Abstract
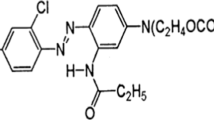
A significant proportion of xenobiotic recalcitrant azo dyes are being released in environment during carpet dyeing. The bacterial strain Stenotrophomonas sp. BHUSSp X2 was isolated from dye contaminated soil of carpet industry, Bhadohi, India. The isolated bacterial strain was identified morphologically, biochemically, and on the basis of 16S rRNA gene sequence. The isolate decolorized 97 % of C.I. Acid Red 1 (Acid RED G) at the concentration of 200 mg/l within 6 h under optimum static conditions (temperature −35 °C, pH 8, and initial cell concentration 7 × 107 cell/ml). Drastic reduction in dye degradation rate was observed beyond initial dye concentration from 500 mg/l (90 %), and it reaches to 25 % at 1000 mg/l under same set of conditions. The analysis related to decolorization and degradation was done using UV-Vis spectrophotometer, HPLC, and FTIR, whereas the GC-MS technique was utilized for the identification of degradation products. Phytotoxicity analysis revealed that degradation products are less toxic as compared to the original dye.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The effluents discharged by industries such as carpet, textile, leather, pharmaceuticals, food, cosmetics, and printing contain azo dye (10–15 %) (Forgacs et al. 2004; Vimonses et al. 2010; Robinson et al. 2001), which are considered to be the most hazardous among xenobiotics. The Acid Red G is one of the popular azo dyes that has been extensively used for the dyeing wool, nylon, and silk fibers (Ozcan et al. 2004; Vimonse et al. 2010). Azo dyes are being recalcitrant and toxic in nature which create havoc to flora and fauna. The discharge of these effluents into nearby water bodies leads to the reduction in sunlight penetration and dissolved oxygen content of waterways; hence, increase in biological oxygen demand and chemical oxygen demand adversely affect the water quality (Jonstrup et al. 2011; Meng et al. 2012). It is also well documented that synthetic dyes and their metabolites are toxic, carcinogenic, and mutagenic in nature (De Aragao Umbuzeiro et al. 2005; Tan et al. 2005). To overcome these problems, effluent standard has to be achieved prior to disposal to surface bodies. There are various physical and chemical methods for treatment such as adsorption, photoionization, electrolysis, oxidation, and neutralization that have limitations like high cost, low efficiency, and production of secondary toxic intermediates (Asad et al. 2007; Zainal et al. 2005; Harrelkas et al. 2008; Asad et al. 2007). Due to cost effectiveness and ecological compatibility, bioremediation of such effluents is most promising technology (Baban et al. 2010; Spagni et al. 2010; Kolekar et al. 2012).
Bhadohi (Uttar Pradesh) carpet cluster of India is one of the oldest clusters in the world, continuously working from the 16th century onward. There are number of dying plants; majority of them are discharging their effluent only after lime treatment. The ground and surface water of the region are badly affected. There is enough literature reported pertaining to dye effluent treatment for textile industries, but very few of them are adding the need of carpet clusters. Few articles available for this cluster have used only fungi. Literally, there is no literature for the treatment of carpet dye effluent using indigenous bacteria. This study is an attempt to identify indigenous bacteria to treat these effluents at lower cost.
The present paper deals with the isolation and identification of bacterial strain BHUSSp X2 and its subsequent application for the bioremediation of carpet dyeing effluent of carpet industries (Bhadohi) Uttar Pradesh, India. Contaminated soil sample from the site was used for isolation of bacterial strain, and their identification was done using 16S rRNA gene sequence technique. Important operating parameters were optimized through aerobic degradation in batch mode. Relative toxicity of degraded products was investigated through phototoxicity analysis of degraded product. GC-MS studies were performed for the detection of biotransformation pathway of RED G dye. The enzyme responsible for azo dye degradation was also identified.
Materials and methods
Media and chemicals
Commercial grade Acid Red G was procured from Local Carpet Industries, Bhadohi (Uttar Pradesh), India. Nutrient broth, nutrient agar, and mineral salt media (MSM media) were obtained from Hi-Media, India. All the chemicals used were of analytical grade. The seed Vigna mungo used for phytoxicity studies was obtained from local market.
Isolation and screening of dye degrading bacteria
Soil and effluents were collected from the carpet dyeing industries located in Bhadohi, India. One hundred milliliters of 1 % soil solution was incubated with 500-mg/l RED G dye in nutrient broth maintained at 35 ± 2 °C for 1 week under static and shaking conditions. The resultant broth sample was streaked on nutrient agar plate having 500-mg/l RED G dye, and this was again incubated at 35 ± 2 °C for a week. Morphologically distinct bacteria were isolated from this agar plate and were again streaked on fresh nutrient agar plate for isolation of pure bacteria. The most promising isolates selected in this manner were tested for decolorization of dye in submerged condition. The dye degrading bacteria were identified on the basis of morphological colony; Gram staining and biochemical test of isolated strain were performed according to the Bergey’s manual and on the basis of 16S rRNA gene sequence analysis. The sequence was compared using BLAST programmed at NCBI server to identify bacteria. The 16S rRNA sequence of isolated bacterial strain and related sequences of NCBI were aligned using Cluster W, and phylogenetic tree was made using neighbor-joining methods of MEGA (Version 6).
Dye decolorization studies
The decolorization experiments were carried out in 250-ml Erlenmeyer flasks containing 100-ml nutrient broth supplemented with RED G dye (100 mg/l). The media were inoculated with respective bacterial strains by addition of inoculums with uniform cell density (O.D. 0.5). Decolorization studies were carried out under static and shaking conditions in 100-ml nutrient broth having dye concentration 200 mg/l dye. All the flasks were incubated under static and shaking (100 rpm) conditions at 35 ± 2 °C for 24 h. Samples were periodically withdrawn after every 2 h, centrifuged (10,000 rpm) to estimate the extent of decolorization using UV-Vis spectrophotometer. Important operating parameters affecting decolorization such as pH (5–10), temperature (20–45 °C), initial dye concentration (100–1000 mg/l), carbon and nitrogen sources, and inoculum size (1, 2, 3, 4, 5, 6, 7, 8, 10 %) were optimized. These investigations were also used for control.
Investigation on capability of isolated microbe for repeated use
A repeated batch of 100-mg/l Red G dye was repeatedly treated at 35 °C under static conditions by using bacterial stain BHUSSp X2. The process was repeated for ten times.
Preparation of cell-free extract and enzyme assays
The isolated bacterial strain BHUSSp X2 was incubated with Red G (200 mg/l) and without dye for 24 h. The resultant biomass was collected by centrifuging the respective broths at 8000 rpm and 4 °C for 15 min. The resultant cell pellets were sonicated using utrasonication probe in 50-mM sodium phosphate buffer (pH 7.4) at 4 °C, with 12 strokes of 30 s, for 1-min intervals each. The sonicated cells were centrifuged, and supernatant was used as the source of intracellular and extracellular enzymes. The enzyme activities were assayed spectrophotometrically for cell-free extracts with and without sonication at room temperature. One unit of enzyme activity is the amount of enzyme required to convert 1 μmol of substrate per min. Azoreductase assays were performed by taking 200 μl of 2 mM NADH, 100-mM sodium phosphate buffer (7.4), 150-μM substrate concentration, and 100-μl enzyme solution. The change in color intensity was monitored by UV-Visible spectrophotometer at 500 nm. The activity of NADH-DCIP reductase was determined by processes reported earlier by Lade et al. (2012). NADH-DCIP reductase activity that was analysis of 2.0-ml reaction mixture was assayed at 500 nm by adding 250 mM NADH, 5-ml reaction mixture containing 25 mM 2,6dichloro-phenol indophenol (DCIP) and 200-μl enzyme solution in potassium phosphate buffer (50 mM, pH 7.4). Tyrosinase activity was measured as described elsewhere (Duckworth and Coleman 1970). Laccase and lignin peroxidase activities were assayed using spectrophotometer in the cell-free extract. Laccase activity, measured as an increase in optical density at 420 nm, was determined in 2-ml mixture containing ABTS (10 %) in 0.1 M acetate buffer pH 4.9 (Hatvani and Mecs 2001; Saratale et al. 2009; Kalyani et al. 2008). Lignin peroxidase (LP) activity was determined by monitoring the propanaldehyde formed at 300 nm in a reaction mixture of 2.5 ml containing 100 mM n-propanol, 250 mM tartaric acid, and 10 mM H2O (Telke et al. 2009). All enzyme assays were carried out at room temperature. All enzyme assays were run in triplicate, and the average activity was calculated.
Analytical investigation
The UV-Vis spectrophotometer (Systronics 2202) was used to determine the extent of decolorization. The degraded metabolites were extracted with equal volumes of ethyl acetate, and this was evaporated to dryness in rotatory evaporator. The extracted metabolites were mixed with HPLC grade potassium bromide (KBr) in the ratio of 5:95 and analyzed at mid IR region (400–4000 cm−1) by using FTIR Perkin Elmer, Spectrophotometer (Perkin Elmer, USA). The intermediates produced after degradation were analyzed by GC-MS.
Results and discussion
Isolation, screening, and identification of bacterial strain
The microorganisms were isolated from the soil sample collected from effluent discharged site of carpet cluster industries as these were well acclimatized with the system having better potential for degradation of dyes. The bacterial strain BHUSSp X2 showed remarkable decolorizing ability for RED G dye, among various strains isolated. The bacterial identification was based on Bergey’s methods employed, and to finalize the species using isolated bacterial strain 16S rRNA, gene sequence analysis was used. The Stenotrophomonas sp. BHUSSp X2 (Fig. 1) thus identified was used for decolorization in present investigation. The isolated gene sequence was deposited in the GeneBank of NCBI having accession number (KJ740220), which has till date not been utilized for decolorization of dye.
Optimization of important operating parameters
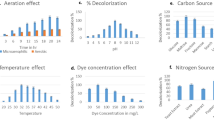
As shown in Fig. 2, 95 % of decolorization was achieved at static condition as compared to 45 % decolorization under shaking condition at 100 rpm. This trend is reversed to the growth of bacterial strain. This observation is well supported by Moosui et al. (2005), Khehra et al. (2006), and Oturkar et al. (2011). This is possibly due to reduced activity of azoreductase with increase dissolved oxygen level under shaking condition whereas at static condition azo, dye itself acts as an electron carrier resulting into increase decolorization efficiency (Pearce et al. 2003). So, optimization of other important operating parameters for dye degradation static conditions was preferred.
The effluent being discharged from a dying plants of carpet industries cluster generally have basic pH (7–10), and this has been validated during the testing of effluent of Bhadohi carpet cluster. Figure 3 shows the effect of pH on decolorization. It was observed from the figure that minimum decolorization achieved at pH 5 was below 23 % whereas when the pH was 7 and 8, decolorization efficiency improved drastically, and more than 95 % decolorization have been achieved. With further increase in pH 9 and 10, efficiency of decolorization again reduced, and only 30 % decolorization was observed. This might be due to the conducing environment of growth of bacterial strain in alkaline environment. However, further increase in pH was again nonfavorable for the system due to the prevailing condition.
Temperature plays a vital role in growth and performance of bacterial strain. So, the performance of BHUSSp X2 was evaluated in the temperature range of 20–45 °C for decolorization as shown in Fig. 4. It is clear from this figure that only 33 % decolorization was obtained at 20 °C. However, with increases in temperature, decolorization efficiency of bacterial strain was improved, and 97 % decolorization was observed at 35 °C. With further increase in temperature, decolorization efficiency started declining resulting only 55 % decolorization at 45 °C. The reduced decolorization efficiency at 20 and 45 °C are probably due to deactivation of enzyme at these extreme conditions. But, further increase in temperature showed adverse impact on decolorization as even less than 55 % decolorization could be achieved at 45 °C. The decolorization rate depends on temperature because at very low and high temperature, deactivation of enzymes takes place.
At lower initial dye concentrations of 100–200 mg/l, more than 95 % decolorization have been achieved within 6 h in Fig. 5. With further increase in dye concentration (300–500 mg/l), reduced and delayed decolorization was observed, and maximum decolorization of 90 % could be achieved after 18 h. Beyond 700 mg/l, the bacterial strain seems to be ineffective as maximum of 30 % decolorization only be achieved even after 24 h. This decrease in decolorization at higher dye concentration can possibly be attributed to lower growth and higher toxicity due to accumulation of large quantity of metabolites.
The nutrient plays a vital role in dye degradation as they control the growth of bacterial strain. The impact of various carbon and nitrogen sources on dye degradation was investigated and summarized in Table 1. Efficacy of agricultural residues such as wheat bran, rice husk, and baggage was also investigated as carbon sources. It can clearly be seen from Table 1 that addition of either one carbon or one nitrogen sources has minimal effect on decolorization with exception of yeast as nitrogen source which rendered 80 % decolorization with 10 h even without any carbon source. The combination of yeast and glucose as nitrogen and carbon sources resulted into 90 % decolorization within 8 h closely followed by ammonium chloride and glucose as carbon source resulting into 82 % decolorization in 12 h, whereas the nutrient broth comprising multiple nitrogen sources and single carbon have rendered 98 % decolorization within 6 h. Agriculture residue such as wheat bran and baggage proved to be worthy of replacing the combination of carbon and nitrogen sources as 85 and 72 % decolorization could be achieved within 8 and 10 h, respectively.
Repeated applicability test of isolated bacteria
The decolorization efficiency of isolated bacterial strain for RED G (200 mg/l) under static condition was measured for ten cycles. Fresh addition of dye was made for every cycle. The result thus obtained shown in Fig. 6. It is obvious from figure that decolorization efficiency decreases with increase in cycle as only 25 % decolorization was observed in 48 h for tenth cycle whereas for first two cycles, more than 95 % decolorization was achieved only in 6 h. The decrease decolorization efficiency on repeated use of isolated bacterial strain could be attributed to reduced ability of nutrient leading to stationary phase and subsequently to death phase (Kolekar et al. 2013).
Enzyme analysis
Enzyme and their activities prior and after decolorization are summarized in Table 2, and values presented are mean of three replicates. Increased amount of enzymes such as laccase, lignin peroxidase, tyrosinase, azoreductase, and NADH reductase in the degraded product suggest their prominent role in the decolorization process. It also suggests that extracellular and intracellular enzyme activities are induced in the presence of dye. Azoreductase seems to be most prominent enzyme in decolorization using Stenotrophomonas sp. BHUSSp X2 followed by laccase and NADH as significant increase in their amount has been observed as compared to control. The relative contribution of these enzymes may vary with the change in bacterial strain. The decolorization observed in the enzymes in present study such as azoredustase, laccase, NADH, tyrosinase, and ligin peroxidase was, respectively, 500, 103, 97, 60, and 17 % reflecting their relative importance. These findings are well supported by Vijaykumar et al. (2007) and Jadhav et al. (2010).
Analytical investigations
UV-Visible spectrometric analysis was made in the range (200–800 nm) to investigate decolorization for the dye samples prior to and after degradation as shown (Fig. S1). Complete disappearance of peak in the visible region after 6 h was observed for bacterially degraded sample, confirming the efficacy of isolated strain BHUSSp X2.
The results of analytical investigation by HPLC (Water HPLC, Model no. 2690) are presented in Fig. S2 (Prior to degradation) and S3 (after degradation). The retention time for one peak observed in case of dye sample before degradation as shown in Fig. S1 was 6 min whereas two peaks were observed at retention times 3.99 and 5.1 min, respectively, for bacterially degraded sample (Fig. S3). These suggest degradation or fragmentation of the compound present in the dye sample.
FTIR spectra of control and bacterial degraded samples are shown in supplementary Figs. S4 and S5. The complete absence of certain peaks and shifting of others in the FTIR spectra of dye degraded sample indicate complete elimination of certain component and degradation of others. The presence of characteristic of peaks 3451.23 and 1597.26 cm–1 indicates the presence of N-H stretching and azo group, respectively, in dye sample prior to degradation (Fig. S4). The absence of peak at 1597.26 cm–1 and shifting of peak 3451.29 to 3414 cm–1 represent complete degradation of azo group and change of N-H stretching to O-H stretching, respectively. The other prominent peaks present in the spectra as shown in Fig. S4 for dye sample before degradation or 1496.81 and 1620.26 cm–1 confirming the presence of aromatic nitro compound, whereas the peaks at 2964.26, 1668, 1411.09, 1319.21, 1151.54, and 839 cm–1 confirm the presence of C-H stretching of CH3 group, aromatic amines, O-H deformation, –SO stretching, and sulfonated groups, respectively. The absence of peaks at 1319.21 21 and 1151.54 cm–1 for FTIR spectra of degraded sample represent the removal of azo bond and sulfonated group; this finding is aligned to Waghmode et al. (2012) and Senan and Abraham (2004), confirming microbial degradation.
Degradation pathway
To understand the degradation pathway, GC-MS analysis of dye samples prior to and after degradation was carried out and presented in Fig. 7. The presence of various groups in the GC-MS spectra of dye sample before degradation confirms the characteristics of ACID RED 1 dye, whereas GC-MS spectra of degraded samples confirms the presence of aniline (M.W. 93 peak at 93 m/z), benzene with M.W. 78 m/z 74, sodium7-amino-6-hydroxynapthalene-2-sulfonate (M.W. 261, m/z 263), 3-hydroxyphthalic acid (M.W. 182, m/z 184), and pyrocatechol (M.W.110, m/z 110). The pathway presented in the present paper suggests that possibly bacterial strain has fragmented the dye initially into aniline and unknown intermediates due to presence of azoreductase enzyme. This is possibly due to the breakage of azo bond. Aniline further breaks into benzene and the unknown intermediate into sodium7-amino-6-hydroxynapthalene-2 possibly through desulphonation and deamination which finally converts to pyrocatechol. Dehydroxylation and decarboxylation in addition to desulphonation and deamination were potential degradation mechanism. The fractions of benzene and pyrocatechol possibly have been mineralizing due to ring fission and then TCA cycle.
Phytotoxicty study
The investigations were made to estimate the phytotoxicity of dye effluents prior to and after degradation. The germination characteristics of gram seeds in pure water, effluents, and extracted metabolites were observed and summarized in Table 3. Nearly similar germination characteristics were observed in case of water and extracted metabolites whereas only 30 % germination where observed in case of effluent confirming reduced/nontoxicity of treated sample. The degraded sample has not only possess the noninhibitory effect but also resultant into the improved stem length reflecting availability of additional nutrients as compared to pure water. Reduction in root length as compared to pure water sample in case of degraded metabolites also confirms the easy availability of nutrient in case of degraded metabolites.
Conclusion
BHUSSp X2 microbial strain was isolated from contaminated sites of carpet cluster Bhadhoi. The bacteria were used for the decolorization of effluent from dying plants of the cluster in Uttar Pradesh, India. Upto 97 % decolorization could be achieved at pH 8, temperature 35 °C under static condition, and initial concentration of 200 mg/l. The UV-Visible spectrophotometer, HPLC, FTIR, and GC-MS investigation confirms the microbial degradation as potential mechanism of color removal. GC-MS analysis also rivals that final degradation products were benzene and pyrocatechol due to azo bond cleavage due to microbial action. Phytotoxicity investigation also confirms the noninhibitory effects of degraded metabolites. So, it can be concluded that BHUSSp X2 is having good potential for the treatment of azo dye being discharged from carpet/textile industries. It was also suggested that degraded metabolites were nontoxic in nature and not harmful to the environment. The attempt will be made to transfer the technology after continuous experiments in suspended as well attached culture mode for decolorization of dye.
References
Asad MA, Amoozegar AA, Pourbabaee MN, Sarbolouki SMM, Dastgheib (2007) Decolorization of textile azo dyes by newly isolated halophilic and halotolerant bacteria. Bioresour Technol 98:2082–2088
Baban A, Yediler A, Avaz G, Hostede SS (2010) Biological and oxidative treatment of cotton textile dye-bath effluents by fixed and fluidized bed reactors. Bioresour Technol 101:1147–1152
de Aragao Umbuzeiro G, Freeman HS, Warren SH, de Oliveira DP, Terao Y, Watanabe T, Claxton LD (2005) The contribution of azo dyes to the mutagenic activity of the Cristais River. Chemosphere 60:55–64
Duckworth HW, Coleman JE (1970) Physicochemical and kinetic properties of mushroom tyrosinase. J Biol Chem 245:1613–1625
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Harrelkas F, Paulo A, Alves MME, Khadir L, Zahraa O, Pons MN, van der Zee FP (2008) Photocatalytic and combined anaerobicphotocatalytic treatment of textile dyes. Chemosphere 72(11):1816–1822
Hatvani N, Mecs I (2001) Production of laccase and manganese peroxidase by Lentinus edodes on malt containing by product of the brewing process. Process Biochem 37:491–496
Jadhav JP, Phugare SS, Dhanve RS, Jadhav SB (2010) Rapid biodegradation and decolorization of Direct Orange 39 (Orange TGLL) by an isolated bacterium Pseudomonas aeruginosa strain BCH. Biodegradation 21:453–463
Jonstrup M, Kumar N, Murto M, Mattiasson B (2011) Sequential anaerobic–aerobic treatment of azo dyes: decolourisation and amine degradability. Desalination 280:339–346
Kalyani DC, Patil PS, Jadhav JP, Govindwar SP (2008) Biodegradation of reactive textile dye Red BLI by an isolated bacterium Pseudomonas sp. SUK1. Bioresour Technol 99:4635-4641
Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2006) Biodegradation of azo dye C.I. Acid Red 88 by an anoxic-aerobic sequential bioreactor. Dyes Pigments 70:1–7
Kolekar MY, Nemade NH, Markad LV, Adav SS, Patole SM, Kodam MK (2012) Decolorization and biodegradation of azo dye, reactive blue 59 by aerobic granules. Bioresour Technol 104:818–822
Kolekar YM, Konde PD, Markad VL, Kulkarni SV, Chaudhari AU, Kodam KM (2013) Effective bioremoval and detoxification of textile dye mixture by Alishewanella sp. KMK6. Appl Microbio Biotechnol 97:881–889
Lade HS, Tatoba RW, Avinash AK, Sanjay PG (2012) Enhanced biodegradation and detoxification of disperse azo dye Rubine GFL and textile industry effluent by defined fungal-bacterial consortium. Int Biodeterior Biodegradation 72:94–107
Meng X, Liu G, Zhou J, Fu QS, Wang G (2012) Azo dye decolorization by Shewanella aquimarina under saline conditions. Bioresour Technol 114:95–101
Moosvi S, Keharia H, Madamwar D (2005) Decolourization of textile dye Reactive Violet 5 by a newly isolated bacterial consortium RVM 11.1. World J Microb Biot 21:667–672
Oturkar CC, Nemade HN, Mulik PM, Patole MS, Hawaldar RR, Gawai KR (2011) Mechanistic investigation of decolorization and degradation of reactive red 120 by Bacillus lentus BI377. Bioresour Technol 102:758–764
Ozcan AS, Erdem B, Ozcan A (2004) Adsorption of Acid Blue 193 from aqueous solutions onto Na-bentonite and DTMA-bentonite. J Colloid Interface Sci 280(1):44–54
Pearce CI, Lloyd JR, Guthrie JT (2003) The removal of color from textile wastewater using whole bacterial cells: a review. Dyes Pigments 58:179–196
Robinson T, McMullan G, Marchant G, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Saratale RG, Saratale GD, Kalyani DC, Chang JS, Govindwar SP (2009) Enhanced decolorization and biodegradation of textile azo dye Scarlet R by using developed microbial consortium-GR. Bioresoure Technol 100:2493–2500
Senan RC, Abraham TE (2004) Bioremediation of textile azo dyes by aerobic bacterial consortium. Biodegradation 15(4):275–280
Spagni A, Casu S, Crispino NA, Farina R, Mattioli D (2010) Filterability in a submerged anaerobic membrane bioreactor. Desalination 250:787–792
Tan NC, van Leeuwen A, van Voorthuizen EM, Slenders P, Prenafeta-Boldu FX, Temmink H, Lettinga G, Field JA (2005) Fate and biodegradability of sulfonated aromatic amines. Biodegradation 16(6):527–537
Telke AA, Kalyani DC, Dawkar VV, Govindwar SP (2009) Influence of organic and inorganic compounds on oxidoreductive decolorization of sulfonated azo dye C.I. Reactive Orange 16. J Hazard Mater 172(1):298–309
Vijaykumar MH, Vaishampayan PA, Shouche YS, Karegoudar TB (2007) Decolourization of naphthalene-containing sulfonated azo dyes by Kerstersia sp. strain VKY1. Enz Microb Tech 40:204–211
Vimonses V, Jin B, Chow CW, Saint C (2010) An adsorption-photocatalysis hybrid process using multi-functional-nanoporous materials for wastewater reclamation. Water Res 44:5385–5397
Waghmode TR, Kurade MB, Kabra AN, Govindwar SP (2012) Degradation of remazol red dye by Galactomyces geotrichum MTCC 1360 leading to increased iron uptake in Sorghum vulgare and Phaseolus mungo from soil. Biotechnol Bioproc Engg 17:117–126
Zainal Z, Hui LK, Hussein MZ, Taufiq-Yap YH, Abdullah AH, Ramli I (2005) Removal of dyes using immobilized titanium dioxide illuminated by fluorescent lamps. J Hazard Mater 125(1–3):113–120
Acknowledgments
Authors wish to express their increase thank to Prof. Gopal Nath, Department of Microbiology, Indian Institute of Medical science, Banaras Hindu University, Varanasi, India, for their help in characterization and identification of isolated bacteria. One of the author (Lata Kumari) is thankful to Ministry of Human Resource Development and Department of Chemistry for providing financial support in the form of Senior Research Fellowship.
Ethical statement
The work presented in this paper is an original work and has not been copied from anywhere. The authors take full responsibility of communicated work. No human activity was taken up to perform the work. This work has not been submitted in any other journal for simultaneous consideration, and also, this work has not been published partly or fully elsewhere. We further wish to declare that no data have been fabricated and manipulated including figures, tables etc. to support our conclusion.
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
• Isolation and identification of bacterial strain BHUSSp X2.
• Application of the isolate for azo dye degradation.
• The detection of biotransformation pathways for Acid Red G dye using GC-MS and identification of enzyme involved in degradation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 349 kb)
Rights and permissions
About this article
Cite this article
Kumari, L., Tiwary, D. & Mishra, P.K. Biodegradation of C.I. Acid Red 1 by indigenous bacteria Stenotrophomonas sp. BHUSSp X2 isolated from dye contaminated soil. Environ Sci Pollut Res 23, 4054–4062 (2016). https://doi.org/10.1007/s11356-015-4351-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4351-8