Abstract
Assessment of exposure and effect of fish to pharmaceuticals that contaminate aquatic environment is a current major issue in ecotoxicology and there is a need to develop specific biological marker to achieve this goal. Benzyloxy-4-trifluoromethylcoumarin-O-debenzyloxylase (BFCOD) enzymatic activity has been commonly used to monitor CYP3A activity in fish. In this study, we assessed the capacity of a panel of toxicologically relevant chemicals to modulate BFCOD activity in fish, by using in vitro and in vivo bioassays based on fish liver cell lines (PLHC-1, ZFL, RTL-W1) and zebrafish embryos, respectively. Basal BFCOD activity was detectable in all biological models and was differently modulated by chemicals. Ligands of human androgens, glucocorticoids, or pregnanes X receptors (i.e., dexamethasone, RU486, rifampicin, SR12813, T0901317, clotrimazole, ketoconazole, testosterone, and dihydrotestosterone) moderately increased or inhibited BFCOD activity, with some variations between the models. No common feature could be drawn by regards to their capacity to bind to these receptors, which contrasts with their known effect on mammalian CYP3A. In contrast, dioxins and polycyclic aromatic hydrocarbons (PAHs) strongly induced BFCOD activity (up to 30-fold) in a time- and concentration-dependent manner, both in vitro in all cell lines and in vivo in zebrafish embryos. These effects were AhR dependent as indicated by suppression of induced BFCOD by the AhR pathway inhibitors 8-methoxypsoralen and α-naphthoflavone. Altogether our result further question the relevance of using liver BFCOD activity as a biomarker of fish exposure to CYP3A-active compounds such as pharmaceuticals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last decade, the effects of aquatic pollutants on endocrine functions in aquatic species have become a major issue in aquatic toxicology (Jobling and Tyler 2003). Disruption of hormonal homeostasis via alteration of cytochrome P450s (CYP) expression has been identified as one mechanism underlying endocrine disruption, among several others (Hotchkiss et al. 2008).
In mammals, CYP3A is one of the most abundant enzymes that plays a crucial role in the metabolism of xenobiotics and endogenous steroids in the liver (Gibson and Plant 2002). In particular, the human CYP3A is able to metabolize 60 % of the currently prescribed drugs (Burk and Wojnowski 2004). Mammalian CYP3A gene transcription is upregulated by many xenobiotics and endogenous substances such as steroids and their metabolites, through their interaction with multiple nuclear receptors (NRs) (Pelkonen et al. 2008). CYP3A is known to be mainly under the control of pregnane X receptor (PXR) but other NRs such as constitutive androstane receptor (CAR), vitamin D receptor (VDR), and glucocorticoid receptor (GR) are also involved in its regulation (Moore and Kliewer 2000; Goodwin et al. 2001; Burk and Wojnowski 2004; Pascussi et al. 2000). Among them, the human PXR is activated by a diversity of environmental pollutants, including numerous pharmaceuticals, and has been proposed as a toxicological target to study the effect of such compounds (Creusot et al. 2010; Sinz et al. 2006; Cui et al. 2008).
Assessment of environmental impacts by pharmaceuticals is a major issue in aquatic ecotoxicology because of their wide occurrence in the aquatic environment (Heberer 2002; Togola and Budzinski 2008; Barcelo and Petrovic 2008) and their potential impacts on aquatic species, notably fish (Laville et al. 2004; Fent et al. 2006). Given that mammalian CYP3A metabolizes various xenobiotics compounds and can be disrupted by pharmaceuticals through activation of PXR and other NRs, the measurement of its expression in fish systems represents a potential biomarker of exposure to such chemicals (Christen et al. 2009; Gagne et al. 2006; Wassmur et al. 2010). However, data are still needed to better characterize the modulation of CYP3A expression by environmental contaminants in fish as interspecies differences may exist. Indeed, recent studies have highlighted strong differences between human and fish PXR activation by various chemicals (Moore et al. 2002; Ekins et al. 2008). Furthermore, up to date, most of investigations in fish have concerned modulation of CYP3A activity and expression by a limited number of chemicals, which are mainly human PXR or GR ligands (e.g., rifampicin, clotrimazole, and dexamethasone) (Li et al. 2008, 2013; Christen et al. 2009; Wassmur et al. 2010; Vaccaro et al. 2007; Smith and Wilson 2010; Thibaut et al. 2009). These studies were conducted in different fish species and showed that modulation of CYP3A regulation may differ between fish species. They also suggested that fish CYP3A is controlled by PXR (Li et al. 2008; Christen et al. 2009) and AhR/ARNT (Tseng et al. 2005; Chang et al. 2013), although the exact underlying mechanism remains unclear.
The 7-benzyloxy-4-trifluoromethylcoumarin-O-debenzyloxylase (BFCOD) assay has been frequently used to monitor CYP3A enzymatic activity (Mensah-Osman et al. 2007; Hasselberg et al. 2005). In particular, some studies have demonstrated that this enzymatic assay is functional in fish cell lines (Christen et al. 2009) or fish liver microsomes (Hegelund et al. 2004; Hasselberg et al. 2008) and has been proposed as a useful and convenient assay to detect CYP3A-active chemicals in fish (Christen et al. 2009). However, recent evidence have also pointed out that benzyloxy-4-[trifluoromethyl]-coumarin (BFC) can be metabolized by a variety of CYPs in zebrafish, including CYP1A (Scornaienchi et al. 2010). Thus, the specificity and limits of this fluorometric assay is still to be better determined with respect to its further use for the screening of environmental chemicals or samples.
The aim of this study was (1) to establish and compare BFCOD assay in different in vitro and in vivo fish models and (2) to assess the effect of different organic chemicals that act through different receptor pathways. Because of potential differential CYP3A-like expression and regulation (i.e., expression of receptors and cofactors) between fish species (Bresolin et al. 2005; Li et al. 2008), three liver cell lines (PLHC-1—topminnow, zebrafish liver cells (ZFL)—zebrafish, and RTL-W1—rainbow trout) that derived from different fish species were used. In addition, in order to take into account pharmacokinetic processes, we developed a bioassay based on BFCOD assessment in living zebrafish embryos. After characterization of basal enzymatic activity in the different cell lines and in zebrafish embryos, we tested the effect of a panel of different chemicals. Test chemicals were selected as typical ligands of human NRs and/or environmental chemicals including persistent organic pollutants, pharmaceuticals, alkylphenols, and steroids.
Materials and methods
Chemicals
Dimethylsulfoxide (DMSO), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 1,2,3,7,8-pentachlorodibenzo-p-dioxin (PCDD), hexachlorodibenzo-p-dioxin (HCDD), benzo[a]pyrene (BaP), dibenzo[a]anthracene (DaA), benzo[a]anthracene (BaA), benzo[k]fluoranhene (BkF), dexamethasone (DEX), rifampicin (RIF), testosterone (T), carbamazepin, SR12813, clotrimazole (CLO), T0901317, ibuprofen, naproxen, bezafibrate, fenofibrate, aspirin, ketoconazole, 8-methoxypsoralen (8-MOP), α-naphthoflavone (αNF), RU486, BFC, 7-hydroxy-4-[trifluoromethyl]-coumarin (HFC), 7-ethoxyresorufin (7-ERF), resorufin ethyl ether, 17β-estradiol (E2), 4-nonylphenol (4-NP), and nonylphenol mixture (mNP) were all purchased from Sigma-Aldrich (St Quentin Fallavier, France).
Cell culture and bioassay
Cell lines and culture condition
Because responsiveness to chemicals can be modulated by fish species differences in terms of receptors/transcriptional cofactors/cytochrome P450s expression, basal enzymatic activity, and inducibility, but also overall metabolic capacity, we used three hepatic cell lines derived from different fish species.
PLHC-1 cells derived from topminnow (Poeciliopsis lucida) hepatoma were obtained from the American Tissue Culture Collection (ATCC # CRL-2406). This cell model expresses single CYP1A isoform, which is strongly inducible by AhR ligands (Hahn et al. 1996). Cells were grown at 30 °C in 5 % CO2 humidified atmosphere in Eagle’s Minimal Essential Medium (E-MEM) supplemented with 10 % of inactivated fetal calf serum (FCS), 1 % nonessential amino acids, penicillin/streptomycin (50 U/mL each).
ZFL were purchased from the ATCC (# CRL2643). They were derived from primary culture of normal adult zebrafish liver and exhibited epithelial morphology (Gosh et al. 1994). ZFL cells were grown at 28 °C in a mixture of Leibovitz’s medium (L15) (50 %, v/v), DMEM (35 %, v/v), and Ham-F12 (15 %, v/v) supplemented with mouse epidermal growth factor (EGF, 20 μg/L), bovine insulin (10 mg/L), penicillin/streptomycin (50 U/mL each), and inactivated FCS (5 %).
RTL-W1 cell line was kindly provided by Dr. Kristin Schirmer (Eawag, Dubendorf, Switzerland). This cell line was derived from a primary culture of normal liver of adult rainbow trout (Lee et al. 1993). These cells were grown in L15 medium supplemented with 7.5 % of FCS. All cell culture reagents were purchased from Invitrogen (Cergy-Pontoise, France).
EROD and BFCOD activity measurement
Cells were seeded into transparent 96-well plates at 1.105 cells per well in 150 μL culture medium. After 24-h enabling cell attachment, cells were exposed to test compounds for 4, 24, and 48 h (from pM to 10 μM range). DMSO was used as carrier solvent and its final concentration was 0.1 % v/v for standards and 0.5 % v/v for sediment extract.
The BFCOD assay was based on BFC metabolism onto fluorescent HFC (Stresser et al. 2000). Assay conditions were first optimized for substrate concentration and kinetics (data not shown) and determined as follows. After cell exposure to chemicals, medium culture was removed and each well was rinsed once with PBS, and refilled with 100 μL of PBS containing 40 μM of BFC. Kinetics of BFC metabolism by CYP3A was then monitored for 15 min in living cells with a spectrofluorophotometer (Saphire II, Tecan, Männedorf, Switzerland) using 410 and 530 nm as excitation and emission wavelengths, respectively (Mensah-Osman et al. 2007). To assess CYP1A activity, ethoxyresorufin-O-deethylase (EROD) assay was performed as previously described by Laville et al. (2004). Briefly, 5.104 cells/well were seeded in transparent 96-well plates and left to grow up for 24 h. After cell exposure, EROD assay was processed in intact cells by the addition of 7-ERF 2 μM (PLHC-1) or 5 μM (RTL-W1 and ZFL).
After fluorescence measurement, cells were washed twice with PBS and plates were frozen for 1 h at −80 °C. Total cellular proteins were then determined with the fluorescamine assay (Lorenzen and Kennedy 1993), using bovine serum albumin (BSA) as a standard. BFCOD and EROD activities were expressed as pmol HFC or resorufin/min/mg protein.
Cytotoxicity assay
The effect of test chemicals on cell viability was assessed by using the 3-(4,5-dimethyl-thiazol-2-yl)-2,5diphenyl tetrazolium bromide (MTT) assay (Mosmann 1983). After cell exposure, culture medium was removed and replaced by 100 μL of medium containing 0.5 mg/mL MTT. Cells were incubated for 3 h. In metabolically active cells, MTT is reduced onto a blue formazan precipitate, which was dissolved by adding 100 μL of isopropanol after removal of MTT containing medium. Plates were then read at 570 nm against a 640-nm reference wavelength on a microplate reader (KC-4, BioTek Instruments, France) and results were expressed as absorbance relative to control cells.
Zebrafish embryos: maintenance, xenobiotic exposure, and BFCOD activity measurement
Sexually mature male and female zebrafish (Danio rerio, ABstrain) were used as breeding stocks. The fish were raised in a recirculated water system at 27 °C under a controlled photoperiod (14 h light/10 h dark cycle). They were fed twice daily with TetraMin™ fish flake and live brine shrimp (Artemia spp.; Ocean Nutrition). For breeding, a spawning tray was placed in an aquarium containing 50 fish (sex ratio 1 female:2 males) and spawning was stimulated by light. The next morning, eggs were collected, cleaned, and randomly distributed into experimental groups. Each group consisted of 15 embryos in 100 mL of water kept in an incubator at 28 °C. Exposure to chemicals was carried out from 0 to 4 days post fertilization (dpf). Control groups were exposed to DMSO alone (0.01 %,v/v). At the end of the exposure period, each zebrafish larvae was manually distributed into black 96-well microplates for fluorescence (Fluotrac 200, Greiner), at a rate of one 4 dpf-old zebrafish per well containing 200 μL of water supplemented with BFC at a final concentration of 100 μM. Kinetics of HFC production by BFCOD was then measured in living zebrafish for 5 h with a spectrofluorophotometer thermostated at 27 °C (Saphire II, Tecan), using 410 and 530 nm as excitation and emission wavelengths, respectively. For each compound, at least three independent experiments were performed with n = 3 to 6 replicates per condition and per assay. BFCOD activities were determined as fluorescence units (FU) per minute and expressed as mean ± SD of fold CYP3A activities above control.
Statistic analysis
Significant effects of chemicals were determined by one-way analysis of variance (ANOVA) followed by a Tukey’s bilateral post hoc test. A value of p < 0.05 was considered significant.
Results
Basal BFCOD and EROD activities in fish cell lines
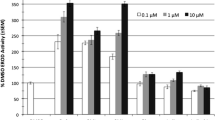
BFCOD basal activity was detected in all three cell lines (Fig. 1a). PLHC-1 and RTL-W1 cells showed the highest basal BFCOD enzymatic activity, with 150 and 135 pmol HFC/min/mg prot, respectively. ZFL cells had a significantly 2-fold lower basal activity than the other cell lines (75 pmol/min/mg prot, p < 0.05). Interestingly, patterns of basal EROD activity differed from that of BFCOD activity between the cell lines. The highest EROD activity was detected in RTL-W1 cells (1.6 pmol/min/mg prot), followed by PLHC-1 (1.0 pmol/min/mg prot) while it was not detectable in ZFL cells (Fig. 1b). Overall, basal EROD activity was lower than BFCOD in all the three cell lines examined.
Effect of AhR and nuclear receptor ligands on BFCOD activity in fish cell lines
Several prototypical or environmental ligands of different nuclear receptors and AhR were tested for their capacity to alter BFCOD activity in fish cell lines (Table 1). A range of concentrations of each substance was tested using three exposure durations. Unless otherwise specified, MTT assays ran in parallel showed no alteration of cell viability at the concentrations described as being active on BFCOD activity.
The results summarized in the Table 1 show that AhR ligands were the strongest BFCOD-inducing compounds in all three cell lines. The highest induction levels (up to 34-fold over control cells) were noted in RTL-W1 cells after 24-h exposure to dioxins (TCDD, PCDD) and PAHs (BkF, BaP, BaA, DaA), while induction levels were slightly lower in PLHC-1 (up to 20-fold) and ZFL (up to 12-fold) cells. In all cell lines, BFCOD induction was maximal after 24 h and persisted after 48 h of exposure. However, some differences were noted between cell lines for certain chemicals. For instance, BaP and BaA induced weakly to moderately BFCOD activity in PLHC-1 and ZFL cells whereas they behave as very strong inducers in RTL-W1 after 24 h (30-fold induction).
To a lesser extent than AhR ligands, other nuclear receptor ligands were also capable of significantly modulating BFCOD activity, either by inducing or inhibiting it. Among the human PXR ligands tested, limited inducing effect was noted. RIF increased enzymatic activity in all the cell lines by two-fold over basal activity after 48 h exposure at high concentrations. The two imidazoles, clotrimazole and ketoconazole, had similar effects as they markedly induced BFCOD activity only in RTL-W1 while they strongly inhibited this activity in the two other cell lines at non-cytotoxic concentrations. Interestingly, the synthetic human PXR ligands (i.e., SR12813, T0901317) as well as carbamazepine led to an inhibition of basal BFCOD activity.
Finally prototypical ligands of steroid receptors were also tested. Among them, the model glucocorticoid DEX, and androgens T and DHT weakly induced the BFCOD activity in all cell lines by 1.7- to 1.9-fold, while RU486, a GR partial agonist, strongly reduced basal enzymatic activity. The estrogenic compounds 17β-E2 and mNP did not modulate BFCOD activity.
Effects of AhR inhibitors on chemical-induced BFCOD activity in fish cell lines
In order to assess the role of AhR in the strong induction by dioxins and PAHs, we evaluated the effect of αNF or 8-MOP, two repressors of AhR signaling pathway, on BFCOD induction by different AhR ligands, namely TCDD, DaA, ketoconazole, and clotrimazole. αNF is an antagonist of AhR, as well as a CYP1A enzyme inhibitor (Scott and Hodson 2008). 8-MOP is known to block AhR transactivation by interfering with the binding of activated AhR to xenobiotic responsive elements in promoter regions of target genes (Navas and Segner 2000). Our results showed that αNF and 8-MOP led to the suppression of BFCOD activity induction by both prototypical (TCDD, DaA) and atypical (clotrimazole, ketoconazole) AhR ligands, hence demonstrating the involvement of AhR in observed inductions (Fig. 2). Interestingly, αNF alone was able to slightly increase BFCOD activity in ZFL cells while it decreased basal activity in RTL-W1 and had no significant effect in PLHC-1. Concerning 8-MOP, it reduced significantly basal BFCOD activity in all cell lines.
Induction of BFCOD activities in PLHC-1, ZFL, and RTL-W cell lines by different AhR activators and its modulation by coexposure with α-naphthoflavone (αNF, 10 μM) or 8-methoxypsoralen (8-MOP, 10 μM). a Effect of TCDD (1 nM for PLHC-1 and RTL-W1, 10 nM for ZFL) and DaA (0.3 μM); *, significantly different (p < 0.05); b Effect of clotrimazole (Clotri, 3 μM) and ketoconazole (Keto) (3 μM) in RTL-W1 cells; expressed as relative activity measured in solvent treated cells
Chemical-induced modulation of BFCOD activity in zebrafish embryos
In 4 dpf-old zebrafish embryos, BFCOD activity was consistently measured in control groups while at the same stage of development, no EROD activity could be detected (data not shown). In vivo screening of dioxins and PAHs AhR agonists revealed a concentration-dependent increase of BFCOD activity for all tested chemicals (Table 2). When compared to in vitro data, in vivo induction levels were weaker than in fish cell lines. Nonetheless, inducing effects by dioxins occurred at lower concentrations since, for instance, PCDD elicited maximal inducing effect at 10 pM in zebrafish embryos against 10 nM in ZFL cells. Interestingly, this higher sensitivity was found for all three tested dioxins (i.e., PCDD, HCDD, and TCDD) but not for PAHs (i.e., BaA, BaP, and diBaA), which were able to induce BFCOD activity but at similar or slightly higher concentrations than in the in vitro assays.
As observed in vitro, coexposure of zebrafish embryos with either αNF or 8-MOP significantly blocked induction of BFCOD activity by AhR ligands, hence showing the involvement of functional AhR in mediating these effects (Fig. 3). Interestingly, both αNF and 8-MOP alone significantly reduced BFCOD activity in comparison to control zebrafish. Although EROD activity was not detected in control fish, all the dioxin-like compounds tested in this study were able to strongly induced EROD activity in a concentration-dependent manner and at similar concentrations than those leading to BFCOD induction. These inducing effects on EROD were also blocked by αNF (data not shown).
Regarding other NR ligands, RU486, clotrimazole, and ketoconazole significantly inhibited BFCOD activity in the 0.1-1 μM range, which is in agreement with the results found in ZFL cells (Fig. 4).
BFCOD activity measured in zebrafish embryos after exposure to a range of concentration of RU486, clotrimazole, and ketoconazole. Results are expressed as relative to the response measured in presence of solvent control (Ctrl); *, significantly different from the control group (p < 0.05, n = 10 embryos per group)
Discussion
In the present study, we successfully applied the BFCOD assay to different in vitro and in vivo fish models to address the question of its modulation by various chemicals including pharmaceuticals in fish. The screening of a panel of selected chemicals revealed BFCOD responsiveness to chemicals from different classes, highlighted major effect of AhR ligands, and suggested possible regulatory mechanisms linked to the known modes of action of test chemicals.
Basal BFCOD activity is detectable in fish cell lines and zebrafish embryos
Significant basal BFCOD activity was quantified in the different in vitro and in vivo fish models, which is in line with previous reports on fish cell lines (Christen et al. 2009; Li et al. 2008) or in vivo measurements in other fish species such as Atlantic cod, killifish, or rainbow trout (Hegelund et al. 2004; Hasselberg et al. 2005, 2008; Smith and Wilson 2010). Some differences were however noted between hepatic cell lines, in line with previous studies that compared ZFL and PLHC-1 cells (Christen et al. 2009) or PLHC-1 and RTL-W1 cells (Thibaut et al. 2009). Overall, these results confirm that fish cell lines are not equivalent in terms of metabolic capacity and that comparisons and species extrapolation between fish species should be done carefully.
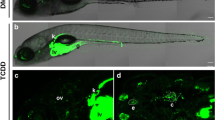
In our study, ZFL cells seemed the less metabolically active ones, as it has the lowest BFCOD and non-detectable EROD basal activities (Fig. 1). Interestingly, the same enzymatic pattern as in ZFL cells was observed in vivo in living zebrafish embryo, which has no detectable basal EROD activity, though highly inducible by dioxin-like compounds (Brion et al., unpublished data). To our knowledge, this is the first study to report in vivo measurement of BFCOD activity in living zebrafish embryos. Our data show that such assay serves as convenient and useful in vivo tool to screen those compounds that interfere with phase I metabolism in fish. In addition, such concordance between in vitro (ZFL) and in vivo (embryo) zebrafish EROD and BFCOD activities argues for the use of zebrafish as a model species for integrative assessment of chemicals on phase I metabolism.
BFCOD activity is modulated by various chemicals that activate different nuclear receptors
Only few or partial data are available on regulatory mechanisms of CYP3A-like transcription and modulation of enzymatic activity in fish although recent evidences suggest the involvement of both PXR and AhR receptors in the regulation of CYP3A65 transcription in the zebrafish (Tseng et al. 2005; Chang et al. 2013). In mammals, CYP3A4 gene transcription involves chemical binding to members of the nuclear receptors family NR1, including PXR, CAR, and VDR (Moore and Kliewer 2000; Pascussi et al. 2000). It is also well established, in human, that GR ligands potentiate CYP3A transcription through cross-talk between GR and other signaling pathways including PXR and CAR (Pascussi et al. 2000). Thus, in this study, different chemical ligands for these receptors were tested in order to assess the potential of BFCOD assay in fish for the detection of such chemicals.
In our experiments, human PXR (hPXR) and GR ligands exerted limited but significant effect on BFCOD in studied fish systems. The reference hPXR agonist RIF induced BFCOD activity in all fish cell lines but had no effect in vivo in zebrafish embryos. The effects of these compounds on CYP3A expression and CYP3A-like activity have been previously addressed in different fish species but not consistent results were sometimes reported. For instance, RIF have been shown to induce CYP3A-like activity in PLHC-1, ZFL, and GCL cells (Christen et al. 2009; Li et al. 2008) but was inactive to modulate CYP3A protein or testosterone hydroxylation in PLHC-1 and RTL-W1 cells (Celander et al. 1996; Thibaut et al. 2009; Wassmur et al. 2013). Recently, Corcoran et al. (2013) also reported induction of CYP3A gene transcription in Carp primary hepatocytes by RIF. In PLHC-1, rainbow trout hepatocytes, and GCL cells, no significant effect of DEX was reported (Celander et al. 1996; Wassmur et al. 2010; Li et al. 2008) while a 2-fold increase of BFCOD activity was reported in our study. In vivo, RIF and DEX failed to induce CYP3A-like activity in killifish or rainbow trout using BFCOD assay (Smith and Wilson 2010). In the same way, no significant in vivo effect of these two compounds could be detected on BFCOD activity measured in living whole zebrafish embryos. Nevertheless, by using in situ hybridization in zebrafish embryos, Tseng et al. (2005) reported a significant upregulation of the cyp3a65 gene transcription by DEX and RIF. The apparent discrepancies may reflect the use of different model species and different endpoints (i.e., gene expression, protein, enzyme), which may not be directly comparable. Notably, in vitro and in vivo assays are not equal in terms of metabolic capacity while different regulatory mechanisms but also marked ligand specificity may occur between species. For instance, PXR ligands identity and responsiveness of PXR to them are known to strongly differ between species (Moore et al. 2002; Ekins et al. 2008; Wassmur et al. 2010; Bainy et al. 2013).
In this regard, DHT, T, and CLO are of particular interest since they have been shown to bind to zebrafish PXR (zfPXR), with CLO having slightly higher potency on zfPXR than on hPXR (Milnes et al. 2008; Ekins et al. 2007; Moore et al. 2002; Lemaire et al. 2006). Our data indicate that all three compounds induced BFCOD activity preferentially in RTL-W1 cells, while CLO inhibited this activity in PLHC-1 and ZFL cells, as well as in vivo in zebrafish embryos. Such inhibition of BFCOD activity by CLO has been previously reported in rainbow trout microsomal fraction (Burkina et al. 2013). Conversely, increased zfPXR and CYP3A mRNA production has been reported in zebrafish liver treated with CLO (Bresolin et al. 2005) while it induced cyp3a65 but not zfPXR mRNA in ZFL cells (Eide et al. 2014). Altogether, our data and others indicate that zebrafish PXR ligands led to different effect on BFCOD activity and behaved as inducing, inhibiting, or inactive compounds depending on the biological model used. Thus, no common feature could be drawn with respect to the capacity of zf and hPXR ligands to modulate BFCOD activity. These results also reflect the lack of knowledge on CYPs modulation in fish. In this way, it cannot be excluded that other receptors (AhR), CYPs (e.g., CYP3B, CYP1A), or mechanisms (e.g., inhibition of enzymatic activity) are involved in the fish BFCOD responses that are measured in this study.
As mentioned in Table 1, several of these compounds are able to interact with different molecular targets. For instance, in fish, CLO has been shown to interact with zfPXR (Moore et al. 2002), AhR (Navas et al. 2004) but also to inhibit various CYP enzymatic activities involved in the synthesis of hormones or in the xenobiotic metabolism (Miranda et al. 1998; Schlenk et al. 2008; Pelkonen et al. 2008; Hegelund et al. 2004; Hasselberg et al. 2008; Hinfray et al. 2006; Burkina et al. 2013). The capacity of CLO to act as direct inhibitor of CYP enzymes might explain its inhibitory effect on BFCOD activity in several of the investigated models in our study. Interestingly, a structurally related compound, ketoconazole, led to similar inhibitory effect in these models while recent study revealed also inhibition of induced CYP3A expression by this compounds (Corcoran et al. 2013) and inhibition of BFC metabolism in PLHC-1 cell lines (Wassmur et al. 2013). Conversely to CLO, ketoconazole was recently described as an antagonist of hPXR (Ekins et al. 2007) and human GR (Duret et al. 2006), but it acts also as an agonist of AhR, as CLO (Korashy et al. 2007; Navas et al. 2004). Thus, in this study, the different responses to CLO and ketoconazole observed between the models, behaved as potent inducers of BFCOD activity in RTL-W1 and inhibitors in PLHC-1, ZFL, and zebrafish embryos, could be the result of the induction or repression and interaction of different signaling pathways combined to direct modulation of the enzymatic activity.
Altogether, these results confirm that BFCOD response is seen as complex because of multiple pathways that are potentially involved in the modulation of its transcription and of its catalytic activity (for review, see Table S1). This is in accordance with the unusually wide substrate specificity of CYP3A described in mammals (Pelkonen et al. 2008). However, our results mostly reflects an overall lack of knowledge about mechanism gene regulation (e.g., transcriptional activation, ligand specificity for nuclear receptors) and the catalytic activity function of many of P450s enzyme in fish (Schlenk et al. 2008). Thus, although BFCOD had been usually used for its potential capacity to report CYP3A activity modulation in response to exposure pharmaceuticals that activate PXR or GR receptors, our results suggest that further investigations are needed to clarify whish fish CYP forms use BFC as a substrate and which receptors are involved in the transcription of these CYPs. According to the current knowledge, BFCOD could be used as a non-specific marker alteration of phase I metabolism, including CYP3A-like enzymes.
The fish BFCOD activity is strongly induced by all AhR ligands in vitro and in vivo
A major outcome of this study is the finding that halogenated aromatic hydrocarbons (HAHs) and polycyclic aromatic hydrocarbons (PAHs) strongly induced BFCOD activity both in vitro and in vivo (Tables 1 and 2, Figs. 2 and 3). In vertebrates, these compounds are well known to induce EROD expression and activity in an AhR-dependent manner. We report here that these compounds are also able to markedly induce BFCOD activity in fish liver through a similar mechanism as αNF and 8-MOP blocked completely the induction. Even if BFCOD was described to report both CYP1A and CYP3A activities (Scornaienchi et al. 2010), our results clearly agree with that of Tseng et al. (2005) who showed that, in early developmental stage of zebrafish, CYP3A65 expression was upregulated by TCDD through a mechanism involving the AhR2 signaling pathway. These authors also pointed out that the AhR2 signaling pathway plays a critical role in constitutive CYP3A65 transcription (Tseng et al. 2005). More recently, Chang et al. (2013) have confirm that the AhR2 ligand-binding domain is crucial for driving basal CYP3A65 transcription, suggesting that AhR2 ligand activation is a critical process for transcription. Accordingly, αNF and 8-MOP, inhibitors of the AhR signaling pathways, decreased basal BFCOD in zebrafish embryos in our study. In other fish species, it is noteworthy that very few information is available regarding direct modulation of CYP3A-like activities by AhR ligands (Smith and Wilson 2010; Schlenk et al. 2008). While these results support that BFCOD responses reflect a modulation of CYP3A by AhR ligands, it cannot be excluded that CYP1A contribute to this response however.
The imidazole compounds clotrimazole and ketoconazole, also known as AhR activators (Navas et al. 2004; Korashy et al. 2007), induced BFCOD activity in RTL-W1 through an AhR-dependent mechanism, as shown by α-NF and 8-MOP coexposure. In the others models, imidazoles inhibit BFCOD activity. Discrepancies between responses of fish cells may rely on transcriptional cofactors expressions that differ between models that could influence the responsiveness of the cell lines (Marty et al. 2010). Differential expression levels of nuclear receptors and AhR may also have favored one signaling pathway more than another one in a given cell line. In this study, differential expression of CYP1A and CYP3A may explain such difference, since both may contribute to BFCOD in fish and in mammals (Scornaienchi et al. 2010; Stresser et al. 2002). In fact, exposure to α-NF led to slight increase of BFC metabolism in ZFL while it decreased basal activity in RTL-W1 and had no significant effect in PLHC-1. α-NF is known to activate CYP3A activities in both mammals and fish (Schlenk et al. 2008). Thus, suppression or absence of response to α-NF in RTL-W1 and PLHC-1 may reflect that these cell lines have no or very little CYP3A expressed and that BFCOD is predominately carried out by CYP1A enzymes. This is in accordance with a recent study (Wassmur et al. 2013) that reported low basal expression of CYP3A in PLHC-1 and concluded that BFCOD is not a convenient tool to assess CYP3A activities in this cell line.
Overall, in vitro and in vivo effect of dioxin-like compounds showed that the AhR signaling pathway is involved in the modulation of BFCOD enzymatic activity in fish. These results were in agreement with recent findings suggesting the involvement of AhR in CYP3A65 regulation in fish (Chang et al. 2013). Nevertheless, contribution of CYP1A in the BFCOD responses cannot be excluded. Altogether, these results confirm the complexity in interpretation of BFCOD catalytic activity, in part because of the overlapping of BFC specificity between CYP1A, CYP3A and maybe others CYPs, but mostly because of the lack of knowledge about the regulation of CYP genes transcription in fish.
Conclusions
In this study, we reported (1) the successful detection and quantification of BFCOD activity in three fish cell lines and in living zebrafish embryos; (2) its modulation by various environmental pollutants, including ligands of different NRs involved in xenobiotic metabolism regulation in humans; (3) and the strong inducing effect of dioxin-like compounds on BFCOD activity, suggesting a potential role of the AhR signaling pathway in its regulation, in accordance with recent findings.
Although BFCOD had been previously used as a marker of CYP3A activity modulation in fish systems exposed to activators of PXR or GR, our results, in line with other studies (Tseng et al. 2005; Chang et al. 2013), suggest that regulation mechanisms are more complex, involving notably other signaling pathways and P450s. For instance, similar responsiveness with EROD activity to dioxin-like compounds suggests that BFCOD could be a marker of both CYP1A and CYP3A and/or of CYP3A65 modulation through AhR activation. Although further investigations are needed to better understand transcriptional regulation mechanisms and CYP450 genes involved in BFCOD activity in fish, it is likely that BFC is not a specific substrate of CYP3A. Hence, we suggest that BFCOD assay cannot be used as a specific marker of exposure to CYP3A modulators in the assessment of complex environmental samples but can usefully inform on the presence of compounds that affect xenobiotic metabolism through either inhibitory or inducing effects.
Finally, our study also underlines that extrapolation from mammals to fish systems and between fish species, should be carefully considered for CYP3A-like responses (e.g., BFCOD). In particular, our results reflect the lack of knowledge as regard to nuclear receptor activation and ligand specificity in fish and thus argues for a better understanding of the molecular mechanisms controlling transcription of CYP3A-like but also other P450s genes in fish and their modulation by environmental contaminants.
References
Bainy ACD, Kubota A, Goldstone JV, Lille-Langøy R, Karchner SI, Celander MC, Hahn ME, Goksõyr A, Stegeman JJ (2013) Functional characterization of a full length pregnane X receptor, expression in vivo, and identification of PXR alleles, in Zebrafish (Danio rerio). Aquat Toxicol 142-143:447–457
Barcelo D, Petrovic M (2008): Emerging contaminants from industrial and municipal waste. The handbook of environmental chemistry
Bresolin T, Rebelo MD, Bainy ACD (2005) Expression of PXR, CYP3A and MDR1 genes in liver of zebrafish. Comp Biochem Physiol C: Toxicol Pharmacol 140:403–407
Burk O, Wojnowski L (2004) Cytochrome P450 3A and their regulation. Naunyn-Schmiedebergs Arch Pharmacol 369:105–124
Burkina V, Zlabek V, Zamaratskaia G (2013) Clotrimazole, but not dexamethasone, is a potent in vitro inhibitor of cytochrome P450 isoforms CYP1A and CYP3A in rainbow trout. Chemosphere
Celander M, Hahn ME, Stegeman JJ (1996) Cytochromes P450 (CYP) in the Poeciliopsis lucida hepatocellular carcinoma cell line (PLHC-1): dose- and time-dependent glucocorticoid potentiation of CYP1A induction without induction of CYP3A. Arch Biochem Biophys 329:113–122
Chang C-T, Chung H-Y, Su H-T, Tseng H-P, Tzou W-S, Hu C-H (2013) Regulation of zebrafish CYP3A65 transcription by AHR2. Toxicol Appl Pharmacol 270:174–184
Christen V, Oggier DM, Fent K (2009) A microtiter-plate-based cytochrome P450 3a activity assay in fish cell lines. Environ Toxicol Chem 28:2632–2638
Corcoran J, Lange A, Winter MJ, Tyler CR (2013) Effects of pharmaceuticals on the expression of genes involved in detoxification in a carp primary hepatocyte model. Environ Sci Technol 46:6306–6314
Creusot N, Kinani S, Balaguer P, Tapie N, LeMenach K, Maillot-Marechal E, Porcher JM, Budzinski H, Ait-Aissa S (2010) Evaluation of an hPXR reporter gene assay for the detection of aquatic emerging pollutants: screening of chemicals and application to water samples. Anal Bioanal Chem 396:569–583
Cui XM, Thomas A, Gerlach V, White RE, Morrison RA, Cheng KC (2008) Application and interpretation of hPXR screening data: validation of reporter signal requirements for prediction of clinically relevant CYP3A4 inducers. Biochem Pharmacol 76:680–689
Duret C, Daujat-Chavanieu M, Pascussi JM, Pichard-Garcia L, Balaguer P, Fabre JM, Vilarem MJ, Maurel P, Gerbal-Chaloin S (2006) Ketoconazole and miconazole are antagonists of the human glucocorticoid receptor: consequences on the expression and function of the constitutive androstane receptor and the pregnane X receptor. Mol Pharmacol 70:329–339
Eide M, Rusten M, Male R, Jensen KHM, Goksoyr A (2014) A characterization of the ZFL cell line and primary hepatocytes as in vitro liver cell models for the zebrafish (Danio rerio). Aquat Toxicol 147:7–17
Ekins S, Chang C, Mani S, Krasowski MD, Reschly EJ, Iyer M, Kholodovych V, Ai N, Welsh WJ, Sinz M, Swaan PW, Patel R, Bachmann K (2007) Human pregnane X receptor antagonists and agonists define molecular requirements for different binding sites. Mol Pharmacol 72:592–603
Ekins S, Reschly EJ, Hagey LR, Krasowski MD (2008) Evolution of pharmacologic specificity in the pregnane X receptor. Bmc Evolutionary Biology 8
Fent K, Escher C, Caminada D (2006) Estrogenic activity of pharmaceuticals and pharmaceutical mixtures in a yeast reporter gene system, 34th annual conference of the European-teratology-society. Pergamon-Elsevier Science Ltd, Abano Terme, pp 175–185
Gagne F, Blaise C, Andre C (2006) Occurrence of pharmaceutical products in a municipal effluent and toxicity to rainbow trout (Oncorhynchus mykiss) hepatocytes. Ecotoxcol Environ Saf 64:329–336
Gibson GG, Plant NJ (2002) Receptor-dependant transcriptional activation of cytochrome P4503A genes: induction mechanisms, species differences and interindividual variation in man. Xenobiotica 32:165–206
Goodwin B, Moore LB, Stoltz CM, McKee DD, Kliewer SA (2001) Regulation of the human CYP2B6 gene by the nuclear pregnane X receptor. Mol Pharmacol 60:427–431
Gosh C, Zhou YL, Collodi P (1994) Derivation and characterization of a zebrafish liver cell line. Cell Biol Toxicol 10:167–176
Hahn ME, Woodward BL, Stegeman JJ, Kennedy SW (1996) Rapid assessment of induced cytochrome P4501A protein and catalytic activity in fish hepatoma cells grown in multiwell plates: response to TCDD, TCDF, and two planar PCBS. Environ Toxicol Chem 15:582–591
Hasselberg L, Grøsvik BE, Goksøyr A, Celander MC (2005) Interactions between xenoestrogens and ketoconazole on hepatic CYP1A and CYP3A, in juvenile Atlantic cod (Gadus morhua). Comparative Hepatology 4
Hasselberg L, Westerberg S, Wassmur B, Celander MC (2008) Ketoconazole, an antifungal imidazole, increases the sensitivity of rainbow trout to 17 alpha-ethynylestradiol exposure. Aquat Toxicol 86:256–264
Heberer T (2002) Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 131:5–17
Hegelund T, Ottosson K, Radinger M, Tomberg P, Celander MC (2004) Effects of the antifungal imidazole ketoconazole on CYPIA and CYP3A in rainbow trout and killifish. Environ Toxicol Chem 23:1326–1334
Hinfray N, Porcher JM, Brion F (2006) Inhibition of rainbow trout (Oncorhynchus mykiss) P450 aromatase activities in brain and ovarian microsomes by various environmental substances. Comp Biochem Physiol C: Toxicol Pharmacol 144:252–262
Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE (2008) Fifteen years after “Wingspread”—environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci 105:235–259
Jobling S, Tyler CR (2003) Endocrine disruption in wild freshwater fish. Pure Appl Chem 75:2219–2234
Korashy HM, Shayeganpour A, Brocks DR, El-Kadi AOS (2007) Induction of cytochrome P450 1A1 by ketoconazole and itraconazole but not fluconazole in murine and human hepatoma cell lines. Toxicol Sci 97:32–43
Laville N, Ait-Aissa S, Gomez E, Casellas C, Porcher JM (2004) Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 196:41–55
Lee LEJ, Clemons JH, Bechtel DG, Caldwell SJ, Han KB, Pasitschniakarts M, Mosser DD, Bols NC (1993) Development and characterization of a rainbow-trout liver-cell line expressing cytochrome P450-dependent monooxygenase activity. Cell Biol Toxicol 9:279–294
Lemaire G, Mnif W, Pascussi JM, Pillon A, Rabenoelina F, Fenet H, Gomez E, Casellas C, Nicolas JC, Cavailles V, Duchesne MJ, Balaguer P (2006) Identification of new human pregnane X receptor ligands among pesticides using a stable reporter cell system. Toxicol Sci 91:501–509
Li D, Yang XL, Zhang SJ, Lin M, Yu WJ, Hu K (2008) Effects of mammalian CYP3A inducers on CYP3A-related enzyme activities in grass carp (Ctenopharyngodon idellus): possible implications for the establishment of a fish CYP3A induction model. Comp Biochem Physiol C: Toxicol Pharmacol 147:17–29
Li X, Ma J, Fang Q, Li Y (2013) Transcription alterations of microRNAs, cytochrome P4501A1 and 3A65, and AhR and PXR in the liver of zebrafish exposed to crude microcystins. Toxicon 73:17–22
Lorenzen A, Kennedy SW (1993) A fluorescence-based protein assay for use with a microplate reader. Anal Biochem 214:346–348
Marty MS, Carney EW, Rowlands JC (2010) Endocrine disruption: historical perspectives and its impact on the future of toxicology testing. Toxicol Sci: S93-S108
Mensah-Osman EJ, Thomas DG, Tabb MM, Larios JM, Hughes DR, Giordano TJ, Lizyness ML, Rae JM, Blumberg B, Hollenberg PF, Baker LH (2007) Expression levels and activation of a PXR variant are directly related to drug resistance in osteosarcoma cell lines. Cancer 109:957–965
Milnes MR, Garcia A, Grossman E, Grun F, Shiotsugu J, Tabb MM, Kawashima Y, Katsu Y, Watanabe H, Iguchi T, Blumberg B (2008) Activation of steroid and xenobiotic receptor (SXR, NR1I2) and its orthologs in laboratory, toxicologic, and genome model species. Environ Health Perspect 116:880–885
Miranda CL, Henderson MC, Buhler DR (1998) Evaluation of chemicals as inhibitors of trout cytochrome P450s. Toxicol Appl Pharmacol 148:237–244
Moore JT, Kliewer SA (2000) Use of the nuclear receptor PXR to predict drug interactions. Toxicology 153:1–10
Moore LB, Maglich JM, McKee DD, Wisely B, Willson TM, Kliewer SA, Lambert MH, Moore JT (2002) Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol 16:977–986
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Navas JM, Chana A, Herradón B, Segner H (2004) Induction of cytochrome P4501A (CYP1A) by clotrimazole, a non-planar aromatic compound. Computational studies on structural features of clotrimazole and related imidazole derivatives. Life Sci 76:699–714
Navas JM, Segner H (2000) Antiestrogenicity of Î2-naphthoflavone and PAHs in cultured rainbow trout hepatocytes: evidence for a role of the arylhydrocarbon receptor. Aquat Toxicol 51:79–92
Pascussi JM, Drocourt L, Fabre JM, Maurel P, Vilarem MJ (2000) Dexamethasone induces pregnane X receptor and retinoid X receptor-alpha expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol 58:361–372
Pelkonen O, Turpeinen M, Hakkola J, Honkakoski P, Hukkanen J, Raunio H (2008) Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol 82:667–715
Schlenk D, Celander M, Gallagher EP, George S, James M, Kullman SW, van den Hurk P, Willett K (2008) Biotansformation in fishes. In: Di Giulio RT, Hinton DE (eds), The toxicology of fishes CRC Press - Taylor & Francis Group: 153–234
Scornaienchi ML, Thornton C, Willett KL, Wilson JY (2010) Cytochrome P450-mediated 17b-estradiol metabolism in zebrafish (Danio rerio). J Endocrinol 206:317–325
Scott JA, Hodson PV (2008) Evidence for multiple mechanisms of toxicity in larval rainbow trout (Oncorhynchus mykiss) co-treated with retene and alpha-naphthoflavone. Aquat Toxicol 88:200–206
Sinz M, Kim S, Zhu ZR, Chen TS, Anthony M, Dickinson K, Rodrigues AD (2006) Evaluation of 170 xenobiotics as transactivators of human pregnane X receptor (hPXR) and correlation to known CYP3A4 drug interactions. Curr Drug Metab 7:375–388
Smith EM, Wilson JY (2010) Assessment of cytochrome P450 fluorometric substrates with rainbow trout and killifish exposed to dexamethasone, pregnenolone-16 alpha-carbonitrile, rifampicin, and beta-naphthoflavone. Aquat Toxicol 97:324–333
Stresser DM, Blanchard AP, Turner SD, Erve JCL, Dandeneau AA, Miller VP, Crespi CL (2000) Substrate-dependent modulation of CYP3A4 catalytic activity: analysis of 27 test compounds with four fluorometric substrates. Drug Metab Dispos 28:1440–1448
Stresser DM, Turner SD, Blanchard AP, Miller VP, Crespi CL (2002) Cytochrome P450 fluorometric substrates: identification of isoform-selective probes for rat CYP2D2 and human CYP3A4. Drug Metab Dispos 30:845–852
Thibaut R, Schnell S, Porte C (2009) Assessment of metabolic capabilities of PLHC-1 and RTL-W1 fish liver cell lines. Cell Biol Toxicol 25:611–622
Togola A, Budzinski H (2008) Multi-residue analysis of pharmaceutical compounds in aqueous samples. J Chromatogr A 1177:150–158
Tseng HP, Hseu TH, Buhler DR, Wang WD, Hu CH (2005) Constitutive and xenobiotics-induced expression of a novel CYP3A gene from zebrafish larva. Toxicol Appl Pharmacol 205:247–258
Vaccaro E, Salvetti A, Del Carratore R, Nencioni S, Longo V, Gervasi PG (2007) Cloning, tissue expression, and inducibility of CYP 3A79 from sea bass (Dicentrarchus labrax). J Biochem Mol Toxicol 21:32–40
Wassmur B, Grans J, Kling P, Celander MC (2010) Interactions of pharmaceuticals and other xenobiotics on hepatic pregnane X receptor and cytochrome P450 3A signaling pathway in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 100:91–100
Wassmur B, Grõns J, Norström E, Wallin M, Celander MC (2013) Interactions of pharmaceuticals and other xenobiotics on key detoxification mechanisms and cytoskeleton in Poeciliopsis lucida hepatocellular carcinoma, PLHC-1 cell line. Toxicol Vitro 27:111–120
Acknowledgments
The authors wish to thank Dr. K. Schirmer and Dr. N.C Bols for the kind gift of RTL-W1 cells. The study was funded by a PhD fellowship from INERIS and ANRT to NC and by the French Ministry of Ecology (P190-Ecotoxicology).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Cinta Porte
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Creusot, N., Brion, F., Piccini, B. et al. BFCOD activity in fish cell lines and zebrafish embryos and its modulation by chemical ligands of human aryl hydrocarbon and nuclear receptors. Environ Sci Pollut Res 22, 16393–16404 (2015). https://doi.org/10.1007/s11356-014-3882-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3882-8