Abstract
From the concentration in water and sediments, bioconcentration and bioaccumulation of copper (Cu), manganese (Mn), zinc (Zn), iron (Fe), cobalt (Co), cadmium (Cd), chrome (Cr), silver (Ag), lead (Pb), nickel (Ni), aluminum (Al), and arsenic (As) were determined in the gills, liver, and muscles of Geophagus brasiliensis in the Alagados Reservoir, Ponta Grossa, Paraná, Brazil. Metals were quantified through AAS, and a study was carried out on the existing relations between metal and body weight, size, and genre of this species. The level of metal in the water of the reservoir was lower than the maximum set forth in the legislation, except for that of Cd and Fe. In sediments, Cu, Cd, Cr, and Ni presented concentrations above the threshold effect level (TEL). Pb and Cr were above the limits for the G. brasiliensis. The tendency of metals present in the muscles of G. brasiliensis was Al > Cu > Zn > Fe > Co > Mn > Cr > Ag > Ni > Pb > Cd > As. In the gills, it was Al > Fe > Zn > Mn > Co > Ag > Cr > Ni > Cu > As > Pb > Cd, and the liver presented Al > Cu > Zn > Co > Fe > Mn > Pb > Ag > Ni > Cr > As > Cd. The bioconcentration and bioaccumulation of metal in the tissues follow the global tendency liver > gills > muscle. The statistical analysis did not point to significant differences in the metal concentration and body weight, size, and gender of the species in the three tissues under analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal contamination in water reservoirs has become an issue of concern along the last decades, not only regarding the threat it poses to the water supply but also for the danger it represents to the human consumption of fish (Terra et al. 2008). Metals are continuously released into the water environment through natural and anthropogenic sources, and they tend to accumulate in the sediment and, depending on the environmental conditions, can also be released into the water column becoming bioavailable. In such conditions, they might affect the biota, be incorporated throughout the food chain, and consequently harm human health (Shrivastava et al. 2003; Khan et al. 2005).
Among the known chemical elements, 53 are described as metals and, from these, only 17 are bioavailable and important for the ecosystem (Carranza-Álvarez et al. 2008). Attention has been drawn recently both in developed and underdeveloped countries to the anomalous distribution of metals in the water, sediments, and fish, which are very important to the comprehension of metal behavior in reservoirs (Singh et al. 2014). In addition to the metal analysis in the water and sediment, it is also important to identify the extension of metal concentration in the biota and consider its potential impact to the trophic chain and the risk to human health, as it is not possible to be absolutely sure that metals are not incorporated to the biota by simply verifying their concentration in the water and sediments.
Bioconcentration and bioaccumulation of metals are indicative of the water body and sediment contamination, respectively, becoming a useful tool to study the biological role of metals present in water organisms, especially fishes that tend to accumulate contaminants in their tissues even when the water presents lower levels of these compounds than those tolerated by the law (Shah et al. 2009).
Each fish species has a particular way of accumulating (and/or eliminating) metals when exposed to such contaminants, species with relatively low trophic levels are exposed to lower contamination, even though they are submitted to the chemical stress that might provoke changes in target organs (Peakall and Burger 2003). Metal bioconcentration and bioaccumulation processes depend on the fish species and their trophic level, the sampling place, the kind of food, the kind of absorption carried out by the organism, the particle size, or the phase in which the metal is (dissolved or particulate) (Asuquo et al. 2004).
Despite its broad geographical distribution all over Brazil, there are few studies analyzing metals in G. brasiliensis. The etymology of the name of the species under study comes from the Greek (geo = earth and phaigen = eating), referring to the fact that this fish revolves the sediment with its mouth. This is a Brazilian omnivorous native species, a natural inhabitant of lentic environments, such as lakes and reservoirs, which adapts very well to hot and cold water regions. The male fish grow faster than the female; they usually build nests and protect their offspring (the female fish protect the larvae in their mouth). Their main characteristics are versatility, territorialism, and resistance, and they are subject to bioconcentration through several kinds of pollutants (Hidetoshi 1984; Kullander 2003; Abelha and Goulart 2004; Rocha et al. 2005; Di Giulio and Hinton 2008).
The Alagados Reservoir provides water for three towns in the state of Paraná, Brazil, and receives pollutants from different sources such as agriculture, cattle breeding, domestic sewage, and building activity on its margins. The choice for this species is suitable due to its living habits, easy capture, and its abundance in the reservoir under study, and it is one of the most consumed species by fishermen in the region. Besides that, it is an environmental contamination bioindicator which presents a varied diet throughout the water column, including sediments, on which a lot of chemicals are deposited (Benincá et al. 2012; Osório et al. 2013).
The objective of this study was to investigate the bioconcentration and bioaccumulation of metals in different tissues of the G. brasiliensis (the liver, muscle, and gills) in the Alagados Reservoir, from the concentration of metals in the water and in the sediments, as well as to verify the existing relations between the metals and body weight, size (length), and genre of this species.
Materials and methods
Area of study
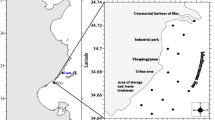
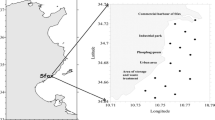
The Alagados water basin is located in the region of Campos Gerais, in the southeast of Paraná, almost entirely in the first plateau, limited by the geographical coordinates 24° 52′ to 25° 05′ latitude S and 49° 46′ to 50° 06′ longitude W of the Greenwich (UTM 592.000 to 624.000 and 7.226.300 to 7.249.800), and it is the water source of the three towns in the region of Campos Gerais in the south of Brazil: Ponta Grossa, Castro, and Carambeí (Clemente et al. 2010) (Fig. 1). The Alagados Reservoir is an artificial dam built on the Pitangui River, between the Jutuva River mouth and the São Jorge River mouth. It was built in 1929 aiming to generate electrical power. However, the reservoir has also gradually become a source of leisure, irrigation, animal water supply, and also, water supply to the population of these towns. Nowadays, its water is collected and treated by the Paraná Sanitation Company (Sanepar). The agriculture and cattle breeding activities, together with unregulated occupation of the reservoir margins, are the main sources of pollution, and along the years, the lake formed became eutrophic (Clemente et al. 2010).
Sampling
Samples were collected in the Summer 2013 according to the technical manual EPA 823-B-01-002 (2001). Water and sediments were collected from four distinct points in the Alagados Reservoir (see Fig. 1), with a total of four water samples and four sediment samples, with one sample from each collection point. These points were selected by seeking a variety of impaction levels that included the point where the reservoir begins (P1 = latitude 24° 59′ 40″ S and longitude 49° 59′ 36″ W), margins in agriculture (P2 = latitude 25° 00′ 23″ S and longitude 50° 00′ 46″ W), margins where some houses are built (P3 = latitude 25° 01′ 03″ S and longitude 50° 02′ 08″ W), and the area closer to the dam, at the point where there are no more houses (P4 = latitude 25° 01′ 08″ S and longitude 50° 03′ 39″ W). An Eckman-Birge collector was used for the sediments, superficial water was collected in sampling bags, and the fish were collected with cast nets mesh 5.
Fifty-five G. brasiliensis were collected from the different points of the reservoir, the fish were weighed and measured before their liver, gills, and muscle were separated, lyophilized (Terroni®), and stored in a desiccator until the analyses were carried out.
In order to compare the metal concentration in G. brasiliensis with the maximum concentration acceptable for human consumption by the regulations considered in this study (FAO/WHO, Agência Nacional de Vigilância Sanitária (ANVISA)), the concentrations were recalculated in micrograms per gram wet weight (w.w.).
Metal analyses
The water samples were submitted to digestion using the Method US EPA 3005A (1992), while the sediment and G. brasiliensis samples were submitted using the Method US EPA 3050B (1996). Digestions were carried out in triplicate with reagent blank, and the precision of the methods was verified from the reference material analysis-certified ERM-CE278 (mussel tissue; European Reference Materials) from the Institute for Reference Materials and Measurements (IRMM) in Europe and MESS-2 (marine sediment) from the National Research Council Canada (NRCC), the Environmental Chemistry Institute in Canada, obtaining recovery percentages over 90 % for the metals under analysis.
Arsenic determinations were carried out using an atomic absorption spectrometer (Varian®, AA 240Z) with electrothermal atomization in a graphite oven (model GTA 120), equipped with a transverse Zeeman corrector for background correction, automatic sampling (model PSD 120). A hollow cathode lamp was used with argon as carrying inert gas with a flow rate of 0.3 L min−1. The pyrolitically covered graphite tubes were used in all determinations.
Determination of the remaining metals was carried out using a flame atomic absorption spectrometer (Varian®, AA 240FS), employing the automatic dilutor system as an accessory, equipped with a deuterium lamp as a background corrector and multielement hollow cathode lamps. A nitrous oxide acetylene flame with flow rates of 10.24 and 6.95 L min−1 was used for the aluminum, and an air oxidant acetylene flame with flow rates of 13.50 and 2.00 L min−1 was used for the remaining metals. Standard solutions were prepared with ultrapure water (reverse osmosis and water filter Gehaka®), and the stock solutions of 1000 mg L−1 Qhemis High Purity® were used for the following metals: copper (Cu), manganese (Mn), zinc (Zn), iron (Fe), cobalt (Co), cadmium (Cd), chrome (Cr), silver (Ag), lead (Pb), nickel (Ni), aluminum (Al), and arsenic (As). The analytical curve was derived from these standards. The limits of detection and quantification of metals are shown in Table 1.
Bioconcentration and bioaccumulation factors
The factors evaluated in this study constitute the terms used to quantify the tendency of a compound/element to concentrate in aquatic organisms. The bioconcentration factor (BCF) is the result of absorption, distribution, and elimination of a substance all over the organism after it has been exposed to the water (Subotić et al. 2013). The calculation was carried out using the ratio of the metal found in the fish tissue (M tissue), expressed in micrograms per gram dry weight, and the metal found in the water (M water), expressed in micrograms per milliliter
The bioaccumulation factor (BAF) is used to determine the tendency of a given compound/element to accumulate from the food or sediment, and it was calculated using the ratio of the concentration of the metal found in the fish tissue (M tissue) and the concentration of the metal found in the sediment (M sediment), expressed in micrograms per gram (Lau et al. 1998)
Statistical analyses
All the statistical analyses were carried out using the software Assistat 7.7 and R Project for Statistical Computing.
The Shapiro-Wilk test was employed to analyze the normality of data distribution. The variance analysis (ANOVA) with completely randomized design (CRD) through Tukey’s test was used to test the significant differences between the average concentrations of metal in the muscles, gills, and liver; body weight; size; and genre of G. brasiliensis. For the data sets that did not follow the normal distribution, the Mann-Whitney nonparametric test was used in order to identify all the possible differences between samples (Giannakopoulou and Neofitou 2014).
Values below 0.05 (5 %) were considered statistically significant (P < 0.05).
Results and discussions
Concentration of metals in water and superficial sediment
In reservoirs, the metal comes from natural sources such as the geological washing of soils and rocks, directly exposed to the water, and through anthropic sources such as domestic and industrial effluents, mining processes, application of fertilizers in crops, and through the rain in areas with polluted air (Pereira et al. 2006; Ebrahimpour and Mushrifah 2008), and over 90 % of the metal load is associated to the particulate material in suspension and the sediment (Zheng et al. 2008). In general, when released in the water body, metals are firstly absorbed by organic or inorganic particles and then incorporated to the sediment through the sedimentation process, resulting in higher levels of metals in this compartment (Botté et al. 2007). Metals are dangerous as once they enter the aquatic environment, they cannot be destroyed (WHO 2011).
The results of metal analyses in superficial water are presented in Fig. 2, and Fig. 3 shows the results of metal analyses in the superficial sediment in the Alagados Reservoir.
By evaluating the general average of metal, regarding the four points sampled, it was seen that only Fe (1.99 ± 0.40) and Cd (0.0026 ± 0.0002) concentrations were above the maximum limit set forth for water class II in the resolution CONAMA 357/2011, with 0.3 and 0.001 mg L−1 of Fe and Cd, respectively. Fe is an essential metal to the organism; however, when it is found in high concentrations such as those in the Alagados Reservoir, it can be harmful to health. No biological function is ascribed to cadmium, but it can present chronic or acute toxic effects (Buratini and Brendelli 2006). The metals Cu, Mn, Zn, Co, Cr, Ag, and Ni presented concentrations below the limits set forth by CONAMA (2011) and US EPA (2014a, b). Metals Pb, Al, and As were not detected with the techniques employed. Therefore, the metal global general tendency in the water was Fe > Mn > Cr > Co > Ag > Cu > Ni > Zn > Cd.
Interstitial water (sediment water) can present high concentrations of metal and be able to influence the metal concentration in superficial water through processes like diffusion, consolidation, and bioturbation (Salomons and Förstner 1984). Thus, the metal concentrations in the sediment are higher than those in the water column. Therefore, the sediment analysis is a fundamental source of data about the pollution in the aquatic environment (Brekhovskikh et al. 2002), and high levels of metal concentration in the sediment might indicate pollution due to anthropogenic influence. Thus, this analysis becomes important because it allows the detection of metals that might be absent or in low concentrations in the water column, and their distribution in the sediments permits a register of the pollution spatial and temporal background in a particular ecosystem (Davies et al. 2006).
According to internationally proposed guidance values for sediments by the Environment Canada (1999), metal concentrations in the Alagados Reservoir samples are below the limits set forth for the metals Zn (65.93 ± 12.82), Pb (27.65 ± 2.72), and As (5.12 ± 2.74). These presented average concentrations below the threshold of adverse effects to the biological community (threshold effect level (TEL)), which are 123, 35, and 5.9 μg g−1 for Zn, Pb, and As, respectively. The metals Cu (40.32 ± 7.44), Cd (2.74 ± 0.89), Cr (40.18 ± 5.02), and Ni (31.97 ± 3.20) presented concentrations above the TEL, these being 35.7, 0.6, 37.3, and 18.0 μg g−1 for Cu, Cd, Cr, and Ni, respectively, suggesting that they might be associated to possible adverse biological effects; however, they are below the level of probable adverse effects to the biological community (probable effect level (PEL)), which are 197, 3.5, 90, and 35.9 μg g−1 for Cu, Cd, Cr, and Ni, respectively.
Cu is commonly added to pig food and eliminated in great amounts by these animals, which might lead to the concentration of this metal in the agriculture soil when these areas are altered by pig manure for several years as in the Alagados Reservoir, which is surrounded by properties where pig, cattle, and poultry are bred (Abel 2002; Davies et al. 2006; Carvalho et al. 2008; IPARDES 2010; Fowler et al. 2011). Similar to a study carried out in Croatia, the increase in Cd concentrations in sediments is correlated to the increase in its concentrations in water, which might have a natural origin. Taking into consideration that the removal of metal traces from the water occurs through the carbonate coprecipitation, only Cd and, partly, Zn contents in sediments can be explained (Vukosav et al. 2014).
The metals Mn, Fe, Co, Ag, and Al do not have reference values for sediments. The general global tendency of metals in the sediment in the Alagados Reservoir was Al > Fe > Mn > Zn > Cu > Cr > Ni > Co > Pb > As > Ag > Cd.
Despite the fact that Al was not found in the water through the techniques employed, in the sediment, this metal reached high concentration. Regarding the metals detected, a tendency to high concentrations of Fe and Mn and low concentrations of Cd in the water and sediment of the Alagados Reservoir could be noticed. Iron and manganese are considered important constituent elements in soil and rocks and might influence the high concentration found in the Alagados Reservoir.
Lately, due to the population growth and the intensification of human activities involving the elements under analysis, the concentration of metals, in general, has increased in water bodies at levels that threaten the aquatic biota as well as the terrestrial organisms, including human beings, who survive on it. Studies have shown that metals such as Zn, Mn, Co, and Pb are present in sediments and originate from the impurities of fertilizers and pesticides applied to the agricultural soils (Ramalho et al. 2000). The Alagados Reservoir has a large agricultural area which constitutes one of the most important sources of contamination through metal (Alloway and Ayres 1997).
The quantification of metals in the Alagados Reservoir is important to understand the levels of metal concentration found in the G. brasiliensis tissue, since the accumulation of metal in fishes is usually associated to water and sediment contamination by these elements, and depending on the animal’s eating habit, it might occur through the trophic chain and finally result in damage to the human health (Förstner and Wittmann 1983).
Metal concentration in the G. brasiliensis tissues
The metal concentration in G. brasiliensis is presented in Table 2. Only the metals Cd, Pb, and As, among those analyzed, have limits for food set forth by FAO/WHO (2014), and regarding these, Cd and As are below the proposed limit of 1.0 μg g−1 and are not detected respectively in relation to the muscle which is the part consumed by humans. Pb presents a concentration above the limit 2 μg g−1, regarding the female animals, and below the limit, considering the G. brasiliensis male samples. Lead is related to neurodegenerative damage and is potentially genotoxic in fish (Martinez et al. 2004; Monteiro et al. 2011). The Decree no. 55.871 dated 26 March 1965 and the Ordinance no. 685 by ANVISA (2014) establish the maximum levels of contaminants in food, and according to those levels, the samples analyzed present Cu, Ni, and Zn concentrations below these levels, which are 30, 5, and 50 μg g−1, respectively. The metal Cr has a maximum level of 0.1 μg g−1 according to ANVISA, and the G. brasiliensis samples presented concentrations above the limit for both male and female animals. Therefore, the fish under analysis were Pb and Cr contaminated.
Fish mainly assimilate metals through the ingestion of particulate material in suspension in the water; food ingestion; ionic exchange of metal dissolved through lipophilic membranes, for example the gills; and the adsorption on the surface of tissues and membrane. The metal distribution among the different tissues depends on the kind of exposure, that is, the diet and/or water exposure, and might be an indicator of pollution (Shah et al. 2009).
The general global tendency of metals in the G. brasiliensis muscle was Al > Cu > Zn > Fe > Co > Mn > Cr > Ag > Ni > Pb > Cd > As. In the gills, it was Al > Fe > Zn > Mn > Co > Ag > Cr > Ni > Cu > As > Pb > Cd, and the liver presented Al > Cu > Zn > Co > Fe > Mn > Pb > Ag > Ni > Cr > As > Cd. A tendency to high concentrations of Al, Zn, Fe, and Mn was noticed in all tissues under analyses as well as in the Alagados Reservoir sediment, suggesting that the G. brasiliensis contamination might occur due to their interaction with the sediment.
For the muscle and gills, the same tendency was related to the metals Co and Cr; regarding the gills and liver, the metal Cd showed the same tendency, and for the muscle and liver, the metals Cu, Mn, and Ni revealed the same relation regarding tendencies.
Fe presented high concentration in the gills when compared to the other tissues of the G. brasiliensis. Due to the Fe essentiality and requirement in the function of transporting oxygen in the blood, this element is found in high concentrations. Therefore, high bioconcentrations are expected in environmental contaminations, and it is one of the means used to inform the water quality criteria (Wood et al. 2011).
The Shapiro-Wilk test indicated the abnormality of data distribution. Thus, the Mann-Whitney U test was applied due to the unmatching data among the levels observed. The comparison between the level pairs (liver × muscle, muscle × gills, and liver × gills) presented P < 0.05, indicating that the metal average results are different in the three tissues of G. brasiliensis, except for Co and Pb that presented P > 0.05 for the muscle and gills, indicating that there was no statistical difference between the concentrations of these metals in such tissues. Similarly, Cr and Al presented P > 0.05 without any statistical difference in these metal concentrations in the liver and gills.
Therefore, the metals Pb and Co statistically presented the same average concentrations in the muscle and gills, being only different from the liver that presented higher average concentrations. The metals Cr and Al statistically presented concentrations similar to those in the liver and gills, only differing from the muscle which presented lower average concentrations. The metals Cu, Pb, Ag, As, Cd, Co, and Ni presented higher average concentrations in the liver, while the metals Fe, Mn, and Zn had higher average concentrations in the G. brasiliensis gills.
Similar to this study, four species of eatable fishes (Tor putitora, Cirrhinus mrigala, Labeo calbasu, and Channa punctatus) from a reservoir in Pakistan presented relatively high metal concentrations in the liver and gills, in comparison with the muscle (Malik et al. 2014). Similarly, the liver tissue of four species of fish from the Titicaca Lake (Odontesthes bonariensis, Orestias luteus, Orestias agassii, and Trichomycterus rivulatus) was identified as the main storage site of all metals analyzed (Cu, Zn, Cd, Pb, Co, Fe) (Monroy et al. 2014).
The liver and gills, as metabolic active organs, are the target organs for the accumulation of metals, while the accumulation in the muscular tissue is lower, this is due to the liver’s higher metabolic activity, once it is involved in storage and excretion activities, acting as a final metal repository. The metals are then transported to the liver from the other tissues for subsequent elimination, hepatocyte kidnapping, transport, and/or excretion of toxic metals (Yilmaz et al. 2007; Poleksić et al. 2010; Višnjić-Jeftić et al. 2010; Jarić et al. 2011).
The tissues under analysis were selected according to their functions. The gills are a multifunctional organ, which takes part in the ion transport, gaseous exchange, acid-base regulation, and excretion. They are also considered as relevant target organs for metal acute intoxication in fishes, since they are important organs in the metal elimination. Both the metal dissolved in water and those originated from the fish diet enter the gills through the blood vessels and are expelled by the gill epithelial cells (Alvorado et al. 2006). The liver, as well as the gills, accumulates pollutants of several kinds in much higher doses than those found in the environment. The literature shows that also, in many cases, the liver has an important role in the storage of contaminants, detoxification, redistribution, and transformation of these elements in the organism (Licata et al. 2005).
Fishes are considered as a very important source of protein for human health, and evaluation of the G. brasiliensis muscle is necessary to know the metal concentration that might be consumed by the population that has access to the Alagados Reservoir fishes. In spite of the G. brasiliensis muscle presenting the lowest metal concentrations when compared to the liver and gills which are not consumed, people who consume this fish are accumulating metal in their organisms, which might result in higher concentrations of these elements in the human organism.
Concentration of metals in relation to body weight, size, and gender
Table 3 presents the intervals of body weight and size (length) of the G. brasiliensis samples as well as the number of male and female animals analyzed.
The statistical analysis indicated an abnormality in the data distribution, with P > 0.05; therefore, no substantial differences were found in the level of metal concentration regarding the gender of G. brasiliensis and the three tissues under analysis. Unlike the results found for the sea species Lethrinus lentjan, average concentrations of metal in the liver and muscle of the female animal were found to be higher when compared to male samples (Al-Yousuf et al. 2000).
The lack of a relation between the genre and metals in the G. brasiliensis tissues observed might be due to the same environmental conditions to which these animals are exposed, and there was only a slight variation in the diet. A study on G. brasiliensis revealed that there are significant differences in diet between genres (male/female) and sexual maturity (immature/mature individuals). Male G. brasiliensis spend more energy to grow, resulting in higher body growth rate and larger sizes than the female ones (Bastos et al. 2011). Therefore, the male should not necessarily present higher concentrations of metal than the female.
Similar to the genre, the relations between the metal concentration in the muscle, gills, and liver and the size (length) of G. brasiliensis did not present significant statistical differences (P > 0.05). A study carried out on sea benthic fish (Mullus barbatus) and on benthic pelagic ones (Pagellus erythrinus) showed that the size of organisms does not have an important role in the metal concentration and that the gender is not statistically significant for any of the species (Giannakopoulou and Neofitou 2014).
Body weight did not show significant statistical difference (P > 0.05) in relation to the concentration of metals under analysis in the G. brasiliensis tissues. In the study carried out on six fish species (Sparus auratus, Atherina hepsetus, Mugil cephalus, Trigla cuculus, Sardina pilchardus, and Scomberesox saurus) from the northeast of the Mediterranean Sea, there was no significant statistical difference in relation to the muscle, liver, and gills and the fish size (length and weight) (Canli and Atli 2003).
Bioconcentration and bioaccumulation of metals in G. brasiliensis
Metal results in the water and sediment samples were used to evaluate the bioconcentration and bioaccumulation of metals, respectively, in G. brasiliensis. Table 4 shows the metal bioaccumulation and bioconcentration factors in the muscle, gills, and liver of G. brasiliensis in the Alagados Reservoir.
Since sediments have great capacity to accumulate metals, even if the amount of these elements in the water is low, animals associated to the bottom, or those that eat waste and benthic organisms, such as the G. brasiliensis, are more susceptible to accumulating them in their tissue, so by knowing the metal concentration in the water and sediment, it is possible to infer the G. brasiliensis contamination in the Alagados Reservoir. The biotic interaction is more intense in closed systems, such as reservoirs, with the occurrence of adverse conditions associated to the environment variables. These factors might contribute to the increase in fish stress, also increasing the occurrence of serious abnormalities as the ones seen in the G. brasiliensis gills in the Lajes Reservoir (Gomes et al. 2012). Thus, the results regarding metal bioaccumulation and bioconcentration in the G. brasiliensis tissue are very important to understand the action of these metals in the water environment and their accumulation in the food chain.
Based on the results obtained from BCF, it is possible to see that the liver has more affinity for metal bioconcentration than the muscle as well as for the bioconcentration of Cu, Co, Cd, Cr, Ag, and Ni than the G. brasiliensis gills. Zn had the highest BCF in the three tissues, being highly superior to the factors found for the remaining metals. In the gills and muscle, the Zn BCF was higher than the other metals analyzed in these two tissues, and in the liver, this value was very close to that found for Cu that reached the highest value in this tissue. Similar to the G. brasiliensis, a study carried out with Sander lucioperca, Silurus glanis, Lota lota, and Cyprinus carpio in the Danube River (Serbia) revealed that Zn presented the highest BCF in the same three tissues of four different species (Subotić et al. 2013).
Despite the Zn concentration being below the limits established for water, sediments, and the Alagados Reservoir G. brasiliensis, as seen in sections “Concentration of metals in water and superficial sediment” and “Metal concentration in the G. brasiliensis tissues”, the Zn bioconcentration in the fish tissue was high, showing that Zn has a higher tendency to concentrate in animal tissues, and its presence in the environment occurs due to possible impurities in fertilizers and waste generated from the intensive breeding of pig and poultry, which are common in the Alagados Reservoir.
The kind of chemical substance, the tissue metabolic properties, and the environmental pollution degree can affect the BCF levels (Uysal et al. 2009). Therefore, the gills had the highest affinity for bioconcentration of Mn, Zn, and Fe, being in accordance with the results obtained in the reservoir water where Fe presented a concentration above the legal limits and Mn was determined with a tendency to high concentrations. The liver had the highest affinity with the metals Cu, Co, Cd, Cr, Ag, and Ni in G. brasiliensis, even if the concentration of these metals was below the limits established for the reservoir water, except for Cd, showing that the bioconcentration in these tissues is more intense even if it presents low levels in the environment. The Cr BCF was studied in carps which, like in G. brasiliensis, presented higher affinity for bioconcentration of this metal in the liver (Palaniappan and Karthikeyan 2009).
The G. brasiliensis muscle has the lowest affinity among all the metals under analysis in relation to the reservoir water, except for Cu that presented the lowest BCF in gills. Pb, Al, and As were not found in the superficial water through the techniques employed; thus, it was not possible to calculate the bioconcentration of these metals in the G. brasiliensis tissues.
The general global tendency of BCF in the metal in the G. brasiliensis muscle was Zn > Cu > Ni > Ag > Co > Cr > Mn > Fe > Cd. It was Zn > Ag > Ni > Mn > Cu > Co > Fe > Cr > Cd in the gills and Cu > Zn > Co > Ag > Ni > Mn > Cr > Cd > Fe in the liver. Regarding muscle and gills of G. brasiliensis, the same tendency was found in BCF of the metals Zn and Ni in the muscle and liver and the Ag BCF revealed the same tendency. Thus, the muscle, the part of the fish which is eaten, is shown to bioconcentrate lower amounts of metal, however, revealing similar tendency as those for the gills and liver, which are target organs for Zn, Ni, and Ag contamination, even if their concentration is low in the reservoir, which constitutes relevant information regarding human consumption, since among these metals, only Zn is essential to the organism, while Ni and Ag are toxic. The gills and liver did not present any tendency in relation to the BCF of all metals.
Therefore, it was possible to verify that the metal bioconcentration in the G. brasiliensis tissues follows the tendency liver > gills > muscle. In fact, it is known that metals present different affinities for specific tissues and the liver is the most appropriate tissue for the evaluation of contamination with metal traces in the whole organism, due to its more efficient capability to accumulate (Papagiannis et al. 2004). The muscles, despite having the lowest bioconcentration of metals than gills and liver, are suitable for assessing contamination and have an important role in human nutrition; thereby, bioconcentration factor as well as their exposure to metals can ensure that the level of metals in tissues is not transferred to the trophic chain (Singh et al. 2012).
BAF results revealed that the liver has higher affinity for the bioaccumulation of metals than the muscle and the higher affinity for the bioaccumulation of Cu, Co, Cd, Cr, Ag, Pb, Ni, and As than the gills in G. brasiliensis. Thus, the liver accumulates a higher amount of metals when compared to the other tissues under analysis, even if the concentration of some metals such as Zn, Pb, and As are below the legal concentrations for sediment in the Alagados Reservoir, similar to Co, which presented low concentration in the reservoir water and had higher BAF in the liver, and this value was above the factors found for the remaining metals in the three tissues under analysis, showing that there is a high tendency of accumulation in this tissue.
Therefore, the G. brasiliensis gills presented higher affinity for the bioaccumulation of Zn and Al, in relation to bioaccumulation in the muscle and liver. As presented before, Al had higher concentration in sediments following the global tendency. The muscle presented the lowest affinity with all the metals under analysis in relation to the reservoir sediment, except for the Cu that presented the lowest BAF in the gills. Cr had the same affinity for the bioaccumulation in the G. brasiliensis gills and liver.
The BAF general global tendency of metals in the G. brasiliensis muscle was Ag > Co > Ni > Cu > Zn > Cr > Al > Pb > Cd > Mn > Fe > As; in the gills, it was As > Ag > Zn > Co > Ni > Al > Cr > Cd > Pb > Cu > Mn > Fe, and the liver presented Co > As > Ag > Ni > Pb > Cu > Cd > Zn > Al > Cr > Mn > Fe. The muscle and gills of the G. brasiliensis did not present any tendency in relation to the BAF of any metals. The gills and liver presented the same BAF tendency of metals Mn and Fe, confirming the results verified for sediments in the reservoir water. Thus, it was possible to verify that the bioaccumulation of metals in the G. brasiliensis tissues follows the global tendency liver > gills > muscle. The muscles are the important part of fish due to its consumption by human beings and to its lipophilic nature. If the metal concentration increases in the muscle according to threshold level, then it can cause serious health effects (Malik et al. 2014).
Taking into consideration the bioconcentration and bioaccumulation factors evaluated in the G. brasiliensis, it was seen that the tissue exposure to metals influences these factors. This data has been investigated and illustrate the importance of absorption routes which might be different among species and for different metals (Marsden et al. 2014). Some parameters influence the accumulation and concentration of pollutant metals in the organism tissues, and the most important to be considered is the concentration of metal to which the organisms are exposed through the water and sediment. However, other factors also influenced the accumulation of metals; the mobility, activity, and bioavailability degrees; seasonal variations such as temperature, pH, and water hardness; competition with other metals; anions bonds; and biotic parameters such as the size and age of individuals (Kehrig et al. 2007; Ebrahimi and Taherianfard 2010).
Conclusions
The level of metal in water of the reservoir was lower than the maximum set forth in the legislation, except for that of Cd and Fe. In sediments, Cu, Cd, Cr, and Ni presented concentrations above the TEL. The Pb and Cr concentrations were above the limits set forth in the law for fish consumption by human beings. Al and Zn presented high concentrations in all tissues and on the superficial sediment. In the gills, the Fe presented a high concentration in relation to the other tissues. The liver presented higher affinity for bioconcentration of metals than the muscle and higher affinity for the bioconcentration of Cu, Co, Cd, Cr, Ag, and Ni than the G. brasiliensis gills. Regarding bioaccumulation, the liver has higher affinity than the muscle and also higher affinity for the bioaccumulation of Cu, Co, Cd, Cr, Ag, Pb, Ni, and As than the gills. Therefore, the liver is the tissue with the highest tendency to bioconcentrate and bioaccumulate metals in G. brasiliensis. The statistical analysis did not identify substantial differences in the levels of metal concentration between the body weight, size (length), and genre of the species and the three tissues under analysis.
References
Abel PD (2002) Water pollution biology. CRC
Abelha MC, Goulart E (2004) Oportunismo trófico de Geophagus brasiliensis (Quoy & Gaimard, 1824) (Osteichthyes, Cichlidae) no reservatório de Capivari, Estado do Paraná, Brasil. Acta Sci Biol Sci 26(1):37–45
Alloway BJ, Ayres DC (1997) Chemical principles of environmental pollution, 2nd edn. Chapman and Hall, p. 395
Alvorado NE, Quesada I, Hylland K, Marigómez I, Soto M (2006) Quantitative changes in metallothionein expression in target cell-types in the gills of turbot (Scophthalmus maximus) exposed to Cd, Cu, Zn and after a depuration treatment. Aquat Toxicol 77:64–77
Al-Yousuf MH, El-Shahawi MS, Al-Ghais SM (2000) Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci Total Environ 256(2–3):87–94
ANVISA (2014) Agência Nacional de Vigilância Sanitária. http://www.anvisa.gov.br/alimentos/legis/especifica/contaminantes.htm.
Asuquo FE, Ewa-Oboho I, Asuquo EF, Udo PJ (2004) Fish species used as biomarker for heavy metal and hydrocarbon contamination for Cross River, Nigeria. Environmentalist 2:29–37
Bastos RF, Condini MV, Varela Junior AS, Garcia AM (2011) Diet and food consumption of the pearl cichlid Geophagus brasiliensis (Teleostei: Cichlidae): relationships with gender and sexual maturity. Neotropical Ichthyol 9(4):825–830
Benincá C, Ramsdorf W, Vicari T, de Oliveira Ribeiro CA, de Almeida MI, de Assis HCS, Cestari MM (2012) Chronic genetic damages in Geophagus brasiliensis exposed to anthropic impact in Estuarine Lakes at Santa Catarina Coast–Southern of Brazil. Environ Monit Assess 184(4):2045–2056
Botté SE, Hugo Freije R, Marcovecchio JE (2007) Dissolved heavy metal (Cd, Pb, Cr, Ni) concentrations in surface water and porewater from Bahia Blanca estuary tidal flats. Bull Environ Contam Toxicol 79:415–421
Brekhovskikh VF, Volkova ZV, Katunin DN, Kazmiruk VD, Kazmiruk TN, Ostrovskaya EV (2002) Heavy metals in bottom sediment in the upper and lower Volga. Water Resour 29(5):539–547
Buratini SV, Brendelli A (2006) Bioacumulação, In: P. A. Zagatto & E. Bertoletti: Ecotoxicologia Aquática Princípios e Aplicações. Rima, pp. 55–87
Canli M, Atli G (2003) The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut 121(1):129–136
Carranza-Álvarez C, Alonso-Castro AJ, Alfaro-de La Torre MC, García-de La Cruz RF (2008) Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial Lagoon in San Luis Potosí, México. Water Air Soil Pollut 188:297–309
Carvalho CEV, Di Beneditto APM, Souza CMM, Ramos RMA, Rezende CE (2008) Heavy metal distribution in two cetacean species from Rio de Janeiro State, south-eastern Brazil. J Mar Biol Assoc U K 88(6):1117–1120
Clemente Z, Busato RH, de Oliveira Ribeiro CA, Cestari MM, Ramsdorf WA, Magalhães VF, Wosiack AC, de Assis HCS (2010) Analyses of paralytic shellfish toxins and biomarkers in a southern Brazilian reservoir. Toxicon 55(2):396–406
CONAMA (2011) Conselho Nacional do Meio Ambiente. Resolução 357, de 17 de março de 2005, Publicada no DOU n° 053, de 18/03/2005, págs. 58–63. Alterada pela Resolução 410/2009 e pela 430/2011. http://www.mma.gov.br/port/conama/res/res05/res35705.pdf.
Davies OA, Allison ME, Uyi HS (2006) Bioaccumulation of heavy metals in water, sediment and periwinkle (Tympanotonus fuscatus var radula) from the Elechi Creek, Niger Delta. Afr J Biotechnol 5(10):968–973
Di Giulio RT, Hinton DE (2008) The toxicology of fishes. CRC
Ebrahimi M, Taherianfard M (2010) Pathological and hormonal changes in freshwater fishes duo to exposure to heavy metals pollutants. Water Air Soil Pollut 217(1):47–55
Ebrahimpour M, Mushrifah I (2008) Heavy metal concentrations (Cd, Cu and Pb) in five aquatic plant species in Tasik Chini, Malaysia. Environ Geol 54:689–698
Environment Canada (1999) Canadian sediment quality guidelines for the protection of aquatic life summary tables. http://www.ec.gc.ca.
FAO/WHO (2014) Food and Agriculture Organization/World Health Organization. Contaminants & food additives. Limit test for heavy metals in food additive specifications—explanatory note. http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/guidelines0/en/
Förstner U, Wittmann GTW (1983) Metal pollution in the aquatic environment, 2ath edn. Springer, Berlin, p 486
Fowler BA, Nordberg GF, Nordberg M, Friberg L (2011) Handbook on the toxicology of metals. Academic
Giannakopoulou L, Neofitou C (2014) Heavy metal concentrations in Mullus barbatus and Pagellus erythrinus in relation to body size, gender, and seasonality. Environ Sci Pollut Res 21(11):7140–53
Gomes ID, Nascimento AA, Sales A, Araújo FG (2012) Can fish gill anomalies be used to assess water quality in freshwater Neotropical systems? Environ Monit Assess 184:5523–5531
Hidetoshi N (1984) Dicionário dos Peixes do Brasil. Editora Editerra, 1° Edição, 482 p
IPARDES (2010) Instituto Paranaense de Desenvolvimento Econômico e Social. Cadernos Municipais – Ponta Grossa. Paraná
Jarić I, Višnjić-Jeftić Ž, Cvijanović G, Gačić Z, Jovanović LJ, Skorić S, Lenhardt M (2011) Determination of differential heavy metal and trace element accumulation in liver, gills, intestine and muscle of starlet (Acipenser ruthenus) from the Danube River in Serbia by ICP-OES. Microchem J 98:77–81
Kehrig HA, Costa M, Malm O (2007) Estudo da contaminação por metais pesados em peixes e mexilhão da Baia de Guanabara—Rio de Janeiro. Trop Oceanogr 35(1):32–50
Khan R, Israili SH, Ahmad H, Mohan A (2005) Heavy metal pollution assessment in surface water bodies and its suitability for irrigation around the Neyevli lignite mines and associated industrial complex, Tamil Nadu, India. Mine Water Environ 24:155–161
Kullander SO, Cichlidae (Cichlids) (2003) In: Checklist of the freshwater fishes of South and Central America, Reis RE, Kullander SO, Ferraris Jr. CJ. Porto Alegre: EDIPUCRS, Brasil, 605–654
Licata P, Trombetta D, Cristiani MT, Naccari C, Martino D, Cal M, Naccari IF (2005) Heavy metals in liver and muscles of bluefin tuna (Thunnus thynnus) caught in the straits of Messina (Sicily, Italy). Environ Monit Assess 107:239–48
Lau S, Mohammed MA, Yen TC, Su’ut S (1998) Accumulation of heavy metals in fresh water molluscs. Sci Total Environ 214:113–121
Malik RN, Hashmi MZ, Huma Y (2014) Heavy metal accumulation in edible fish species from Rawal Lake Reservoir, Pakistan. Environ Sci Pollut Res 21:1188–1196
Marsden ID, Smith BD, Rainbow PS (2014) Effects of environmental and physiological variables on the accumulated concentrations of trace metals in the New Zealand cockle Austrovenus stutchburyi. Sci Total Environ 470–471:324–339
Martinez CBR, Nagae MY, ZaiaCTBV ZDAM (2004) Acute morphological and physiological effects of lead in the neotropical fish Prochilodus lineatus. Braz J Biol 64:797–807
Monroy M, Maceda-Veiga A, de Sostoa A (2014) Metal concentration in water, sediment and four fish species from Lake Titicaca reveals a large-scale environmental concern. Sci Total Environ 487(1):233–244
Monteiro V, Cavalcante DGSM, Viléla MBFA, Sfia SH, Martinez CBR (2011) In vivo and in vitro exposures for the evaluation of the genotoxic effects of lead on the Neotropical freshwater fish Prochilodus lineatus. Aquat Toxicol 104:291–298
Osório FHT, Silva LFO, Piancini LDS, Azevedo ACB, Liebel S, Yamamoto FY, Philippi VP, Silva Oliveira ML, Ortolani-Machado CF, Filipak Neto F, Cestari MM, Silva de Assis HC, Oliveira Ribeiro CA (2013) Water quality assessment of the Tubarão River through chemical analysis and biomarkers in the Neotropical fish Geophagus brasiliensis. Environ Sci Pollut Res. doi:10.1007/s11356-013-1512-5
Palaniappan PL, Karthikeyan S (2009) Bioaccumulation and depuration of chromium in the selected organs and whole body tissues of freshwater fish Cirrhinus mrigala individually and in a binary solution with nickel. J Environ Sci 21:229–236
Papagiannis I, Kagalou I, Leonardos J, Petridis D, Kalfakakou V (2004) Copper and zinc in four freshwater fish species from Lake Pamvotis (Greece). Environ Int 30:357–362
Peakall D, Burger J (2003) Methodologies for assessing exposure to metals: speciation, bioavailability of metals, and ecological host factors. Ecotoxicol Environ Saf 56:110–121
Pereira MO, Calza C, Anjos MJ, Lopes RT, Araújo FG (2006) Metal concentrations in surface sediments of Paraíba do Sul River (Brazil). J Radioanal Nucl Chem 269(3):707–709
Poleksić V, Lenhardt M, Jarić I, Đorđević D, Gačić Z, Cvijanović G, Rašković B (2010) Liver, gills, and skin histopathology and heavy metal content of the Danube starlet (Acipenser ruthenus Linnaeus, 1758). Environ Toxicol Chem 29(3):515–521
Ramalho JFGP, Amaral Sobrinho NMB, Velloso ACX (2000) Contaminação da microbacia de Caetés com metais pesados pelo uso de agroquímicos. Pesq Agrop Brasileira 35(7):1289–1303
Rocha O, Espíndola ELG, Fenerich-Verani N, Verani JR, Rietzler AC (2005) Espécies invasoras em águas doces: estudos de caso e propostas de manejo, 416 p
Salomons W, Förstner U (1984) Metals in the hydrocycle. Springer. p. 349
Shah AQ, Kazi TG, Arain MB, Jamali MK, Afridi HI, Jalbani N, Baig JA, Kandhro GA (2009) Accumulation of arsenic in different fresh water fish species—potential contribution to high arsenic intakes. Food Chem 112:520–524
Shrivastava P, Saxena A, Swarup A (2003) Heavy metal pollution in a sewage-fed lake of Bhopal, (M. P.) India. Lakes Reserv Res Manag 8:1–4
Singh AK, Srivastava SC, Ansari A, Kumar D, Singh R (2012) Environmental monitoring and health risk assessment of African catfish Clarias gariepinus (Burchell, 1822) cultured in rural ponds, India. Bull Environ Contam Toxicol 89:1142–1147
Singh AK, Srivastava SC, Verma P, Ansari A, Verma A (2014) Hazard assessment of metals in invasive fish species of the Yamuna River, India in relation to bioaccumulation factor and exposure concentration for human health Implications. Environ Monit Assess 2186(6):3823–36
Subotić S, Spasić S, Višnjić-Jeftić Z, Hegediš A, Krpo-Ćetković J, Mićković B, Skorić S, Lenhardt M (2013) Heavy metal and trace element bioaccumulation in target tissues of four edible fish species from the Danube River (Serbia). Ecotoxicol Environ Saf 98:196–202
Terra BF, Araújo FG, Calza CF, Lopes RT, Teixeira P (2008) Heavy metal in tissues of three fish species from different trophic levels in a tropical Brazilian River. Water Air Soil Pollut 187:275–284
US EPA (1992) Environmental Protection Agency of United States. Acid digestion of waters for total recoverable or dissolved metals for analysis by FLAA or ICP spectroscopy. Method EPA-3005A. http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/3005a.pdf
US EPA (1996) Environmental Protection Agency of United States. Acid digestion of sediments, sludges and soils. Method EPA-3050B. http://www.epa.gov/wastes/hazard/testmethods/sw846/pdfs/3050b.pdf
US EPA (2001). Methods for collection, storage and manipulation of sediments for chemical and toxicological analyses: technical manual. EPA 823-B-01-002. U.S. Environmental Protection Agency, Office of Water, Washington, DC. http://water.epa.gov/polwaste/sediments/cs/upload/toc.pdf
US EPA (2014a) Environmental Protection Agency of United States. Ecological screening values for surface water, sediment, and soil. WSRC-TR-98–00110, http://www.osti.gov/bridge/purl.cover.jsp?purl=/47642uJvjR/webviewable/4764.PDF.
US EPA (2014b) Environmental Protection Agency of United States. Water quality criteria. National recommended water quality criteria. http://water.epa.gov/scitech/swgidance/standards/criteria/current/index.cfm#C.
Uysal K, Köse E, Bülbül M, Dönmez M, Erdoğan Y, Koyun M, Ömeroğlu C, Özmal F (2009) The comparison of heavy metal accumulation ratios of some fish species in Enne Dame Lake (Kütahya/Turkey). Environ Monit Assess 157:355–362
Višnjić-Jeftić Ž, Jari I, Jovanović LJ, Skorić S, Smederevac-Lalić M, Nikčević M, Lenhardt M (2010) Heavy metal and trace element accumulation in muscle, liver and gills of the Ponticshad (Alosa immaculata Bennet 1835) from the Danube River (Serbia). Microchem J 95:341–344
Vukosav P, Mlakar M, Cukrov N, Kwokal Z, Pižeta I, Pavlus N, Špoljarić I, Vurnek M, Brozinčević A, Omanović D (2014) Heavy metal contents in water, sediment and fish in a karst aquatic ecosystem of the Plitvice Lakes National Park (Croatia). Environ Sci Pollut Res 21:3826–3839
WHO (2011) World Health Organization. Guidelines for drinking water quality, 4th edn. WHO, 564
Wood CM, Farrell AP, Brauner CJ (2011) Fish physiology: homeostasis and toxicology of essential metals, Vol. 31. Academic
Yilmaz F, Özdemir N, Demirak A, LeventTuna A (2007) Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chem 100:830–835
Zheng N, Wang Q, Liang Z, Zheng D (2008) Characterization of heavy metal concentrations in the sediments of three freshwater rivers in Huludao City, Northeast China. Environ Pollut 154:135–142
Acknowledgments
The authors thank the Araucaria Foundation by funding the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
Bioaccumulation and bioconcentration of 12 metals in the Geophagus brasiliensis in the reservoir in the state of Paraná, Brazil were determined.
The muscle showed lower bioconcentration and bioaccumulation of metals when compared to the gills and liver.
The liver presented higher bioconcentration of metals Cu, Co, Cd, Cr, Ag, and Ni and higher bioaccumulation of metals Cu, Co, Cd, Cr, Ag, Pb, Ni, and As when compared to the gills.
The gills presented higher bioaccumulation of Zn and Al and higher bioconcentration of Zn when compared to the muscle and liver.
Rights and permissions
About this article
Cite this article
Voigt, C.L., da Silva, C.P., Doria, H.B. et al. Bioconcentration and bioaccumulation of metal in freshwater Neotropical fish Geophagus brasiliensis . Environ Sci Pollut Res 22, 8242–8252 (2015). https://doi.org/10.1007/s11356-014-3967-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3967-4