Abstract
A hydroponics trial was employed to study the effects of Pseudomonas putida CZ1 (CZ1), a heavy-metal-resistant bacterial strain isolated from the rhizosphere of Elsholtzia splendens (E. splendens), on the uptake and translocation of copper (Cu) in E. splendens. Significant promotion of plant growth coupled with the obvious plant-growth-promoting (PGP) characters of the bacteria suggested that CZ1 would be a plant-growth-promoting rhizobacterium (PGPR) to E. splendens under Cu stress condition. The results of inductively coupled plasma optical emission spectrometry (ICP-OES) showed that CZ1 increased the concentration of Cu in the shoots (up to 211.6 % compared to non-inoculation treatment) and translocation factor (TF) (from 0.56 to 1.83 %) of those exposed to Cu. The distribution of Cu in root cross section measured by synchrotron-based X-ray fluorescence microscopy (SRXRF) indicated that CZ1 promoted the transport of Cu from cortex to xylem in roots, which contributed to the accumulation of Cu in shoots. Furthermore, CZ1 improved the uptake of nutrient elements by plants to oppose to the toxicity of Cu. In summary, P. putida CZ1 acted as a PGPR in resistance to Cu and promoted the accumulation and translocation of Cu from root to shoot by element redistribution in plant root; hence, CZ1 is a promising assistance to phytoremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ecosystems are being contaminated with heavy metals (HMs) due to various human activities, which possesses significant risks to the environment as well as to human health (Abou-Shanab et al. 2006). The cleanup of heavy-metal-contaminated soils is utmost necessary but intractable for their potential toxicity and high persistence (Song et al. 2012). Compared to the conventional physical and chemical methods, phytoremediation has emerged to be a cost-effective, efficient, and environmentally friendly remediation technology, which presents a promising alternative for in situ cleanup of large field application at low to moderate concentration of contaminants (Wei et al. 2010; Kim and Lee 2010). Phytoextraction is one of the key processes of phytoremediation which refers to the use of metal-accumulating or metal-tolerant plants to remove metals from soil by concentrating them in aboveground biomass, i.e., shoots. However, shallow root penetration, low biomass, and slow growth of hyperaccumulators or the low concentration of HMs accumulated in harvestable parts of tolerant plants make the phytoextraction a non-cure-all for contaminated soils (Khan et al. 2000). Moreover, even the growth of metal-tolerant or metal-accumulating plants can be severely inhibited when the concentrations of available metals in the contaminated soil are sufficiently high. Therefore, improvement of plant growth and HM accumulation under stressed growth conditions is critical to the optimum performance of phytoremediation of soils.
An effective way to relieve the toxicity of HMs to plants and enhance the extraction efficiency might involve the combination of rhizosphere microbes with some beneficial effects, such as the plant-growth-promoting rhizobacteria (PGPR) (Glick 1995; Glick et al. 1999). Most of these strains are characterized for traits to cope with contaminants and contribute to the tolerance of their host plants to HMs when present at toxic levels (Hoflich and Metz 1997; Belimov et al. 2005), which may be achieved by bacterial sequestration or the production of enzyme ACC deaminase and siderophores (Sessitsch et al. 2013). Moreover, plant growth promotion plays a major role in the extraction and removal of HMs since a simple improvement in biomass results in an increase in the overall HM yield. Mechanisms such as nitrogen fixation, solubilization of minerals, production of siderophores, and transformation of nutrient elements by bacteria have profound enhancement on plant growth (Glick 2003), and many studies have confirmed the positive effects of PGPR (Abou-Shanab et al. 2006; Guo et al. 2011). Apart from the plant biomass, the success of phytoextraction depends on factors including HM availability to the roots and the ability of the plant to intercept, take up, and accumulate HMs in the shoots (Ernst 2000). The two main bottlenecks in phytoremediation indeed are HM availability in the soil and HM uptake and translocation by the plants. Reports suggested that most metal-resistant rhizobacteria associated with plant-growth-promoting (PGP) characters could improve the availability of heavy metals for plant uptake through solubilization or mobilization and can be effectively utilized for phytoremediation (Sheng and Xia 2006; Ma et al. 2009a). Although there are numbers of studies available describing the influence of free-living and symbiotic microbes on metal mobilization and interactions, there have been few studies referring to contributory microbial processes (Gadd 2010; Rajkumar et al. 2012). With the limitations of traditional techniques, the translocation and interaction of trace elements in plant tissues are rarely studied. In recent years, the utilities of synchrotron-based techniques such as synchrotron radiation X-ray fluorescence (SRXRF) have assisted to localize elements in organisms in situ and explained the tolerant mechanisms of HMs (Tian et al. 2010). Therefore, the application of this new method is prized in the observation and explanation of element distribution and translocation in plants associated with rhizosphere bacteria.
In a previous study, an extremely heavy-metal-resistant rhizobacterium Pseudomonas putida CZ1 (CZ1) was isolated from the rhizosphere of Elsholtzia splendens (a Cu-tolerant plant) grown in heavy-metal-contaminated soil (Chen et al. 2006). CZ1 exhibited high minimal inhibitory concentration value for Cu with about 3 mmol L−1 and was capable of removing about 87.2 % of Cu in aqueous solutions, with specific biosorption capacities of 24.2 mg L−1 Cu (Chen et al. 2006). The ability of CZ1 to regulate the mobility of heavy metals in soil was also studied (Chen et al. 2009). However, how significant CZ1 affect the growth and the HM acquisition of its host plant is still unknown. In this study, a hydroponics trial was employed instead of a pot experiment in order to eliminate the complex distractions of the soils, which was commonly used in the previous studies (Ghosh et al. 2011; Star et al. 2012). The main purposes of this work were to assess the roles and mechanisms of P. putida CZ1 in metal uptake and translocation in Cu-tolerant plant E. splendens and to explore a feasible way to raise efficiency of phytoremediation.
Materials and methods
Bacteria and media
P. putida CZ1, isolated from metal-contaminated soil from mining activities in Zhuji County of Zhejiang province of China, was used as a Cu-tolerant strain in this study. CZ1 was grown and maintained on nutrient broth medium which contained 5.0 g beef extract, 10.0 g peptone, and 5.0 g sodium chloride per liter with an initial pH of 7.0. CZ1 cells were inoculated into 250-mL Erlenmeyer flasks containing 150 mL of sterile medium and cultivated aerobically in an orbital rotary shaker (200 rpm) at 30 °C for 24 h.
Plant culture and treatment
Seeds of E. splendens were soaked in 70 % ethanol for 30 s, surface-sterilized in 10 % (v/v) NaClO4 solution, and washed thoroughly with sterilized deionized water, subsequently germinated in moist gauze immerged in deionized water in controlled dark conditions (25 °C) for 1 week. After germination, seedlings were transferred into nutrient solutions until the four-leaf stage, and then, the uniform seedlings were transplanted to 3-L vessels filled with nutrient solution. The nutrient solution with pH adjusted to 6.0 contained macronutrients (in mM), 1.0 Ca(NO3)2, 0.5 MgSO4, and 0.5 K2HPO4, and micronutrients (in μM), 27.0 Fe(III)–EDTA, 23.0 H3BO3, 0.8 CuSO4, 0.5 Na2MoO4, 0.5 ZnSO4, and 4.5 MnSO4 (Shi et al. 2008). Four seedlings were cultured in each vessel for 1 week before being amended.
About 20-day uniform seedlings were then transferred to new nutrient solutions containing the following: no additive as control (T-CK), 100 μM CuSO4 (T-Cu), 1/1000 CZ1 (v/v) (T-CZ1), 100 μM CuSO4 + 1/1000 CZ1 (v/v) (T-Cu + CZ1), and grown for another 14 days. During the cultivation, plants were grown in a controlled environment growth chamber with a 16-h, 25 °C day and 8-h, 20 °C night regime, and 60–70 % relative humidity. Light conditions in the growth chamber were fixed to 230–240 lE m−2 s−1. The nutrient solutions were aerated continuously and renewed every 3 days to sustain the growth of plants (Shi et al. 2008).
Growth parameter measurement of plant
The plants were harvested after 14-day treatment, and the shoots and roots were washed with deionized water. The lengths of roots and shoots were directly measured with a ruler, and the fresh weights (FW) and the dry weights (DW) of roots and shoots were also recorded before and after being oven-dried at 70 °C for 48 h.
PGP characters of the bacterium
All the PGP characterizations of CZ1 were conducted in both Cu-free and 100 μM CuSO4 media.
The phosphorus solubilization of the strain was detected in Pikovskaya medium where in tricalcium phosphate was used as described by Gaur (1990). The agars inoculated with CZ1 were incubated in the dark at 30 °C for 3 days, and the inoculation of pure broth culture was set as control. Transparent halos around the colonies on agar were indicative of phosphorus solubilization.
A modified MSA-CAS method was used to detect the siderophore secretion by the strains (Zhao et al. 2006). The blue agars inoculated with CZ1 were incubated in the dark at 30 °C for 2 days, and the inoculation of pure broth culture was set as control. Orange halos around the colonies on blue agar were indicative of siderophore excretion. The ratio (D/d) of the orange halo diameters (D) to the colony diameters (d) was calculated to evaluate the siderophore production by CZ1.
The excretion of indicative of indole acetic acid (IAA) was determined according to Libbert and Risch (1969). The tested bacterial strains were cultured in Luria-Bertani (LB) medium amended with 100 mg L−1 l-tryptophan at 30 °C, 100 rpm for 2 days. After incubation, a 50-μL cell suspension was transferred into a plate and mixed with 50 μL of Salkowski’s reagent (50 mL 35 % HClO4 + l mL 0.5 M FeCl3), and allowed to stand at room temperature for 25 min. Pink colors were indicative of IAA excretion. Fifty milligrams per liter of pure IAA was set as the positive control, and the incubation without l-tryptophan was set as the negative control. LB medium without bacterial inoculation was set as control. The same procedures were conducted, with a 2-mL cell suspension mixed with 4 mL of Salkowski’s reagent, to quantify the IAA production. The absorbance of pink color developed after 25-min incubation was read at 530 nm. The IAA concentration in culture was determined using a calibration curve of pure IAA as a standard following the linear regression analysis.
Sample preparation and X-ray imaging
Uniform roots under different treatments were selected, flushed cleanly with the deionized water and dried surface of water by filter paper. Maturation zone of roots were embedded in the cryostat chuck, and subsequent 60-μm-thick cross sections from the roots were achieved using a low-profile microtome blade (Paracut; McCormick Scientific). Then, the slices were placed on 3 M adhesive tapes (Scotch tape 810; 3M Corporation) and examined via microscopy to confirm the integrated structure of roots sections. Well-structured sections were selected and stored at −20 °C until analysis.
Specimens were imaged with the scanning X-ray microprobe at beamline 15U1 at the SSRF (Shanghai, China) according to Chen et al. (2011). Undulator-generated X-rays of 10-keV incident energy were monochromatized with a single bounce Si (ӀӀӀ) monochromator and focused to a measured spot size of 0.3 × 0.3 μm using Fresnel zone plate optics (X-radia, Concord, CA). Roots were raster-scanned in 1.0-μm steps, and fluorescence spectra were collected for 1- to 2-s dwell times by a three-element UltraLE Ge-detector (Canberra, Meridien, CT) (Finney et al. 2007). Quantitation and image processing were performed with Matlab; standardization to convert the fluorescence signal to a two-dimensional concentration in micrograms per square centimeter was achieved by fitting spectra against the signal derived from standards.
ICP-OES analysis of metal concentrations of plant
Dried samples of shoot and root tissues were triturated and homogenized with mortars and pestles. A final sample of 0.5 g was digested by adding 10 mL HNO3-HCl mixture (3:1, v/v) in a microwave digestion unit at 200 °C and 90-bar pressure for 30 min. After the digestion, the sample was diluted to 50 mL with 1 % HCl and directly analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES). The translocation factor (TF) was also calculated as follows to evaluate the metal translocation in plants (Zacchini et al. 2009):
where Mshoot is the metal accumulation in plant shoots (mg kg−1) and Mroot is the metal accumulation in plant roots (mg kg−1). The TF value is represented in percent.
Statistical analysis
All of the experiments were conducted in triplicate, and mean values were used in the analysis of data. Statistical analysis was performed using Oringin 8.0 for Windows. The treatment effects were calculated using one-way analysis of variance (ANOVA) and the least-significant difference (LSD) multiple-range test in Oringin 8.0. Differences with values of p < 0.05 were considered statistically significant.
Results
Effects of metal-resistant bacterium on plant growth
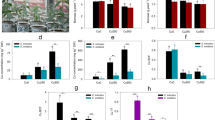
The effects of CZ1 on plant growth were observed (Fig. 1) and characterized by measuring length and biomass of plant tissues under different treatments (Fig. 2). Without additional Cu amendment, CZ1 showed rather low to no promoting effects on shoot length (Fig. 2a) and biomass production (Fig. 2b) of E. splendens, except for the decreased root length (Fig. 2a). Although the length and biomass of plant tissues were not significantly (p < 0.05) affected by 100-μM Cu pollutions in nutrient solutions, E. splendens did present a slight toxic symptom: leaves becoming light yellow in color (Fig. 1c, d) which suggested a lack of chlorophyll content. Then, the inoculation of CZ1 into the Cu stress solutions significantly (p < 0.05) enhanced both the shoot length (from 31.56 to 39.98 cm) and the shoot biomass (FW from 5.93 to 12.83 g, and DW from 1.10 to 2.40 g) in T-Cu + CZ1 compared to those in T-Cu. Meanwhile, CZ1 promoted the root biomass production (FW from 2.75 to 5.07 g, and DW from 0.23 to 0.41 g) of plants under Cu stress condition.
Stem and root length (a) and biomass (b) of E. splendens grown in different solutions (FW fresh weight, DW dry weight). The values were given as mean ± SD of triplicate samples with four seedlings each. Different letters represent different statistical groups suggested by the LSD multiple comparisons at p < 0.05 level
PGP characters of bacterium
The phosphorus solubilization and the siderophores and IAA productions were determined with or without Cu amendment (Fig. 3). The unapparent transparent halos in both the Cu-free and Cu-amended phosphate media indicated that CZ1 had little ability to solubilize phosphates (Fig. 3a). The orange halos indicating the siderophores excretion were observed in both the Cu-free and 100 μM Cu-amended CAS agars (Fig. 3b). The ratio (D/d) of siderophores produced by Cu-incubated bacteria was significant larger than that of Cu-free bacteria (Fig. 3c), which suggested a higher excretion of siderophores by CZ1 in Cu-amended culture. Both the Cu-free and 100 μM CuSO4-incubated bacterial suspensions presented pink colors, while the latter showed darker in color (Fig. 3d). The production level of IAA increased significantly with the Cu amendment (from 7.44 mg L−1 in the Cu-free culture to 11.15 mg L−1 in the Cu-amended culture) (Fig. 3e).
PGP characters of CZ1 in different culture media, including (a) phosphorus solubilization (CK, axenic control; Cu−, Cu-free incubation; Cu+, 100 μM CuSO4 incubation). Transparent halos around the colonies on agar are indicative of phosphorus solubilization; (b, c) siderophore production (CK, axenic control; Cu−, Cu-free incubation; Cu+, 100 μM CuSO4 incubation) (orange halos around the colonies on blue agar are indicative of siderophore excretion); (d, e) IAA production (1–4, reagent control; 5 and 6, negative control; 7, Cu-free incubation; 8, 100 μM CuSO4 incubation; 9, 50 mg L−1 IAA as positive control; L−, without l-tryptophan; L+, 100 mg L−1 l-tryptophan; Cu−, without Cu; Cu+, 100 μM CuSO4). Pink colors were indicative of IAA excretion. The values were given as mean ± SD of triplicate samples with four colonies for siderophores and triplicate samples for IAA. Different letters represent different statistical groups suggested by the LSD multiple comparisons at p < 0.05 level
Effects of metal-resistant bacterium on metal accumulation in plant
The Cu accumulation and TF of plants are shown in Fig. 4 and Table 1. The inoculation of CZ1 significantly increased (p < 0.05) the Cu concentration in the plant shoots, with or without Cu stress, while the considerable microbial-induced promoting effect on Cu accumulation in roots was only observed under non-stress condition (Fig. 4). Combined with the biomass productions, the total Cu contents in the shoots and roots of plants in T-CZ1 were up to 3.5-fold and 35.6-fold, respectively, larger than the axenic control (Table 1). With increasing concentration of Cu in nutrient solutions, the Cu accumulation in plant shoots treated with both Cu and CZ1 were nearly 6.8 times higher than that of those exposed to Cu only, while the Cu accumulation in roots was merely 1.7-fold in comparison (Table 1). TF indicates the efficiency of the plant in translocating the accumulated metal from its roots to shoots. It is obvious that the inoculation of CZ1 reduced the TF value from 7.79 % in T-CK to 0.65 % in T-CZ1 (Fig. 4). Contrarily, as contamination level increased, the TF value switched to 1.83 % with bioaugmentation, which was only 0.56 % in the axenic plants (Fig. 4).
Concentrations of other elements (including Fe, Mn, Zn, Ca, and P) were also measured, and the results are listed in Table 2. Most of the metal element accumulation decreased in the plants treated with 100 μM Cu (except for Mn and Zn in shoots). In contrast, concentration of P in plants increased significantly (p < 0.05) coupled with the elevated Cu content in nutrient solutions, which was similar to Li et al. (2007) and Guo et al. (2011). The inoculation of CZ1 exhibited different effects on the uptake, transport, and accumulation of trace elements in the plants treated with or without Cu amendment. We found an overall reduction in metal accumulations in the roots of plants in T-CZ1 with respect to those in the controls, while the metal accumulations in shoots were not notably affected. However, when treated with 100 μM Cu, CZ1 largely enhanced the accumulation of all elements in both shoots and roots. It is worth noting that the concentration of Fe in the shoots and Ca in the roots of plants in T-Cu + CZ1 could be 10.3 and 35.9 times higher than that in the corresponding tissues of plants in T-Cu. In addition, inoculation with CZ1 enhanced the P accumulation under Cu stress much more profoundly than that in non-stress condition.
Effects of metal-resistant bacterium on spatial distributions of elements in roots
Due to the rotational symmetry character of plant roots, we chose half of the cross section of roots to be analyzed by SRXRF. As illustrated in Fig. 5, Cu, Fe, Mn, Zn, and Ca mainly accumulated in the epidermis and/or stele of E. splendens root. Compared with the control, bioaugmentation roots in stress-free treatment obtained a relative higher Cu accumulation in both periphery and stele. The root exposed to 100 μM Cu obtained a high Cu accumulation in the periphery, indicating a great adhesion of Cu onto the root surface. Coupled with the roughly consistent Cu concentrations in the roots of T-Cu and T-Cu + CZ1, the comparable higher Cu accumulation in the stele of bioaugmentation roots under Cu stress suggested that CZ1 had a positive effect on the transferring of Cu from the epidermis to the xylem; hence, the Cu accumulation in shoots increased. Spatial distributions of other elements are also illustrated in Fig. 5, and the image showed high correlations between Cu and any other element in distribution (especially Fe).
Discussion
E. splendens has been widely used in the phytoremediation of Cu-contaminated sites (Chen et al. 2005; Song et al. 2004). However, the growth and development of E. splendens will be negatively affected as a result of exposure to heavy metal contaminants with high toxicity (Jiang et al. 2004), which was confirmed by the reduced chlorophyll content, nutrient uptake, and thus the decreased biomass production of plants exposed to 100 μM Cu in this study. Certain characters have severely limited the application of phytoextraction from polluted sites (Khan et al. 2000; Quartacci et al. 2006). CZ1 was identified as metal-resistant bacteria, which were commonly suggested to be able to proliferate and promote plant growth in some PGPR ways in presence of high levels of toxic metals (Ma et al. 2009b; Rani et al. 2008). Apparently, the boom of both shoots and roots of bacterial inoculated plants under Cu stress condition implies that CZ1 resembles a kind of PGPR and makes contribution to the antagonism of E. splendens with toxic effect. The absence of stimulating effects on E. splendens growth following inoculation of CZ1 without Cu amendment also agreed with the saying that PGPR typically have little or no measurable effect on plant growth when the plants are cultivated under optimal and stress-free conditions (Glick 2010). In particular, CZ1 significantly reduced the plant root length in unpolluted sites, which was in remission coupled with the Cu amendment. Indeed, the maintenance of a metal-resistant microbial community was likely to depend on the presence of metal in the growth medium (Mahler et al. 1986). Thus, the absence of Cu in the growth medium could inhibit the growth of CZ1, which was also confirmed by our previous study (Chen et al. 2006). When the bacteria are under growth restriction condition, more nutrients are required to maintain the basic growth and metabolism of bacterial cells and consequently increased the nutrient competition between bacteria and plants. In addition, the deficiency of nutrient contents in the bioaugmentation roots under stress-free condition can support the saying of nutrient competition.
In order to explain the specific mechanisms of the growth-promoting effect of CZ1, we tested the phosphate-solubilizing capacity of CZ1 and also detected the production of siderophores and the secretion of IAA, with or without Cu amendment. The lack of strong phosphorus solubilization of CZ1 was consistent with the major Cu specie as Cu3(PO4)2 in CZ1 biofilms (Chen et al. 2011), which implied that phosphate solubilization is not the major mechanism adopted by CZ1 toward heavy metals. Generally, heavy metal contamination is often associated with negative effects on plant mineral nutrition, particularly the iron deficiencies, thus affecting plant growth (Parida et al. 2003). Siderophore complexes feature importantly in the successful survival and growth of plants under metal stress by supplying the plant with nutrients, particularly iron, thus alleviating the metal toxicity (Bar-Ness et al. 1992; Dimkpa et al. 2008). Up to now, many species of metal-resistant bacteria have been demonstrated to have the ability to produce siderophores and contribute to the stimulation of plant growth (Rajkumar et al. 2010). The siderophore excretions of CZ1 with or without Cu amendment were consistent with the results of plant growth in the analogical treatments, which suggested that CZ1 might cope with Cu stress by siderophore excretion. Meanwhile, the biosynthesis of IAA with their excretion is been well documented to make contribution to the bacterial plant-growth-promoting effect (Lambrecht et al. 2000). Commonly, heavy metals, such as Cu2+ and Cd2+, can suppressed the production of IAA by bacteria (Kamnev et al. 2005), which was opposite to our results. This might be attributed to the metal-resistant characteristic of CZ1. The higher production level of IAA excreted by CZ1 in the Cu-amended culture coupled with the stimulated plant growth under Cu stress, demonstrating that CZ1 facilitated the plant growth by excreting IAA in heavy-metal-polluted sites. Meanwhile, the siderophores could promote bacterial IAA synthesis by reducing the detrimental effects of heavy metals through chelation reaction (Dimkpa et al. 2008), which was confirmed by the corresponding increase in the siderophores and IAA excretion of CZ1 under Cu stress. Furthermore, the rhizosphere microbes influenced trace element speciation and mobility through processes such as chemical transformation, chelation, and protonation (Sessitsch et al. 2013), resulting in a high acquisition by plants. For example, rhizobacteria can enhance tolerance of host plants by improving the P absorption (Davies et al. 2001; Liu et al. 2000), which was also observed in this study. The enhanced uptake of several vital nutrient elements by inoculating plants in this study clearly indicate the potential of CZ1 to promote plant growth under Cu stress, thus potentially helping to overcome the most crucial limitations of phytoremediation.
As a metal-tolerant plant, E. splendens suffered from limitations including less accumulation of the target heavy metals from the culture medium and low translocation of the accumulated heavy metals from roots to shoots (Song et al. 2004). Under stress-free condition, E. splendens could still absorb Cu from the micro-Cu contained and continuously renewed nutrient solutions during the cultivation period, and accumulated parts of Cu in both shoots and roots. CZ1 cells were free-living once applied into the nutrient solutions and could adsorb amounts of Cu from the aqueous solutions. Then, as time goes on, the Cu-rich bacterial cells would like to cluster or adhere to the plant roots, in order to get access to the carbon source and other nutrient acquisitions from the root exudates (Lugtenberg et al. 2001). These procedures might explain the profound increase in the root Cu accumulation in the plants exposed to bacteria without Cu amendment. However, because of the achievement of maximum adsorption capacity in the roots (Keskinkan et al. 2004), no obvious improvement of root Cu accumulation was observed with bioaugmentation in the presence of 100 μM Cu. As a matter of fact, the rhizobateria secretions, such as phosphate solubilization, IAA, and siderophores, were evidenced to be involved in the increasing bioavailability and facilitating root absorption of heavy metals (Abou-Shanab et al. 2005). In this study, the IAA and siderophore production of CZ1 were related to the metal accumulations in bioaugmentation plants, especially in the Cu-amended media, which suggested that CZ1 might contributed to the heavy metal uptake of plants due to their PGP characters.
Metal translocation to the plant shoots is crucially important in an effective phytoextraction due to the unfeasible harvest of the root biomass (Zacchini et al. 2009; Tangahu et al. 2011). Compared to the axenic treatments, the bacterial inoculated shoots yielded significantly higher Cu accumulations. The microbial-induced enhancement on shoot Cu accumulation was more remarkable in Cu-contaminated sites, which can also be evidenced by the changing TF values. The reduced TF value of bioaugmentation plants in the stress-free treatment could be proved by the similar study that Cd was immobilized by the bacteria on the root surface and was not easily available for the plants, even when the Cd content in the roots of barley plants inoculated with Azospirillum lipoferum 137 was increased (Belimov and Dietz 2000). Previously performed studies also supported the results by providing the Cu speciation bound by CZ1 cells and biofilms, which was Cu-oxalate and Cu3(PO4)2, respectively (Chen et al. 2007; Chen et al. 2011), being insoluble and unavailable to plants. However, when the plants were exposed to 100 μM Cu, CZ1 did have the potential to increase the Cu translocation from plant roots to shoots, which was common to most selected metal-resistant rhizosphere bacteria (Cabello-Conejo et al. 2011). Scientists demonstrated that the release of orgainc acids from both plant roots and bacterial cells was stimulated by the presence of trace elements (Li et al. 2010; Ghosh et al. 2011), which will in turn mobilize and solubilize the immobilized Cu by CZ1. Meanwhile, in some plant-rhizobia interactions, invasion can happen through fissures at the lateral root base and at cortical, intercellular crack entry (Goormachtig et al. 2004). Then, bacteria can penetrate into the root xylem vessels and continue their progression to reach vegetative plant parts (Compant et al. 2010). Generally, plants under heavy metal stress have more cracks in the roots, resulting in the invasion much easier. Since about 50 % of the Cu were actively taken up by CZ1, with the remainder being passively bound to the bacterium (Chen et al. 2006; Chen et al. 2008), we speculated that the microbial-induced TF enhancement under Cu stress might partly attribute to the invasion of CZ1 to the inner roots of E. splendens. Certainly, techniques based on fluorescence staining were required in the further studies to observe the bacterial colonization and movement in the plants.
To further explain the influence of CZ1 on the uptake and translocation of HMs in plants, we employed SRXRF to observe the heavy metal distribution in the cross section of E. splendens roots. Without Cu amendment, CZ1 with Cu adsorption from the nutrient solutions adhered onto the root surface, resulting in a higher Cu accumulation in the root periphery and consequently promoted the Cu transferring into the stele and the xylem. These results were consistent with the results of Cu content in plant tissues. The amended Cu in the nutrient solutions were strongly retained in root cell walls to prevent the uptake and transport to cell components (Clemens 2001; Hall 2002). Element translocation from root to shoot requires a membrane transport step from root symplast into xylem apoplast to cross the impermeable Casparian strip in the endodermis (Pilon-Smits 2005). This symplastic transport is an energy-dependent saturable process, which usually has a large negative resting potential inside the membrane (Bubb and Lester 1991). The charged CZ1 cells and their extracellular compounds might alter the potential and accelerated the transport of Cu through symplast pathway, leading to a higher accumulation into the root xylem. Besides, the obvious high correlation between Cu and other trace elements in root distribution (for instance Fe, which was consistent with our previous study on CZ1 biofilms (Chen et al. 2011)) might attribute to the lack of selectivity in transmembrane ion transports (Ghosh and Singh 2005), which suggested a positive operation of elements co-absorption in the microbe-associated plant system. So far, there are few reports discussing the direct evidences of root cross-sectional metal translocation by rhizobacteria, and the mechanisms are still under debate.
Conclusion
As CZ1 showed stimulation effects on plant growth and Cu accumulation in E. splendens under Cu stress and these effects were consistent with some PGP characters of CZ1, we suggest that CZ1 acted as Cu-resistant bacteria coupled with PGPR and contribute to the uptake and translocation of Cu by E. splendens. With SRXRF, we observed that the elemental transferring is the aggregation of CZ1 to the root surface with amounts of Cu absorbed or adsorbed, followed by the redistribution of Cu from cortex to xylem. In conclusion, inoculation with CZ1 could be a promising approach to increase the efficiency of the phytoextraction of Cu by E. splendens. The development of this approach in future studies will include investigations of metal mobilization in the rhizosphere of plants in field study and transfer mechanisms with inoculation at the molecular level, and the colonization of CZ1 to plant root as well.
References
Abou-Shanab R, Ghozlan H, Ghanem K, Moawad H (2005) Behaviour of bacterial populations isolated from rhizosphere of Diplachne fusca dominant in industrial sites. World J Microbiol Biotechnol 21(6–7):1095–1101
Abou-Shanab R, Angle J, Chaney R (2006) Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biol Biochem 38(9):2882–2889
Bar-Ness E, Hadar Y, Chen Y, Shanzer A, Libman J (1992) Iron uptake by plants from microbial siderophores a study with 7-nitrobenz-2 oxa-1, 3-diazole-desferrioxamine as fluorescent ferrioxamine B analog. Plant Physiol 99(4):1329–1335
Belimov AA, Dietz KJ (2000) Effect of associative bacteria on element composition of barley seedlings grown in solution culture at toxic cadmium concentrations. Microbiol Res 155(2):113–121
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard ( Brassica juncea L. Czern.). Soil Biol Biochem 37(2):241–250
Bubb J, Lester J (1991) The impact of heavy metals on lowland rivers and the implications for man and the environment. Sci Total Environ 100:207–233
Cabello-Conejo MI, Becerra-Castro C, Monterroso C, Prieto-Fernández A, Mench M, Kidd PS (2011) Effects of rhizobacterial inoculation on biomass and nickel concentration in Alyssum pintodasilvae. Paper presented at the In: Proceedings of the 11th In-ternational Conference on the Biogeochemistry of Trace Elements (ICOBTE), Florence, Italy, 4–7th July 2011
Chen YX, Wang YP, Lin Q, Luo YM (2005) Effect of copper-tolerant rhizosphere bacteria on mobility of copper in soil and copper accumulation by Elsholtzia splendens. Environ Int 31(6):861–866
Chen XC, Shi JY, Chen YX, Xu XH, Xu SY, Wang YP (2006) Tolerance and biosorption of copper and zinc by Pseudomonas putida CZ1 isolated from metal-polluted soil. Can J Microbiol 52(4):308–316
Chen XC, Shi JY, Chen YX, Xu XH, Chen LT, Wang HP, Hu TD (2007) Determination of copper binding in Pseudomonas putida CZ1 by chemical modifications and X-ray absorption spectroscopy. Appl Microbiol Biotechnol 74(4):881–889
Chen XC, Chen LT, Shi JY, Wu WX, Chen YX (2008) Immobilization of heavy metals by Pseudomonas putida CZ1/goethite composites from solution. Colloids Surf B: Biointerfaces 61(2):170–175
Chen XC, Hu SP, Shen CF, Dou CM, Shi JY, Chen YX (2009) Interaction of Pseudomonas putida CZ1 with clays and ability of the composite to immobilize copper and zinc from solution. Bioresour Technol 100(1):330–337
Chen GC, Chen XC, Yang YQ, Hay AG, Yu XH, Chen YX (2011) Sorption and distribution of copper in unsaturated Pseudomonas putida CZ1 biofilms as determined by X-ray fluorescence microscopy. Appl Environ Microbiol 77(14):4719–4727
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212(4):475–486
Compant S, Clement C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42(5):669–678
Davies FT, Puryear JD, Newton RJ, Egilla JN, Grossi JAS (2001) Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus). J Plant Physiol 158(6):777–786
Dimkpa CO, Svatos A, Dabrowska P, Schmidt A, Boland W, Kothe E (2008) Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 74(1):19–25
Ernst WH (2000) Evolution of metal hyperaccumulation and phytoremediation hype. New Phytol 146(3):357–358
Finney L, Mandava S, Ursos L, Zhang W, Rodi D, Vogt S, Legnini D, Maser J, Ikpatt F, Olopade OI, Glesne D (2007) X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. PNAS 104(7):2247–2252
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156(3):609–643
Gaur A (1990) Phosphate solubilizing micro-organisms as biofertilizer. Omega Scientific Publishers, New Delhi, pp 16–72
Ghosh M, Singh S (2005) A review on phytoremediation of heavy metals and utilization of its by products. Asian J Energy Environ 6(4):18
Ghosh P, Rathinasabapathi B, Ma LQ (2011) Arsenic-resistant bacteria solubilized arsenic in the growth media and increased growth of arsenic hyperaccumulator Pteris vittata L. Bioresour Technol 102(19):8756–8761
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41(2):109–117
Glick BR (2003) Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21(5):383–393
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28(3):367–374
Glick BR, Patten CL, Holguin G, Penrose DM (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. Imperial Collage Press, London
Goormachtig S, Capoen W, James EK, Holsters M (2004) Switch from intracellular to intercellular invasion during water stress-tolerant legume nodulation. PNAS 101(16):6303–6308
Guo J, Tang S, Ju X, Ding Y, Liao S, Song N (2011) Effects of inoculation of a plant growth promoting rhizobacterium Burkholderia sp. D54 on plant growth and metal uptake by a hyperaccumulator Sedum alfredii Hance grown on multiple metal contaminated soil. World J Microbiol Biotechnol 27(12):2835–2844
Hall J (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53(366):1–11
Hoflich G, Metz R (1997) Interactions of plant-microorganism-associations in heavy metal containing soils from sewage farms. Bodenkultur 48(4):239–247
Jiang LY, Yang X, He Z (2004) Growth response and phytoextraction of copper at different levels in soils by Elsholtzia splendens. Chemosphere 55(9):1179–1187
Kamnev AA, Tugarova AV, Antonyuk LP, Tarantilis PA, Polissiou MG, Gardiner PH (2005) Effects of heavy metals on plant-associated rhizobacteria: comparison of endophytic and non-endophytic strains of Azospirillum brasilense. J Trace Elem Med Biol 19(1):91–95
Keskinkan O, Goksu MZL, Basibuyuk M, Forster CF (2004) Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresour Technol 92(2):197–200
Khan AG, Kuek C, Chaudhry TM, Khoo CS, Hayes WJ (2000) Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 41(1–2):197–207
Kim SH, Lee IS (2010) Comparison of the ability of organic acids and EDTA to enhance the phytoextraction of metals from a multi-metal contaminated soil. Bull Environ Contam Toxicol 84(2):255–259
Lambrecht M, Okon Y, Vande Broek A, Vanderleyden J (2000) Indole-3-acetic acid: a reciprocal signalling molecule in bacteria–plant interactions. Trends Microbiol 8(7):298–300
Li WC, Ye ZH, Wong MH (2007) Effects of bacteria on enhanced metal uptake of the Cd/Zn-hyperaccumulating plant, Sedum alfredii. J Exp Bot 58(15–16):4173–4182
Li WC, Ye ZH, Wong MH (2010) Metal mobilization and production of short-chain organic acids by rhizosphere bacteria associated with a Cd/Zn hyperaccumulating plant, Sedum alfredii. Plant Soil 326(1–2):453–467
Libbert E, Risch H (1969) Interactions between plants and epiphytic bacteria regarding their auxin metabolism. Physiol Plant 22(1):51–58
Liu A, Hamel C, Hamilton RI, Ma BL, Smith DL (2000) Acquisition of Cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels. Mycorrhiza 9(6):331–336
Lugtenberg BJ, Dekkers L, Bloemberg GV (2001) Molecular determinants of rhizosphere colonization by Pseudomonas. Annu Rev Phytopathol 39(1):461–490
Ma Y, Rajkumar M, Freitas H (2009a) Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J Hazard Mater 166(2):1154–1161
Ma Y, Rajkumar M, Freitas H (2009b) Inoculation of plant growth promoting bacterium Achromobacter xylosoxidans strain Ax10 for the improvement of copper phytoextraction by Brassica juncea. J Environ Manag 90(2):831–837
Mahler I, Levinson HS, Wang Y, Halvorson HO (1986) Cadmium-and mercury-resistant bacillus strains from a salt marsh and from Boston Harbor. Appl Environ Microbiol 52(6):1293–1298
Parida BK, Chhibba IM, Nayyar VK (2003) Influence of nickel-contaminated soils on fenugreek (Trigonella corniculata L.) growth and mineral composition. Sci Hortic 98(2):113–119
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39
Quartacci MF, Argilla A, Baker AJM, Navari-Izzo F (2006) Phytoextraction of metals from a multiply contaminated soil by Indian mustard. Chemosphere 63(6):918–925
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28(3):142–149
Rajkumar M, Sandhya S, Prasad M, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30(6):1562–1574
Rani A, Shouche YS, Goel R (2008) Declination of copper toxicity in pigeon pea and soil system by growth-promoting Proteus vulgaris KNP3 strain. Curr Microbiol 57(1):78–82
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel W, Fallmann K, Puschenreiter M (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194
Sheng XF, Xia JJ (2006) Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 64(6):1036–1042
Shi JY, Wu B, Yuan XF, Cao YY, Chen XC, Chen YX, Hu TD (2008) An X-ray absorption spectroscopy investigation of speciation and biotransformation of copper in Elsholtzia splendens. Plant Soil 302(1–2):163–174
Song J, Zhao FJ, Luo YM, McGrath SP, Zhang H (2004) Copper uptake by Elsholtzia splendens and Silene vulgaris and assessment of copper phytoavailability in contaminated soils. Environ Pollut 128(3):307–315
Song X, Hu X, Ji P, Li Y, Chi G, Song Y (2012) Phytoremediation of cadmium-contaminated farmland soil by the hyperaccumulator Beta vulgaris L. var. cicla. Bull Environ Contam Toxicol 88(4):623–626
Star L, Matan O, Dardanelli MS, Kapulnik Y, Burdman S, Okon Y (2012) The Vicia sativa spp. nigra-Rhizobium leguminosarum bv. viciae symbiotic interaction is improved by Azospirillum brasilense. Plant Soil 356(1–2):165–174
Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng 2011
Tian S, Lu L, Yang X, Webb SM, Du Y, Brown PH (2010) Spatial imaging and speciation of lead in the accumulator plant Sedum alfredii by microscopically focused synchrotron X-ray investigation. Environ Sci Technol 44(15):5920–5926
Wei S, Zhou Q, Zhan J, Wu Z, Sun T, Lyubu Y, Prasad MNV (2010) Poultry manured Bidens tripartite L. extracting Cd from soil—potential for phytoremediating Cd contaminated soil. Bioresour Technol 101(22):8907–8910
Zacchini M, Pietrini F, Mugnozza GSM, Iori V, Pietrosanti L, Massacci A (2009) Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollut 197(1–4):23–34
Zhao X, Chen S, Xie Z, Shen P (2006) Isolation, identification and over-siderophores production of Pseudomonas fluorescens sp-f. Acta Microbiol Sin 46(5):691–695
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant Nos. 21177109 and 11179025), Natural Science Foundation for Distinguished Young Scholars of Zhejiang Province of China (Grant No. R5110031), and Zhejiang Provincial Science and Technology Program of China (Grant No. 2010c33057). We sincerely thank the beamline 15U1 in the Shanghai Synchrotron Radiation Facility (SSRF) for the SRXRF data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Xu, C., Chen, X., Duan, D. et al. Effect of heavy-metal-resistant bacteria on enhanced metal uptake and translocation of the Cu-tolerant plant, Elsholtzia splendens . Environ Sci Pollut Res 22, 5070–5081 (2015). https://doi.org/10.1007/s11356-014-3931-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3931-3