investigate the effect of soil moisture on As immobilization, a series of immobilization tests were carried out at the soil moisture varying from 30 to 120 % of the water-holding capacity when the ratio of FeOS-to-soil maintained at 4 %. Under different soil moisture conditions, no significant fluctuation was observed on water-soluble As immobilization (Fig. 8a). The immobilization efficiency of water-soluble As maintained above 97.5 %, as soil moisture varied from 60 to 80 %. Negligible effect on water-soluble As immobilization may be due to minor amounts of the water-soluble As present in the soil. However, a significant effect was found on NaHCO3-extractable arsenic immobilization by soils moisture. With increasing soil moisture, the immobilization percentage of NaHCO3-extractable As in soil presented an increase trend and reached the highest value at 80 % of water-holding capacity. Thereafter, immobilization percentage of NaHCO3-extractable As declined again when soil moisture was higher than 100 %. The date clearly demonstrated that when soil moisture was less than 70 %, the immobilization percentage of NaHCO3-extractable As was lower than 35 %. This phenomenon could be explained as that little moisture is not conducive for As to migrate onto FeOS.
Sequential chemical extraction of As fractionation
In order to better evaluate As stabilization in soils, the sequential chemical extraction procedure was employed for As fractionation. As concentration in each extraction step was converted to the percentage of the sum amount extracted by all steps. As shown in Fig. 9, after FeOS application, non-specifically adsorbed and specifically adsorbed As fractions distinctly reduced. Compared to the untreated soils, the percentage of the non-specifically adsorbed and specifically adsorbed As fractions in FeOS-treated soils decreased from 3.64 to 0.09 % and 17.69 to 11.58 %, respectively.
A significant increase in As percentage was observed in the poorly crystalline hydrous Fe and Al oxides-bonded fraction. For instance, As percentage in the above fraction increased from 68.34 to 76.24 % after FeOS addition. There were no pronounced change for As percentage between crystalline hydrous Fe and Al oxides-bonded fraction and residue fraction. The results indicated that a significant amount of As were associated with amorphous or poorly crystalline iron oxides after FeOS treatment, resulting in the decrease of NaHCO3-extractable As.
As release in FeOS-treated soil under leaching with simulated acid rain
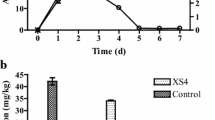
The concentration of As in leachate with simulated acid rain leaching is shown in Fig. 10a. After leaching for 12 times of pore volumes, As concentrations in leachates of FeOS-treated soil reached the maximum of 0.4 mg/l. For the untreated soil, the higher As concentration (21.7 mg/l) in leachate was observed at 14 times of pore volumes. After leaching at approximate 35 times of pore volumes, the release of As reached a steady-state with As concentrations of 3 mg/l for untreated soil and 0.03 mg/l for FeOS-treated soil. This tendency of As release was consistent with several previous research results (Chiang et al. 2010; Ko et al. 2012). Figure 10b shows the accumulation of As concentrations in leachates after leaching with simulated acid rain at 60 times pore volumes. Accumulated amount of As release in leachates of untreated soil and FeOS-amended soil were 98.4 and 1.2 mg, respectively, which correspond to 7.69 and 0.09 % of total As amounts in soil.
Conclusions
A poorly crystalline Fe-oxyhydroxy sulfate was prepared in this study. The FeOS was a ~500-nm spheroids with about 25-nm “microvillus”. Because of its good efficiency of As immobilization and slightly influence on soil acidity, FeOS can be used as an As immobilizer in soils.
Arsenic immobilization in soils was affected by FeOS dosage and reaction time. The immobilization percentages of water-soluble and NaHCO3-extractable arsenic in soil increased with increasing FeOS dosage. The highest immobilization efficiencies for water-soluble and NaHCO3-extractable arsenic were 99.8 and 73.3 %, respectively. More FeOS addition led to less time for both water-soluble and NaHCO3-extractable As immobilization. The effect of soil moisture on water-soluble As immobilization was not obvious. However, there was considerable influence of soil moisture on NaHCO3-extractable As and the optimal soil moisture was 80 % of field holding capacity. As immobilization in soils contributed to As association with poorly crystalline iron oxides after FeOS treatment, resulting in a decrease of bioavailable As. Under leaching with simulated acid rain, soil amended with FeOS was resistant to As release as compared to untreated soil. The results suggest that FeOS could be used as a potential effective As fixative in soils.
References
Antelo J, Fiol S, Gondar D, López R, Arce F (2012) Comparison of arsenate, chromate and molybdate binding on schwertmannite: surface adsorption vs anion-exchange. J Colloid Interface Sci 386:338–343
Ascher J, Ceccherini MT, Landi L, Mench M, Pietramellara G, Nannipieri P, Renella G (2009) Composition, biomass and activity of microflora, and leaf yields and foliar elemental concentrations of lettuce, after in situ stabilization of an arsenic-contaminated soil. Appl Soil Ecol 41:351–359
Blgham JM, Schwertmann U, Carlson L, Murad E (1990) A poorly crystallized oxyhydroxysulfate of iron formed by bacterial oxidation of Fe(II) in acid mine waters. Geochim Cosmochim Acta 54:2743–2758
Boily JF, Gassman PL, Peretyazhko T, Szanyi J, Zachara JM (2010) FTIR spectral components of schwertmannite. Environ Sci Technol 44:1185–1190
Burton ED, Bush RT, Johnston SG, Watling KM, Hocking RK, Sullivan LA, Parker GK (2009) Sorption of arsenic(V) and arsenic(III) to schwertmannite. Environ Sci Technol 43:9202–9207
Carbonell-Barrachina AA, Rocamora A, García-Gomis C, Martínez-Sánchez F, Burló F (2004) Arsenic and zinc biogeochemistry in pyrite mine waste from the Aznalcóllar environmental disaster. Geoderma 122:195–203
Chiang KY, Lin KC, Lin SC, Chang TK, Wang MK (2010) Arsenic and lead (beudantite) contamination of agricultural rice soils in the Guandu Plain of northern Taiwan. J Hazard Mater 181:1066–1071
García-Sanchez A, Alvarez-Ayuso E, Rodriguez-Martin F (2002) Sorption of As(V) by some oxyhydroxides and clay minerals. Application to its immobilization in two polluted mining soils. Clay Miner 37:187–194
Guo X, Du Y, Chen F, Park HS, Xie Y (2007) Mechanism of removal of arsenic by bead cellulose loaded with iron oxyhydroxide (β-FeOOH): EXAFS study. J Colloid Interface Sci 314:427–433
Hartley W, Edwards R, Lepp NW (2004) Arsenic and heavy metal mobility in iron oxide-amended contaminated soils as evaluated by short- and long-term leaching tests. Environ Pollut 131:495–504
Jomova K et al (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107
Jönsson J, Persson P, Sjöberg S, Lövgren L (2005) Schwertmannite precipitated from acid mine drainage: phase transformation, sulphate release and surface properties. Appl Geochem 20:179–191
Kim JY, Davis AP, Kim KW (2003) Stabilization of available arsenic in highly contaminated mine tailings using iron. Environ Sci Technol 37:189–195
Kim KR, Lee BT, Kim KW (2012) Arsenic stabilization in mine tailings using nano-sized magnetite and zero valent iron with the enhancement of mobility by surface coating. J Geochem Explor 113:124–129
Ko MS, Kim JY, Bang S, Lee JS, Ko JI, Kim KW (2012) Stabilization of the As-contaminated soil from the metal mining areas in Korea. Environ Geochem Health 34:143–149
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides—a review. Environ Pollut 172:9–22
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manag 28:215–225
Kumpiene J, Fitts JP, Mench M (2012) Arsenic fractionation in mine spoils 10 years after aided phytostabilization. Environ Pollut 166:82–88
Lee JE, Kim Y (2008) A quantitative estimation of the factors affecting pH changes using simple geochemical data from acid mine drainage. Environ Geol 55:65–75
Liao Y, Liang J, Zhou L (2011) Adsorptive removal of As(III) by biogenic schwertmannite from simulated As-contaminated groundwater. Chemosphere 83:295–301
Mench M, Vangronsveld J, Clijsters H, Lepp NW, Edwards R (1999) In situ metal immobilization and phytostabilization of contaminated soils. In: Phytoremediation of contaminated soil and water. CRC Press, Boca Raton, p 323
Moore TJ, Rightmire CM, Vempati RK (2000) Ferrous iron treatment of soils contaminated with arsenic-containing wood-preserving solution. Soil Sediment Contam 9:375–405
Ng JC, Wang J, Shraim A (2003) A global health problem caused by arsenic from natural sources. Chemosphere 52:1353–1359
Nielsen SS, Petersen LR, Kjeldsen P, Jakobsen R (2011) Amendment of arsenic and chromium polluted soil from wood preservation by iron residues from water treatment. Chemosphere 84:383–389
Paikaray S, Göttlicher J, Peiffer S (2011) Removal of As(III) from acidic waters using schwertmannite: surface speciation and effect of synthesis pathway. Chem Geol 283:134–142
Regenspurg S, Brand A, Peiffer S (2004) Formation and stability of schwertmannite in acidic mining lakes. Geochim Cosmochim Acta 68:1185–1197
Subacz JL, Barnett MO, Jardine PM, Stewart MA (2007) Decreasing arsenic bioaccessibility/bioavailability in soils with iron amendments. J Environ Sci Health A 42:1317–1329
Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323
Xenidis A, Stouraiti C, Papassiopi N (2010) Stabilization of Pb and As in soils by applying combined treatment with phosphates and ferrous iron. J Hazard Mater 177:929–937
Zhu YG, Williams PN, Meharg AA (2008) Exposure to inorganic arsenic from rice: a global health issue? Environ Pollut 154:169–171
Acknowledgments
The authors gratefully acknowledge National Natural Science Foundation of China (51304251) and the Key Project of Science and Technology of Hunan Province, China (2012FJ1010), for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Yang, Z., Liu, L., Chai, L. et al. Arsenic immobilization in the contaminated soil using poorly crystalline Fe-oxyhydroxy sulfate. Environ Sci Pollut Res 22, 12624–12632 (2015). https://doi.org/10.1007/s11356-015-4455-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4455-1