Abstract
The coffee processing industry is one of the major agro-based industries contributing significantly in international and national growth. Coffee fruits are processed by two methods, wet and dry process. In wet processing, coffee fruits generate enormous quantities of high strength wastewater requiring systematic treatment prior to disposal. Different method approach is used to treat the wastewater. Many researchers have attempted to assess the efficiency of batch aeration as posttreatment of coffee processing wastewater from an upflow anaerobic hybrid reactor (UAHR)-continuous and intermittent aeration system. However, wet coffee processing requires a high degree of processing know-how and produces large amounts of effluents which have the potential to damage the environment. Characteristics of wastewater from coffee processing has a biological oxygen demand (BOD) of up to 20,000 mg/l and a chemical oxygen demand (COD) of up to 50,000 mg/l as well as the acidity of pH below 4. In this review paper, various methods are discussed to treat coffee processing wastewaters; the constitution of wastewater is presented and the technical solutions for wastewater treatment are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Scientifically called as genus Coffea, coffee belongs to a group of flowering plants whose seeds, called coffee beans, are used to make coffee. It is a member of the Rubiaceae family and are shrubs or small trees native to tropical and southern Africa and tropical Asia (Purseglove 1976). Coffee contains large amount of caffeine, the effects of which have always been an important element in the drink’s popularity. Coffee drinking began in the fifteenth-century in Arabia and it reached Europe by the mid-seventeenth century and immediately became hugely popular (Reich 2010). Coffee is the second most important commercial product in the world, after oil (Ponte 2002) and is now consumed by about one third of the world’s population, thus, there is a growing need to determine the environmental effects caused by a solid and liquid waste generated by the processing of coffee. Moreover, majority of the coffee growers apply fertilizers and agrochemicals that can easily make the water quality unfavourable if these chemicals return to streams or infiltrate the groundwater. In many places of the world, coffee replaces tropical forests, thus this conversion can be detrimental to watershed functions, particularly if it contributes to higher soil erosion (Verbist et al. 2005).
The Central Pollution Control Board (CPCB) of India had suggested a technical solution based on the National Environmental Engineering Research Institute (NEERI) design for treatment of coffee effluents. The CPCB option consists of three phases: a neutralization phase in which the acidic effluent is neutralized with lime, followed by anaerobic digestion (in the lagoon) and finally an aerobic phase (Narasimha et al. 2004). Currently, coffee pulp constitutes a source of serious contamination and environmental problem which due to lack of technology and bulkiness eventually end up in polluting rivers, generating offensive odours and encouraging proliferation of flies (Alemayehu and Devi 2007).
Types of coffee beans
There are a number of coffee varieties but usually these are categorized as Coffea arabica and Coffea robusta. The main varieties of Coffea Arabica have distinct characteristics and are described as having an excellent cup quality like typica, bourbon, Caturra, Catuai, Pache comum, Pache colis, Catimor, Kent, Mundo Novo, Maragogype, Amarello, Blue mountain and some C. robusta varieties like Indonesian Kopi Luwak and the Philippine Kape Alamid. Other coffee varieties include Barako coffee and Coffea liberica. However, only C. arabica, C. canephora (of which C. robusta is a major variety) and C. liberica are of commercial importance (Schenker 2000). Different coffee varieties contribute to the distinctive aromatic compounds which are unique to each type or origin of green coffee (Bhumiratana et al. 2011).
Arabica beans have a sweeter, softer taste, with high amount of sugar, fruit and berries. Their acidity is higher, with a winey taste that characterizes the coffee with an excellent acidity. Robusta, however, has a stronger, harsher taste, with a grain-like overtone and peanutty aftertaste. They contain twice as much the caffeine as arabica beans, and they are generally considered to be of inferior quality compared to arabica. Some robustas, however, are of high quality and valued especially in espressos for their deep flavour and good crema. Arabica is more valuable because it produces a better tasting beverage which is therefore more expensive than the robusta coffee (Brohan et al. 2009).
Monsooned coffee is a specialty coffee in which dry green arabica and robusta seeds of good quality are naturally cured for 3–4 months by exposure to the moist monsoon winds prevailing in the west coast of Southern India (Malabar coast), especially in the regions of Mangalore and Tellicherry (Variyar et al. 2003; Coffee Board of India 2007). Thus, from these two main coffee varieties, arabica monsooned and robusta monsooned coffees are processed, which are unique specialty coffees in the world specialties and are exclusively prepared and exported from India to cater to the demands of other countries in the world market (Frisullo et al. 2012). It was also reported that monsooned coffees have markedly different chemical and organoleptic quality characteristics from that of the natural coffee (Ahmad and Magan 2002).
Some coffee hybrids have also been developed and it was found that the chemical composition of hybrid coffee seeds is similar to that of the parent species. For example, hybrids of C. arabica and C. canephora are Timor hybrid and Catimor (cross between Timor hybrid with C. arabica cv. Caturra), which tends to exhibit intermediate characteristics (Duarte et al. 2009; Clifford and Ramirez-Martinez 1991, Clifford and Kazi 1987).
World production of coffee
The main producers of coffee in the world are Brazil followed by Vietnam, Columbia, Indonesia, Ethiopia and India (International Coffee Organization 2014). The total coffee production has increased from 128,636,000 bags from 2008 to 145,775,000 bags in 2013 (Table 1). In both Australia (Datamonitor 2010) and Japan (Specialty Coffee Association of Japan 2010), the demand for specialty coffee has increased as the global consumer culture has grown. Thus, it can be said that no national borders exist in the consumer’s demands for a special experience through superior cup quality. Coffee is a traded commodity on major futures and commodity exchanges, most importantly in London and New York (ICO 2011).
February 2014 had witnessed some significant developments in the coffee market, with price shooting upwards at a startling rate. The ICO composite daily price has increased from under 100 US cents/lb in November 2013 to a high of 176.37 on 11 March 2014. This has been driven by a serious drought in Brazil, with several coffee growing regions receiving little or no rain in the critical development months of January. There had been a rapid growth in coffee consumption per capita from 3.00 kg/capita in 2007 to 3.82 kg/capita in 2010 (ICO 2011; World Resources Institute 2011).
Arabica accounts for approximately 64 % while robusta accounts for about 35 % of the world’s production while the other species with not much commercial value like C. liberica and Coffea excelsa represent only 1 % (Rubayiza and Meurens 2005). Moreover, C. arabica is also responsible for approximately 70 % of the global coffee market, and Coffea canephora or robusta coffee accounts for the rest (International Coffee Organization ICO Statistics 2011; ABIC 2011). From the green robusta coffee beans of around 1 kg, the relevant type in the world manufacture of soluble coffee, about 0.33 to 0.45 kg of instant coffee (3 % moisture) is produced. This process generates large amounts of dark coloured waste (550 to 670 g/kg coffee beans) known as spent coffee grounds (SCG), which contains 15.2–17.9 % of lipids depending on the coffee species (Fan and Soccol 2005).
Processing of coffee cherries and wastewater generated
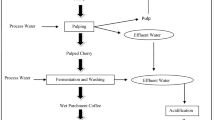
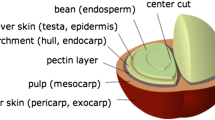
Coffee seeds are generally planted in large beds in shaded nurseries. After sprouting, the seedlings are removed from the seed bed to be planted in individual pots in formulated soils. The coffee cherries are brought in bags to the de-pulping machines after their grading. In the de‐pulping machines, the cherries are selected based on their size and de‐pulped, which is the process in which the pulp and the outer skin are removed. There remains a slimy layer around the coffee bean with a varying thickness of 0.5 to 2 mm. The separated pulp is then used for a variety of purposes or discarded as junk after which the grains are transported to a fermentation reservoir. The coffee is then moved with water to ceramic-lined bins where they will ferment for 12–48 h. This water not only moves the coffee but also removes most of the sticky substance that surrounds each coffee bean. The purpose of this process is to remove the slick layer of mucilage (called the parenchyma) that is still attached to the parchment; while resting in the tanks, naturally occurring enzymes will cause this layer to dissolve. Then, the beans are sent for drying and hulling. The coffee drying process is a fundamental aspect and determinant for the quality of the beverage, such that critical care taken cultivation, harvesting and processing of the fruits may be lost if the drying process is not carried out correctly (Rendo’n et al. 2013). Figure 1 shows a generalized flowchart to distinguish between the three processes that take place for the processing of coffee beans.
But, although there is a large consumption of this beverage, yet more than 50 % of the coffee fruit is not used for coffee production and is discarded during processing (Esquivel and Jiménez 2012).
Composition of coffee waste
The composition of the coffee waste was studied by Pujol et al. (2013). They analyzed the coffee waste generated in a soluble coffee industry and found that exhausted coffee wastes showed characteristics for various potential applications such as biodiesel production, as a source of antioxidants and as a biosorbent of hydrophobic pollutants
Wet coffee processing produces a slightly different by-product. Pressing the wet fruit through a screen leaves part of the pulp, the mucilage and the parchment still attached to the seeds (Belitz et al. 2009). The following (Tables 2, 3 and 4) show the composition of coffee pulp, mucilage and characteristics of wastewater but the composition of coffee pulps and husks can vary depending on the processing mode, cultivar, soil type and other factors (Pandey et al. 2000).
In most of the coffee processing industries, water is used for de-pulping and the vast majority of mills use water to channel the pulp directly into rivers. The oxygen required for the breakdown of pulp is so high that rivers with high amounts of pulp can be depleted of oxygen due to decomposition processes. The main component is organic matter, stemming from de-pulping and mucilage removal (Enden et al. 2002a). Generally, 42, 48 and 57 g roasted, ground coffee are used typically per litre of water in the USA, UK and Europe, respectively, and some 150 ml of the water are retained in the spent coffee grounds (ICO 2011).
Moreover, the water waste from coffee is broadly divided into two forms: the pulping water with a high content of quickly fermenting sugars and the wastewater generated from the processing applications. The main pollution in coffee wastewater occurs when the organic matter that set free during pulping becomes particularly difficult to degrade mucilage layer surrounding the beans. The mucilage contains mainly proteins, sugars and pectins and thus adds up to its better utilization.
Table 5 shows the physico-chemical properties of coffee wastewater as compared to ISI standards. Other constituents of wastewater from coffee processing include toxic chemicals like tannins, caffeine and polyphenols. Thus, the presence of protein, sugars, minerals and high water contents of wet processed coffee pulp makes it an excellent substrate for the growth of microorganisms and fast rate of spoilage (Pandey et al. 2000).
Thus, some compounds that we can obtain from coffee waste include the following:
-
1.
Unrefined pectins, (i.e. crude) soluble dietary fibre or SDF—mainly from the mucilage
-
2.
Naturally occurring coffee fruit sugars—mainly from the recycled pulping water
-
3.
Antioxidants and flavonoids—mainly from the skins but also some from the de-esterified mucilage.
-
4.
Extraction of some colour chemicals
Thus, from an industrial point of view the extraction of these materials could also substantially reduce the wastewater and pollution problems from our largest factories. Due to the great demand of this product, large amount of residues are generated in the coffee industry, which are toxic and represent serious environmental effects (Mussatto et al. 2011).
Environmental effects of coffee wastewater
The effluents from washed and semi-washed methods are loaded with organic matter and are high in toxicity. The results can lead to degradation of the level of oxygen in water, which can kill off virtually all aquatic life. Moreover, the total suspended solids in the effluents are high, in particular, the digested mucilage, when precipitated out of the solution, builds a crust on the surface, clogging up waterways and further contributing to the anaerobic conditions. Similarly, removal of phosphorus from wastewater is of great interest due to the limited nutrient status, and its disposal in water bodies causes adverse effects such as eutrophication (O’Neill et al. 2011).
Water is degraded when it is used in the fermentation of cherries, where it becomes highly acidic. It is clear from the above discussion that water is used for de-pulping, and the vast majority of mills use water to channel pulp directly into rivers. The oxygen required for the breakdown of pulp is so high that rivers with high amounts of pulp can be depleted of oxygen due to decomposition processes which makes the low availability of oxygen to the aquatic life and thus reducing the self purification capacity of rivers making it more pollutant. Moreover, the amount of oxygen required for biological breakdown exceeds the oxygen present in water creating an anaerobic atmosphere which turns out to be a growing place for health threatening bacteria with bad smell and dark appearance caused by tannins.
The amount of oxygen needed biologically to break down organic wastes diluted in water, that is, the biological oxygen demand (BOD), could be as high as 15,000 mg/l, while the amount of dissolved oxygen required to combine with chemicals in the wastewater, that is, the chemical oxygen demand (COD), could be between 15,000 and 25,000 mg/l.
Another environmental problem is the high requirement of water for coffee processing; as much as 15,000 L per tonne of cherries (coffee fruit) can be used, if there is no recycling and reuse.
Traditional methods of wastewater treatment
The most obvious and troublesome method of coffee wastewater management involves simply dumping it into the river. Another method involves the creation of a large holding tank where the wastewater is deposited and treated with talc to reach the desired pH. The major problem with these is that during the coffee season, the quantity of water is too great for the size of the tanks, and overflow instantly reaches water sources. Recycling requires skills because temperature, pH and bacteria level of the processing water need to be monitored and kept at optimum levels (Enden and Calvert 2002a). Several studies have also been carried out on the use of coffee pulp as food for animals (Braham and Bressani 1979; Cabezas et al. 1987).
Towards a new approach
Coffee wastes and its by-products form a source of severe contamination and a serious environmental problem. Many efforts have been made by researchers to develop methods for its utilization as a raw material for the production of feeds, beverages, vinegar, biogas, caffeine, pectin, pectic enzymes, protein and compost. The use of fresh or processed coffee pulp has been the subject of numerous studies which, in general, lead to the conclusion that coffee by-products and wastes can be used in a variety of ways, some of which are summarized here.
Utilization of coffee wastewater
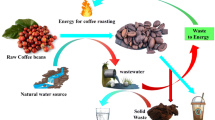
In a study conducted by Narasimha et al. 2004, they developed a bioreactor-biogas route of effluent treatment that provides not only a solution to waste disposal, but also an alternative fuel for electricity generation. They considered a waste-to-energy conversion route for coffee effluent treatment which includes pH neutralization of the effluents followed by anaerobic digestion and collection of the biogas so generated in gasbags which can be further used as a source of dual-fuel engines for electricity generation. The flow diagram for this process can be constructed as in Fig. 2.
The financially quantifiable benefit is the replacement of diesel with biogas in a dual-fuel engine. Based on the research work carried out by the IEI (2003), the estimation of the avoided cost of diesel is represented (Table 6). Based on the availability of biogas from the processing of 8 t of coffee fruit per day, the total gas generation possible during the 5-month season would be about 12,000 m3. Used at 0.918 m3 per kilowatt-hour, biogas has been shown to replace 77 % of the diesel required for the generation of electricity. The diesel replacement by biogas, for the generation of electricity, increases at the rate of 77 % with average use of 0.381 L/kWh.
Similarly, another author (Selvamurugan et al. 2009) developed an upflow anaerobic hybrid reactor (UAHR) to treat the coffee processing wastewater which offers the advantage of both suspended and attached growth anaerobic reactor systems. A UAHR of 4 mm thick clear acrylic sheet with volume of 19.25 L and a gas-liquid-solid separator installed at the top was constructed. The reactor was then seeded using coffee pulp and coffee effluent. For initial start-up of the reactor, coffee wastewater was fed with hydraulic retention time (HRT) of 24 h continuously and then it was operated at different HRTs as 24, 18, 12 and 6 h and the biomethanation potential for the reactor was assessed in terms of BOD and COD reduction, methane and total gas production. As a result of this, pH ranged from 3.88 to 4.21 because of the presence of organic acids in the berry skin and pulp and electrical conductivity ranged from 0.96 to 1.20 μm/cm2, which could be due to the presence of nutrients. The reactor performed better at HRT of 18 h with a short period of time. At 18 h retention time, reduction of chemical oxygen demand, biochemical oxygen demand and total solids were 61.0, 66.0 and 58.0 %, respectively, with organic loading rate of 9.55 kg/m3/day. The maximum quantity of biogas produced was 840, 775 and 430 L/kg of total solids (TS), BOD and COD removal, respectively; with the methane content of 60.7 % at HRT of 18 h, the study revealed the efficiency in treatment of coffee processing wastewater under different HRT and organic loading rates (Table 7).
An attempt was also made by Selvamurugan et al. (2010) to assess the efficiency of batch aeration as posttreatment of coffee processing wastewater from a UAHR by continuous and intermittent aeration system. Results showed the maximum BOD, COD and TS removal efficiency of 74.5, 68.6 and 49.3 %, respectively, which were recorded in biomethanated CPWW aerated continuously (Table 8).
The study was carried out by Mohana et al. (2011) to characterize the coffee wastewater and to know the effect of treated and untreated coffee wastewater on growth, yield and other properties of palmarosa grass. Coffee wastewater from coffee pulping and processing units were collected and analyzed for physico-chemical properties like colour, odour, pH, EC, TSS, TDS, BOD, COD, N, P, K, Ca, Mg and S. The height, number of tillers/clump, leaf area, plant spread, leaf to stem ratio, green forage, dry matter production, chlorophyll content, total crude protein and total fibre content of the plant was also analyzed. The study revealed that coffee wastewater can be successfully utilized for irrigation after suitable treatments and dilutions because the growth studies indicate that the treated effluent samples showed better results than the raw effluent (untreated). As coffee wastewater is highly acidic and possess all the qualities of polluted water, the quality can be improved by different ameliorative techniques.
Etiégni et al. (2011) used electrocoagulation technique for the removal of pollutants from wastewater which involves the application of current across electrodes in water which causes the dissolution of the anode to form hydroxides which complexes with and/or absorb contaminants and precipitate out. The precipitate with the contaminants can then be removed from the wastewater by settling and decantation or filtration. As a result of this, it was found that the consumption of power decreased by 57 % when electrocoagulation combined with wood ash leachate (ELCAS) was applied to the wastewater and 58 % when electrocoagulation was combined with leachate from coffee husks ash (ELCHAS).
Teresa et al. (2007) studied the reduction of COD, colour and turbidity of coffee wastewater using coagulation-flocculation and photo-degradation processes in which they first studied the coagulation-flocculation of coffee wastewater and then tested the photo-oxidation processes under acidic conditions. The COD of samples was then measured along with the turbidity and colour. As a result, the initial COD of raw industrial wastewater was found to be 4000–4600 mg/L, which got reduced by 55–60 % when treated using chemical coagulation-flocculation processes (Table 9). In the case of the flocculant 6705 with coagulant T-1, the COD is reduced by approximately 33 %, while use of flocculant 6708 with coagulant T-1 reduced the COD by approximately 34 %. The maximum reduction in COD (58 %) was obtained using the flocculant 6260 with coagulant T-1, which is the combination used in the coagulation-flocculation process used in the industrial coffee plant.
It was also found that the combination of lime (1.0 g) and coagulant T-1 (8 ml) at pH 4.6 gave an even greater reduction in COD (about 67 %). The use of chemical coagulation-flocculation treatment in conjunction with UV/H2O2 photo-oxidation achieved an 86 % reduction in COD but required a long irradiation time of 120 min.
Bio-refining of waste biomass involving the integrated production of chemicals, materials and bio-energy is a potential alternative for adding significant economic value added to the waste as a bio-resource. Coffee processing done by wet and dry methods discard away 99 % of the biomass generated by the coffee plants at different stages from harvesting to consumption level. The waste generated during the process includes cherry wastes, coffee parchment husks, sliver skin, coffee spent grounds, coffee leaves and wastewater that are produced in various stages. Wet processing uses up to 15 m3 of water to produce 1 t of clean beans (Hue et al. 2004) and for every ton of beans produced, about 1 t of husks are generated. It is estimated that coffee processing is generating about 9,000,000 m3 of wastewater, and 600,000 t of husks annually in the EA region.
Coffee waste contains high amounts of organic substrates including carbohydrates, proteins, pectins, fibres and fat for bioconversion into value added bio-products (Gathuo et al. 1991). However, large-scale utilization and management of coffee wastes around the world still remains a challenge due to caffeine, free phenols and tannins (polyphenols) which are known to be very toxic to many life processes (Fan et al. 2003) Previous studies have confirmed that toxic materials can be minimized by hot water pre-treatment, microbial biodegradation and aerobic fermentation (Gaime-Perraud et al. 1993; Martínez-Carrera et al. 2000). To that effect, production of bio-products such as silage, biogas, worms, animal feed, ethanol, vinegar, single-cell protein, enzymes, bio-pesticides and probiotics have only been established at a small scale; thus, demonstration of the technology at a pilot scale is yet to be achieved (Neves et al. 2006; Murthy et al. 2012).
The mechanism involved in UV/H2O2/O3 reduction of COD in coffee wastewater
The photo-oxidation and mineralization of organic pollutants with hydroxyl radicals involves a mixture of oxidants such as hydrogen peroxide and ozone in the presence of UV radiation. H2O2 can initiate the decomposition of O3 by electron transfer (Huang et al. 1993) and the resulting reaction generates OH radicals, consuming H2O2 and O3 as in Equation (1), and producing a chain mechanism, as shown in Equations from (2) to (9) (Domenech et al. 2001).
Good management practice for effective effluent treatment for coffee wastewater
It can be seen from the flow diagram (Fig. 3) that the coffee beans are fed into a depulper where the outer shells are removed. The pulp thus obtained can be further used for the production of compost, thus, is sent to the compost area. In the fermentation unit, most of the sticky substances attached to the coffee beans are removed and the wastewater is collected in bins for further treatments of methane gas production. The treatment unit for effluent treatment plant which comprises of the neutralization tank, equalization basin and others units are discussed. In the equalization basin, the flow is regularized, aerobic and anaerobic treatments are given for the production of methane and the wastewater is passed to lagoons which have a biofilter. Here, the influent is treated in oxidation pond making sure that the BOD and COD levels are regulated. On the other hand, the water contains elevated levels of plant nutrients, K and P. This may be beneficial (irrigation reuse) or detrimental (disposal/discharge).
Thus, the finding of the studies indicated the efficiency of batch aeration in treating wastewater as posttreatment of previously treated coffee processing wastewater by the upflow anaerobic hybrid reactor (UAHR) and is considered as a suitable treatment system for the coffee processing wastewater as an eco-friendly approach.
Utilization of coffee pulp
The water used for de-pulping of the coffee cherries is known as pulping water (Enden and Calvert 2002a), and it accounts for over half of the water used in this process (Enden and Calvert Ken 2002b). Coffee pulp is the main residue obtained during wet and semi-dry processing and represents 29 % dry weight of the whole berry (Murthy and Madhava Naidu 2012) making it rich in nutrients (Menezes et al. 2013). In a study, coffee pulp and coffee wastewater were used as a substrate for the production of ethanol and volatile compounds by using eight yeast strains for fermentation (Bonilla-Hermosa et al. 2014) as a result of which it was found that yeast strain H. uvarum UFLACAF76 presented the best fermentation performance and this improvement was achieved with 12 % w/v of coffee pulp, 1 g/L of yeast extract and 0.3 g/L of inoculum. Under these conditions, interesting results of ethanol yield, productivity and efficiency were obtained which proved that coffee pulp and wastewaters have a large potential as raw material for bioethanol and volatile compounds production.
Generally, the effluents from pulps, during the fermentation process, hold in fermentation tanks and mechanical mucilage recovery takes place. Sugars will ferment in the presence of yeasts to alcohol and CO2. In this situation, the alcohol is quickly converted to vinegar or acetic acid in the fermented pulping water. Thus, the chemical reaction for biological fermentation of six carbon sugars by yeasts to ethanol is as follows:
Fructose to ethanol reaction:
Then, the ethanol formed is quickly broken down by bacteria into acetic acids. This complex enzymatic catalyzed reaction is simplified as follows:
The acidification of sugars will drop the pH to around 4, and the digested mucilage will be precipitated out of the solution and will build a thick crust on the surface of the wastewater, black on top and slimy orange/brown in colour. If this is not separated from the wastewater, this crust will quickly clog up waterways and further contribute to the anaerobic conditions in the receiving stream.
Kumar et al. (2012) carried on a study to treat coffee pulp wastewater using Fenton’s oxidation process in which the effect of different operating conditions like Fenton dosage, mixing speed, pH and reaction time was evaluated. As a result of which they evaluated the percentage removal of pollutant parameters like ammonia nitrogen, nitrate nitrogen and phosphorus to be 90.75, 57.5 and 80 %, respectively. This study was done to minimize the impact of coffee pulping wastewater discharge on lagoons and natural water courses. Ferrous sulphate hepta-hydrate was used as source of iron and performed the tests on Fenton dosage varied from of 15, 25 and 50 % of hydrogen peroxide demand.
Thus, it is clear from Table 10 that there has been a decrease in the COD and BOD levels after Fenton treatment along with a decrease in acidity.
Utilization of coffee mucilage
According to (Carbonell and Vilanova 1974), coffee mucilage contains water, pectins, sugars and organic acids. During maturation, calcium pectate in the middle lamella and protopectin from the cellular wall converted into pectins by hydrolyzing result in disintegration of the cellular wall to free. The plasma which contains sugars and organic acids is derived from metabolism and conversion of starches. Mucilage contains neither tannins nor caffeine, but contains pectin-degrading enzymes that can hydrolyze pectic constituents in this material and become important in fermentation. Thus, the composition of mucilage was studied by them as shown in Table 11.
They investigated the prebiotic properties of coffee mucilage through batch culture fermentation and fluorescent in situ hybridization for counting the cells.
Coffee mucilage can also serve as a novel substrate for hydrogen production (Hernández et al. 2014). As a result of this technique, the hydrogen production was improved by a C/N ratio of 53.4 which indicates a high hydrogen potential compared to substrates such as POME and wheat starch. The biogas composition was found to be 0.1, 50.6 and 39.0 % of methane, carbon dioxide and hydrogen, respectively. Hence, this study established a direct relationship between coffee mucilage, biogas and cumulative hydrogen volume.
Conclusion
Coffee processing water, especially from small-scale facilities that use the wet fermentation processes, is quite high in BOD. Such high BOD levels must be lowered to <300 mg L−1. Electro Fenton and others such as anaerobic methods are useful for the degradation of organic matters. The removal of the natural organic matter present in coffee processing wastewater through chemical coagulation, flocculation and advanced oxidation processes (AOP) under acidic conditions was found to be an effective treatment. Among the advanced oxidation process schemes, the UV/H2O2/O3 process was the most effective in reducing the COD, colour and turbidity of coffee wastewater. The reduction of COD was 84 %. Finally, the treatment combined with biological and chemical treatment is the most effective treatment for coffee waste discharged. This combined method is an effective approach for industrial coffee wastewaters for reducing COD, and consequently greater degradation of organic material. Moreover, it also presented a generalized view of utilizing the coffee mucilage and pulp, which not only forms an essential by-product but also helps in the reduction of coffee pollutants.
References
ABIC (2011) Brazilian Association of Coffee Industry (Technical information)
Ahmad R, Magan N (2002) Microfloral contamination and hydrolytic enzymes differences between monsooned and nonmonsooned coffees. Lett Appl Microbiol 34:279–282
Alemayehu H, Devi R (2007) Effect of effluent generated from coffee processing plant on the water bodies and human health in its vicinity. J Hazard Mater 152:259–262
Belitz H D, Grosch W, Schieberle P (2009) Food chemistry (4th ed.) Heidelberg: Springer Chapter 21
Bhumiratana N, Adhikari K, Chambers E IV (2011) Evolution of sensory aroma attributes from coffee beans to brewed coffee. Food Sci Technol 44:2185–2192
Bonilla-Hermosa VA, Duarte WF, Schwan RF (2014) Utilization of coffee by-products obtained from semi-washed process for production of value-added compounds. Bioresour Technol 166:142–150
Braham JE, Bressani R (1979) Coffee pulp composition technologies utilization. INCAP, Bogota, Colombia
Brohan M, Huybrighs T, Wouters C, Bruggen BV (2009) Influence of storage conditions on aroma compounds in coffee pads using static headspace GC-MS. Food Chem 116:480–483
Cabezas MT, Flores A, Egana JI (1987) Use of coffee pulp in ruminant feeding: composition, technology and utilization. Institute of Nutrition of Central America and Panama, Guatemala City, pp 25–38
Carbonell AJ, Vilanova M (1974) Beneficiado rápido y eficiente del café mediante el uso de Soda Caustica. Cited by Cleves, Rodrigo. In Justificación de un proyecto para investigar Ia obtención de pectina a partir del mucIlago del café. Departamento de studios técnicos y diversificación. Proyecto 1. Subproyecto 5. Oficina de Café, San José, Costa Rica
Central Pollution Control Board (CPCB), Ministry of Environment & Forests www.cpcb.nicia
Clifford MN, Kazi T (1987) The influence of coffee seed maturity on the content of chlorogenic acids, caffeine and trigonelline. Food Chem 26:59–69
Clifford MN, Ramirez-Martinez JR (1991) Phenols and caffeine in wet-processed coffee seeds and coffee pulp. Food Chem 40:35–42
Coffee Board of India http://www.indiacoffee.org/default.htm. (accessed December 3, 2007)
Datamonitor (2010) http://www.ausfoodnews.com.au/2010/03/04/aussie-cafe-culture-accounts-for-biggest growth-in-coffee.html
Domenech X, Jardim WF, Litter M (2001) Elimination of pollutants by heterogeneous photocatalysis. Latin-american cooperation CYTED. Sci & Tech for the Development, Buenos Aires, Argentina, Chapter 1, 15
Duarte G, Pereira A, Marques V, Farah A (2009) Comparison of chlorogenic acids contents in Coffea arabica, Coffea canephora and hybrids resistant to Meloidogyne exigua. Proc. 22rd Int. Conf. Coffee Sci. ASIC, Trieste, Italy 508–512
Enden VJC, Calvert KC (2002a) Limit Environmental Damage By Basic Knowledge of Coffee Waste Waters. GTZ-PPP Project-Improvement of coffee quality and sustainability of coffee production in Vietnam. <http://en.wikipedia.org/wiki/Coffee_wastewater>
Enden V J C, Calvert Ken C (2002b) Review of Coffee Waste Water Characteristics and Approaches to Treatment. GTZ-PPP Project “Improvement of coffee quality and sustainability of coffee production in Vietnam”
Esquivel P, Jiménez VM (2012) Functional properties of coffee and coffee by-products. Food Res Int 46:488–495
Etiégni L, Orori B O, Senelwa K, Mwamburi M M, Balozi B K, Maghanga J K (2011) Ash leachate used as supporting electrolyte during wastewater treatment by electrocoagulation. Geophy Res Abs 13
Fan L, Soccol CR (2005) Coffee residues. Shiitake Bag Cultivation. Chapter 4. Mushroom Grower’s Handbook 2: 92–95
Fan L, Soccol AT, Pandey A, Soccol CR (2003) Cultivation of Pleurotus mushrooms on Brazilian coffee husk and effects of caffeine and tannic acid. Micol Appl Int 15(1):15–21
Frisulloa P, Laversea J, Barnabà M, Navarini L, Del Nobilea MA (2012) Coffee beans microstructural changes induced by cultivation processing: an X-ray microtomographic investigation. J Food Eng 109:175–181
Gaime-Perraud I, Roussos S, Martínez Carrera D (1993) Natural microorganisms of the fresh coffee pulp. Micol Neotrop Appl 6:95–103
Gathuo B, Rantala P, Maatta R (1991) Coffee industry wastes. Water Water Sci Technol 24(1):53–60
Hernández MA, Rodríguez Susa M, Andres Y (2014) Use of coffee mucilage as a new substrate for hydrogen production in anaerobic co-digestion with swine manure. Bioresour Technol 168:112–118
Huang CP, Dong C, Tang Z (1993) Advanced chemical oxidation: its present role and potential future in hazardous waste treatment. Waste Manag 13:361–377
Hue N V, Bittenbender H C, Ortiz-Escobar M E (2004) Managing coffee processing water in Hawaii, Department of Tropical Plant and Soil Sciences, College of Tropical Agriculture and Human Resources, University of Hawaii, Manoa, Honolulu, HI 96822 USA
International Coffee Organization, 2014 (ICO) http://www.ico.org/
International Coffee Organization (ICO) Statistics (2011) Breakdown of exports of green Arabica and green Robusta of countries exporting significant volumes of both types of coffee. www.ico.org
International Energy Initiative (IEI), 2003
Kumar MB, Ulavi SU, Ramesh HS, Asha G, Pallavi R (2012) Pretreatment of coffee pulping wastewater by Fenton’s reagent. Indian J Chem Technol 19:213–217
Martínez-Carrera D, Aguilar A, Martínez W, Bonilla M, Morales P and Sobal M (2000) Commercial Production and Marketing of Edible Mushrooms Cultivated on Coffee Pulp in Mexico Coffee Biotechnology and Quality 471–488
Menezes EGT, Do-Carmo JR, Menezes AGT, Alves JGLF, Pimenta CJ, Queiroz F (2013) Use of different extracts of coffee pulp for the production of bioethanol. Appl Biochem Biotechnol 169:673–687
Mohana VS, Nandini N, Pramila CK, Manu KJ (2011) Effect of treated and untreated coffee wastewater on growth, yield and quality of palmarosa grass (Cymbopogon martini L.) var. motia. IJRCE 1(2):111–117
Murthy PS, Madhava Naidu M (2012) Sustainable management of coffee industry by-products and value addition—a review. Resour Conserv Recycl 66:45–58
Mussatto SI, Machado EMS, Martins S, Teixeira AJ (2011) Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol 4:661–672
Narasimha Murthy KV, Antonette D’Sa, Gaurav Kapur (2004) An effluent treatment-cum-electricity generation option at coffee estates: is it financially feasible?. Draft version, International Energy Initiative, Bangalore
Neves L, Oliveira R, Alves MM (2006) Anaerobic co-digestion of coffee waste and sewage sludge 26(2) :176–181
O’Neill A, Foy RH, Phillips DH (2011) Phosphorus retention in a constructed wetland system used to treat dairy wastewater. Bioresour Technol 102:5024–5031
Pandey A, Soccol CR, Nigam P, Brand D, Mohan R, Roussos S (2000) Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem Eng J 6:153–162
Ponte S (2002) The ‘Latte Revolution’? Regulation, markets and consumption in the global coffee chain. World Dev 30(7):1099–1122
Pujola D, Liua C, Gominhoc J, Olivellab MÀ, Fiola N, Villaescusaa I, Pereirac H (2013) The chemical composition of exhausted coffee waste. Ind Crop Prod 50:423–429
Purseglove JW (ed.) (1976) Rubiacae In: Tropical Crops: Vol 1: Dicotyledons. Longman, London, 458–492
Reich A (2010) Coffee & tea: history in a cup. The Herbanist 76:9–15
Rendo’n MY, Grata˜o PL, Salva TJG, Azevedo RA, Bragagnol N (2013) Antioxidant enzyme activity and hydrogen peroxide content during the drying of arabica coffee beans. Eur Food Res Technol 236:753–758
RubayizA AB, Meurens M (2005) Chemical discrimination of arabica and robusta coffees by Fourier transform Raman spectroscopy. J Agr Food Chem 53(12):4654–4659
Schenker S R (2000) Investigations on the Hot Air Roasting of Coffee Beans. Swiss Federal Institute of Technology
Selvamurugan M, Doraisamy P, Maheswari M, Nandakumar NB (2009) High rate anaerobic treatment of coffee processing wastewater using upflow anaerobic hybrid reactor. IJEHSE 7(2):129–136
Selvamurugan M, Doraisamy P, Maheswari M, Nandakumar NB (2010) Evaluation of batch aeration as a post treatment for reducing the pollution load of biomethanated coffee processing waste water. Global J Environ Res 4(1):31–33
Specialty Coffee Association of Japan (SCAJ) http://www.scaj.org/
Teresa ZP, Gunther G, Fernando H (2007) Chemical oxygen demand reduction in coffee wastewater through chemical flocculation and advanced oxidation processes. J Environ Sci 19:300–305
Variyar PS, Ahmad R, Bhat R, Niyas Z, Sharma A (2003) Flavouring components of raw monsooned arabica coffee and their changes during radiation processing. J Agric Food Chem 51:7945–7950
Verbist B, Putra AED, Budidarsono S (2005) Factors driving land use change: effects on watershed functions in a coffee agroforestry system in Lampung, Sumatra. Agric Syst 5(3):254–270
World Resource Institute (2011) www.wri.org
Acknowledgments
The authors thank the director of CSIR-CFTRI for giving kind permission to publish this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Rattan, S., Parande, A.K., Nagaraju, V.D. et al. A comprehensive review on utilization of wastewater from coffee processing. Environ Sci Pollut Res 22, 6461–6472 (2015). https://doi.org/10.1007/s11356-015-4079-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4079-5