Abstract
Concentrations of heavy metals (Cd, Cr, Cu, Ni, Pb, and Zn) were measured in water, sediments, Ceratophyllum (hornwort), and Bellamya sp. (edible snail) from residential, mixed (industrial and commercial), and agricultural areas with rural rivers in the Taihu Lake region, China. Zn concentrations were the highest, whereas Cd concentrations were the lowest among the six metals in water, sediments, and aquatic organisms. Cd was mainly present in the acid-soluble fraction, Cr in the residual fraction, and Pb in the reducible fraction of sediments. Heavy metal concentrations in water, sediments, and aquatic organisms in the three areas followed the order of the mixed area > residential area > agricultural area. Heavy metal concentrations in aquatic organisms were not only related to total metal concentrations in water and sediments but also to metal speciation concentrations in sediments. In addition, the bio-concentration factor (BCF) values of Cr, Cu, Pb, and Zn for Bellamya sp. were higher than those for Ceratophyllum, whereas the BCF values of Cd and Ni for Bellamya sp. were lower than those for Ceratophyllum. An ecological risk assessment of heavy metals in sediments showed that Cd posed the highest ecological risk to the environment. A health risk assessment showed that consuming Bellamya sp. from the mixed area could cause a potential health risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Taihu Lake region, one of the most economically developed regions in China, is located in the Yangtze River Delta (Yu et al. 2012). It covers 36,900 km2 and has the highest population density, exceeding 1000 persons/km2 (Zhang et al. 2007). Rivers are spread across most of the region with a total length of 1.2 × 105 km. Rivers play an extremely important role in the economic and social development in the region (Jiao et al. 2010).
The rapid development of industrialization and urbanization in recent decades has resulted in a substantial volume of untreated or inadequately treated wastewaters from industrial, agricultural, and domestic discharge into rivers, which has deteriorated the water quality and produced significant negative impacts on aquatic ecosystems (Lin et al. 2010; Wang et al. 2008). Heavy metals from these anthropogenic sources pose serious threats to the environment and human health and have become a widespread concern due to their toxicity, persistence, abiotic degradation, and bioaccumulation (Bonanno and Lo Giudice 2010). Sediments are both sinks and sources of heavy metals in water and also provide habitats and food sources for benthic organisms. However, heavy metals accumulate to toxic levels in aquatic flora and fauna under certain conditions, leading to ecological damage and endangering human health through the food chain (Götze et al. 2014). Investigation of heavy metals in water, sediments, and aquatic organisms are indispensable for assessing heavy metal contamination and its potential risks to aquatic ecosystems and human health.
In this study, we examined the behavior of six heavy metals in water, sediments, and aquatic organisms from residential, mixed, and agricultural areas with rural rivers in the Taihu Lake region, China. We also explored the relationships among heavy metal concentrations in water, sediments, and aquatic organisms. In addition, we also assessed potential heavy metal ecological and health risks in sediments and aquatic organisms from these three areas with rural rivers. Our results will provide the direct evidence needed by local environmental authorities to warn about the potential ecological risks caused by heavy metals in sediments and the potential health risks caused by consuming aquatic products.
Materials and methods
Study area and sampling
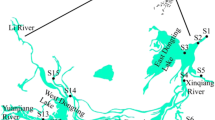
The study area was located in Yangyuan town, Changshu city in southern Jiangsu Province, China (120° 38′ N, 31° 33′ E) (Fig. 1). Thirty-four sampling sites were selected along rivers that run through residential, mixed (industrial and commercial), and agricultural areas. The R1–R7 sampling sites were located in the residential area. Pollutants at these seven sites were mainly from domestic village wastewaters. The M8–M27 sampling sites were situated in a mixed industrial and commercial area (mixed area). This area includes several industrial factories (three printing and dyeing mills, three chemical plants, five garment factories, and three machinery factories), commercial shops, and villages. Therefore, a considerable amount of industrial and domestic wastewater drains directly into the rivers. The remaining sampling sites (A28–A34) were located in an agricultural area where agricultural runoff could increase pollutant loads.
Sampling was conducted in November 2012. Sediment and water samples from the rivers were collected at the corresponding sampling locations. Ceratophyllum (hornwort) and Bellamya sp. (edible snail) of similar sizes (2–3-cm shell length, 20 snails/site) were obtained at every second site. Surface sediment (0–10 cm) samples were collected using a core sampler equipped with Perspex tubes (8-cm inner diameter, 30 cm long). Water samples were collected at a depth of 50 cm below the water surface, filtered through a 0.45-μm membrane filter, and maintained in 1 % nitric acid in polyethylene bottles. The water, sediments, and aquatic organism samples were kept in plastic bags and transported to the laboratory at 4 °C until analysis.
Sample preparation and analysis
The sediment samples were freeze dried, homogenized, and sieved through a 63-μm sieve. Total heavy metals were extracted using a mixture of HF-HNO3-HClO4 in an open system. A 0.5-g dry sediment sample was digested in 15 mL of HF and 10 mL of a 1:1 (v/v) mixture of HNO3 and HClO4. After digestion, the final residue was dissolved in 5 mL of 2 M HCl and the volume was brought up to 25 mL with deionized water.
DTPA-extractable metals were obtained from all sediment samples using 0.005 M DTPA +0.01 M CaCl2 + 0.1 M triethanolamine at pH 7.3 (Lindsay and Norvell 1978). The Community Bureau of Reference sequential extraction procedure (BCR-SEP) was applied to all sediment samples using the conditions given in Table 1 (Rauret et al. 1999; Sahuquillo et al. 1999; Ure et al. 1993).
The hornwort samples were washed with tap water, rinsed with deionized water, dried at 80 °C, and pulverized. The samples (0.8 g) were weighed and digested with 20 mL of HNO3 and 1 mL of HClO4 until digestion was completed. The solution was cooled at room temperature, diluted, and adjusted to 25 mL with deionized water.
The foot muscle and visceral mass were dissected from Bellamya sp., dried at 80 °C, and pulverized. The snail samples (0.5 g) were weighed and digested with 10 mL of HNO3 and 1 mL of HClO4. The solution was cooled at room temperature, diluted, and adjusted to 25 mL with deionized water.
Three replicate samples were measured in all cases. All reagents used were analytical grade or better, and double-deionized water was used for preparing the solutions and all dilutions. Heavy metals were determined by a flame atomic absorbance spectrophotometer equipped with a heated graphite tube atomizer (Hitachi Z-2000, Tokyo, Japan) for Cd, Cr, Cu, Ni, Pb, and Zn. The absorption wavelengths and detection limits were 228.8 nm for Cd, 359.3 nm for Cr, 324.8 nm for Cu, 232.0 nm for Ni, 283.3 nm for Pb, and 213.8 nm for Zn.
Two standard reference materials (GSS3 and GSS27) were used for total metal analysis, and a certified sediment reference material (BCR-701) was analyzed using the BCR-SEP to ensure good quality results. Recoveries were 87–101 % for GSS3, 98–114 % for GSS27, and 94–111 % for BCR-701.
Bio-concentration factor
The bio-concentration factor (BCF) of a heavy metal from the sediment to an aquatic organism is defined as the ratio of the metal concentration in the aquatic organism to that in the corresponding sediment (Agoramoorthy et al. 2008). The BCF is used to evaluate the potential ability of an aquatic organism to accumulate a heavy metal from the sediment.
The BCF was computed as follows:
Where C org and C s represent the metal concentrations in the aquatic organism and sediment extracts, respectively, on a dry weight basis.
Potential ecological risk index
The risk index (RI) was applied to assess the potential ecological risk of heavy metals in sediments. The RI was originally proposed by Hakanson (1980) and is widely used in many studies. The metal RI was defined as follows:
Where E r i is the potential ecological risk factor for a given contaminant, T r i is the toxic-response factor for a given contaminant, C b i is the background heavy metal concentration in Jiangsu Province soil (Wei et al. 1990), and C m i is the measured heavy metal concentration in sediments. T r i for the eight metals is described as follows: Hg = 40, Cd = 30, As = 10, Cu = Pb = Ni = 5, Cr = 2, and Zn = 1 (Hakanson 1980; Sheykhi and Moore 2013).
According to the literature (Hakanson 1980), the values used to describe the risk factor E r i and the RI are as follows: E r i < 40 and RI < 150, low potential ecological risk; 40 ≤ E r i < 80 and 150 < RI < 300, moderate potential ecological risk; 80 ≤ E r i < 160 and 300 ≤ RI < 600, considerable potential ecological risk; 160 ≤ E r i < 320, high potential ecological risk; E r i ≥ 320 and RI ≥ 600, very high ecological risk.
Target hazard quotient
The potential health risk from consuming Bellamya sp. by local people was characterized using the target hazard quotient (THQ) and total THQ (TTHQ) of heavy metals. The THQ and TTHQ were calculated using the following equations:
Where E F is the exposure frequency (365 days/year); E D is exposure duration (70 years); F IR is food ingestion rate, considered to be 16.7 g/person/day for adults and 9.0 g/person/day for children (Yu et al. 2012); C is the metal concentration in food (mg/kg, wet weight); R f D is the oral reference dose (mg/kg/day); BW is the average body weight (kg/person), considered to be 63 kg for adults and 33 kg for children (Yu et al. 2012); and AT is the average time for non-carcinogens (365 day/year × exposure years, assuming 70 years). The R f D values for Cd, Cr, Cu, Ni, Pb, and Zn are 0.001, 1.5, 0.04, 0.02, 0.0035, and 0.3 mg/kg/day, respectively (Hang et al. 2009). If the THQ or TTHQ is <1, there is no obvious risk, whereas there is a potential health risk if the THQ or TTHQ is ≥1 (Zheng et al. 2007).
Statistical analysis
The means and standard errors of the metal concentrations in water, sediment, and aquatic organisms were calculated. Two-way analysis of variance followed by least significant difference tests (SPSS 13.0; SPSS, Inc., Chicago, IL, USA) was used to analyze differences in the heavy metal concentrations at the different areas. Pearson’s bivariate correlation analysis was used to evaluate the relationships among heavy metal concentrations in water, sediments, and aquatic organisms. A p <0.05 was considered significant.
Results and discussion
Heavy metal concentrations in water
The heavy metal concentrations in water from the residential, mixed, and agricultural areas are presented in Table 2. Zn, Ni, and Zn were the heavy metals at the highest concentrations in the residential, mixed, and agricultural areas, respectively. Cd was the heavy metal at the lowest concentration in all three areas. The mean heavy metal concentrations in water were in the following decreasing order: Zn≈Ni > Cr > Cu > Pb > Cd in the residential, mixed, and agricultural areas. In addition, mean Cd concentrations in the residential, mixed, and agricultural areas were 4.0, 6.0, and 2.5 times higher than the background values, respectively. Moreover, mean Ni concentrations in the residential, mixed, and agricultural areas were 7.7, 9.1, and 7.3 times higher than the background values, respectively. However, none of the heavy metals exceeded the safe limit in irrigation water for agricultural purposes. Samples from the rural rivers in the three areas were slightly polluted based on a comparison of the heavy metal concentrations in these rivers in our study with values in other rivers (Abdel-Baki et al. 2013; Ahmed et al. 2012; Aktar et al. 2010; Fernandes et al. 2008; Klavinš et al. 2000).
Heavy metal concentrations in sediments
Total heavy metal concentrations
Sediments in the mixed area had the highest mean concentrations of Cr, Cu, Ni, and Pb, whereas Zn and Cd were highest in sediments in the residential area (Table 3). Mean concentrations of heavy metals were lowest in sediments in the agricultural area. Heavy metal concentrations in sediments displayed the following decreasing order: Zn > Cr > Cu > Ni > Pb > Cd in the residential and mixed areas, whereas in the mixed area, they display the following order: Cr≈Zn > Pb≈Ni > Cu > Cd. Mean concentrations of Cd, Cr, Cu, Ni, Pb, and Zn in the residential area were 78.6, 1.6, 3.6, 2.7, 2.1, and 6.8 times higher than the background values in the Jiangsu Province soil (Wei et al. 1990), respectively. Mean concentrations of Cd, Cr, Cu, Ni, Pb, and Zn in the mixed area were 52.0, 2.6, 7.5, 3.9, 3.3, and 7.1 times higher than the background values (Wei et al. 1990), respectively. However, heavy metal concentrations in the agricultural area were less than two times higher than the background values (Wei et al. 1990). Cd, Cr, and Zn concentrations in river sediments from the residential and mixed areas were significantly higher than those in other selected rivers around the world (Liu et al. 2009; Niu et al. 2009; Sheykhi and Moore 2013; Suthar et al. 2009; Varol 2011).
The different metal accumulation behavior may be related to the intensity of human activities, such as the volume of domestic sewage and industrial wastewater discharge (Long et al. 2009; Ongley ED and Tao 2010), limited wastewater treatment capacity (Wang et al. 2008), and increased heavy traffic activity (Pérez et al. 2008). The high levels of Cd could be due to excess use of Cd-based products (pesticides) in chemical plants and an electroplating factory, which have been relocated. In addition, a large amount of domestic sewage and industrial wastewater is discharged into rivers, which might explain the high level of Cu and Zn accumulation in sediments (Dummee et al. 2012). Furthermore, interactions among dissolved oxygen, temperature, salinity, and detritus may have had a significant effect on heavy metal accumulation and their potential ecological risks (Guhathakurta and Kaviraj 2004; Karadede-Akin and Ünlü 2007), which will be discussed in the next sections.
Metal speciation
Metal speciation in sediments was divided via the BCR-SEP, as shown in Fig. 2. Cd was mainly present in the acid-soluble fraction (Fig. 2-Cd), indicating extraordinary mobility and bioavailability (Yu et al. 2010), which was similar to Cd concentrations described by Yang et al. (2009) in sediments from a Yangtze River catchment. For example, the percentages of Cd in the acid-soluble fraction from the three areas followed the order of mixed area (64.8 %) > residential area (56.8 %) > agricultural area (32.4 %). Furthermore, 33.5, 29.4, and 40.3 % of total Cd in the sediment samples was found in the reducible fraction from the residential area, mixed area, and agricultural area, respectively. In addition, <2 % of total Cd was found in the oxidizable fraction.
More than 75 % of the total Cr was found in the residual fraction (Fig. 2-Cr), indicating relatively less bioavailability and potential risk to the environment (Yu et al. 2010), which was similar to Cr found by Nemati et al. (2011) in sediments from Sungai Buloh. The proportions of Cr in the residual fraction followed the decreasing order of the agricultural area (91.8 %) > residential area (80.6 %) > mixed area (75.2 %). Moreover, <2 and 10 % of total Cr was associated with the acid-soluble and reducible fractions, respectively.
The proportions of Cu in each fraction were relatively distributed evenly in sediments of the three areas, with slightly higher proportions of Cu being found in the reducible and residual fractions than in the acid-soluble and oxidizable fractions (Fig. 2-Cu).
The residual fraction contained a mean of 48.0, 37.5, and 58.4 % of the total Cr in the residential, mixed, and agricultural areas, respectively. Less than 13 % of total Ni was found in the oxidizable fraction. The rest of the Ni was distributed equally in the acid-soluble and reducible fractions (Fig. 2-Ni).
The reducible fraction contained most of the Pb, with mean values of 61.8, 68.6, and 55.2 % of total Pb in the residential, mixed, and agricultural areas, respectively (Fig. 2-Pb), indicating relatively higher mobility and bioavailability under reducing conditions (Passos et al. 2010). These results were in agreement with the Pb concentrations observed in sediment from the Yamuna River by Jha et al. (1990) and Jain (2004). No more than 4 and 9 % of total Pb was found in the acid-soluble and oxidizable fractions, respectively.
The behavior of the four Zn fractions revealed a similar distribution pattern (Fig. 2-Zn). In addition, Zn was mainly associated with the acid-soluble (34.9 and 36.8 %), reducible (23.4 and 24.8 %), and residual fractions (37.6 and 34.2 %) in the residential and mixed areas, respectively. However, Zn was mainly associated with the residual fraction (70.1 %) in the agricultural area.
Heavy metal concentrations in aquatic organisms
Submerged plants in rural rivers in Yangyuan town are dominated by Ceratophyllum. The heavy metal concentrations in Ceratophyllum from the three areas are given in Table 4. Zn concentrations were the highest, whereas Cd concentrations were the lowest among the six metals in Ceratophyllum from the three areas, which was similar to the results reported for plankton from Taihu Lake (Yu et al. 2012). Ni was the second highest element in Ceratophyllum, varying from 20.4–197 mg/kg in the residential area, 20.2–149 mg/kg in the mixed area, and 9.58–19.3 mg/kg in the agricultural area. In general, metal concentrations appeared to decrease in the order of Zn > Ni > Cu > Cr > Pb > Cd in the residential and mixed areas and Zn > Ni > Cr > Cu > Pb > Cd in the agricultural area. Additionally, the concentrations of Cd, Cr, Cu, and Pb in the three areas followed the order of the mixed area > residential area > agricultural area, whereas the concentrations of Ni and Zn in the three areas followed the order of the residential area > mixed area > agricultural area.
Bellamya sp. is a major macro-benthic species in rural rivers in Taihu Lake region, China, and it can survive in polluted environments (Yu et al. 2012). Heavy metal concentrations detected in Bellamya sp. are shown in Table 4. Among the six metals, Zn had the highest concentrations and Cd had the lowest concentrations in all Bellamya sp. samples, which were similar to the results obtained for Ceratophyllum. Cu was the second highest element in Bellamya sp., with an average of 165, 264, and 44.6 mg/kg in the residential, mixed, and agricultural areas, respectively, which was contrary to the findings in Ceratophyllum. The metal concentrations were observed in the order of Zn > Cu > Ni > Pb > Cr > Cd in the residential area and Zn > Cu > Pb > Ni > Cr > Cd in the mixed and agricultural areas. Additionally, the concentrations of Cd, Cr, Cu, Ni, and Pb in the three areas followed the order of the mixed area > residential area > agricultural area, whereas the Zn concentrations in the three areas followed the order of the residential area > mixed area > agricultural area.
Heavy metal concentrations in aquatic organisms in the three areas varied widely depending on the distribution of heavy metals in sediments and where the organisms were caught, because sediments are the major sink for heavy metals in water and play an important role in heavy metal uptake by aquatic organisms (Yi et al. 2011).
Relationships among heavy metals in aquatic organisms, water, and sediments
Significant relationships were observed between the concentration of Cd or Pb in Ceratophyllum and Bellamya sp. and that in water and between the total concentrations of Cd, Cu, and Zn in Ceratophyllum and Bellamya sp. and those in sediments (Table 5). Additionally, significant relationships for Zn were found between Ceratophyllum and water and for Pb were found between Ceratophyllum and sediments. These results suggest that Ceratophyllum is more suitable as a metal pollution biomonitor in water and sediments. Moreover, significant relationships were observed between the concentrations of Cd and Zn in Ceratophyllum and Bellamya sp. and DTPA-extractable Cd and Zn in sediments, indicating that metal concentrations in aquatic organisms were closely related to available metals in sediments. In addition, the Cd concentrations in Ceratophyllum and Bellamya sp. were significantly related to the acid-soluble, reducible, and residual fractions. The Cu concentrations in Ceratophyllum and Bellamya sp. were significantly related to the acid-soluble and residual fractions. The Ni concentrations in Ceratophyllum and Bellamya sp. were significantly related to the reducible fraction. The Zn concentrations in Ceratophyllum and Bellamya sp. were significantly related to the acid-soluble and reducible fractions. Significant relationships for Pb were found between Ceratophyllum and the acid-soluble and reducible fractions.
Heavy metal concentrations in aquatic organisms were not only related to total metal concentrations in water and sediments but also to metal speciation concentrations in sediments (Pempkowiak et al. 1999; Yap et al. 2002). As a consequence, controlling the heavy metal sources in water and sediments in an aquatic system is a key method for protecting aquatic plants and animals.
Bioaccumulation of heavy metals in aquatic organisms
The BCF of Zn was the highest of the six metals for Ceratophyllum (Table 6), indicating the strong capability of Ceratophyllum to enrich Zn and suggesting that Ceratophyllum is suitable for treating Zn-polluted rivers. The BCF of other metals for Ceratophyllum displayed the decreasing order of Ni > Cd > Cu > Pb > Cr. The BCF of Cu and Zn exceeded 1 for Bellamya sp., indicating the high tolerance of Bellamya sp. for Cu and Zn. In addition, the BCF of Zn was the highest, whereas the BCF of Cd was the lowest for Bellamya sp. Moreover, the BCF of Cr, Cu, Pb, and Zn for Bellamya sp. was higher than that for Ceratophyllum, and the BCF of Cd and Ni for Bellamya sp. was lower than that for Ceratophyllum because of selective accumulation of heavy metals by aquatic organisms (Nakajima and Sakaguchi 1986).
Ecological risk and health risk assessment
Potential ecological risk assessment
Comparing the heavy metal E r i criterion, Cd posed a very high ecological risk at four sites in the residential area and 18 sites in the mixed area, but a low ecological risk at five sites in the agricultural area (Table 7). Additionally, E r i of Cr, Cu, Ni, Pb, and Zn at all sampling sites was <40, except site 17 for Pb and sites 13–17 and 21 for Cu, indicating a low potential ecological risk at these sites. The average E r i of the six heavy metals in sediments decreased in the order of Cd > Cu > Ni > Pb > Zn > Cr.
Based on the RI values, 28.6, 14.3, and 57.1 % of all samples in the residential area were classified to have low, high, and very high potential ecological risks, respectively, and 10.0, 10.0, and 80.0 % of all samples in the mixed area were classified to have moderate, high, and very high potential ecological risks, respectively. In addition, all samples in the agricultural area were in the low ecological risk category. The RI values of heavy metals in the residential and mixed areas were higher than those found in the Yangtze River (Yi et al. 2011) and Kor River (Sheykhi and Moore 2013).
Potential health risk assessment
Bellamya sp. is a favorite daily food for local people. Therefore, it is necessary to assess the potential health risk caused by consuming Bellamya sp. The THQ values of individual metals and the TTHQ values of all metals from Bellamya sp. consumption for the different exposure groups are listed in Table 8.
THQ value of none of the metals >1 for children as well as adults, suggesting that the potential health risk was lower for ingesting heavy metals by consuming Bellamya sp. Furthermore, the TTHQ values were <1 in the residential and agricultural areas. However, the TTHQ values in the mixed area were >1, which exhibited potential health risks for children and adults. The health risk caused by consuming Bellamya sp. in the three areas followed the order of the mixed area > residential area > agricultural area.
The heavy metal levels in Bellamya sp. from rural rivers in the three areas and the international standards are listed in Table 9. Cd concentrations in the three areas and Cr concentrations in the agricultural area were safe in terms of the international standards (Demirak et al. 2006; Yu et al. 2012). Moreover, Cu concentrations were safe in comparison with the Chinese limit (Yu et al. 2012). However, the concentrations of the remaining metals exceeded the international standards (Demirak et al. 2006; Yu et al. 2012), indicating that local people should be cautious when consuming Bellamya sp.
Conclusions
Heavy metal concentrations in water, sediments, and aquatic organisms in the three areas with rural rivers followed the order of the mixed area > residential area > agricultural area. In addition, the Zn concentration was the highest, whereas the Cd concentration was the lowest among the six metals in water, sediments, and aquatic organisms in the rural rivers. Heavy metal accumulation in aquatic organisms was not only related to total heavy metal concentrations in water and sediments but also to metal speciation concentrations in sediments. Cd in sediments posed the highest ecological risk to the environment. Furthermore, considerable attention should also be paid to the potential health risks of heavy metals through aquatic product consumption.
References
Abdel-Baki AS, Dkhil MA, Al-Quraishy S (2013) Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of Wadi Hanifah, Saudi Arabia. Afr J Biotechnol 10:2541–2547
Agoramoorthy G, Chen FA, Hsu MJ (2008) Threat of heavy metal pollution in halophytic and mangrove plants of Tamil Nadu, India. Environ Pollut 155:320–326
Ahmad JU, Goni MA (2010) Heavy metal contamination in water, soil, and vegetables of the industrial areas in Dhaka, Bangladesh. Environ Monit Assess 166:347–357
Ahmed ATA, Mandal S, Chowdhury DA, Tareq ARM, Rahman MM (2012) Bioaccumulation of some heavy metals in Ayre Fish (Sperata Aor Hamilton, 1822), sediment and water of Dhaleshwari River in dry season. Bangladesh J Zool 40:147–153
Aktar MW, Paramasivam M, Ganguly M, Purkait S, Sengupta D (2010) Assessment and occurrence of various heavy metals in surface water of Ganga river around Kolkata: a study for toxicity and ecological impact. Environ Monit Assess 160:207–213
Bonanno G, Lo Giudice R (2010) Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol Indic 10:639–645
Demirak A, Yilmaz F, Levent Tuna A, Ozdemir N (2006) Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere 63:1451–1458
Dummee V, Kruatrachue M, Trinachartvanit W, Tanhan P, Pokethitiyook P, Damrongphol P (2012) Bioaccumulation of heavy metals in water, sediments, aquatic plant and histopathological effects on the golden apple snail in Beung Boraphet reservoir, Thailand. Ecotox Environ Safe 86:204–212
Fernandes C, Fontaínhas-Fernandes A, Cabral D, Salgado MA (2008) Heavy metals in water, sediment and tissues of Liza saliens from Esmoriz–Paramos lagoon, Portugal. Environ Monit Assess 136:267–275
Götze S, Bose A, Sokolova IM, Abele D, Saborowski R (2014) The proteasomes of two marine decapod crustaceans, European lobster (Homarus gammarus) and Edible crab (Cancer pagurus), are differently impaired by heavy metals. Comp Biochem Phys 162:62–69
Guhathakurta H, Kaviraj A (2004) Effects of salinity and mangrove detritus on desorption of metals from brackish water desorption of metals from brackish water and shrimp. Acta Hydrochimica Et Hydrobiologica 32:411–418
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hang X, Wang H, Zhou J, Ma C, Du C, Chen X (2009) Risk assessment of potentially toxic element pollution in soils and rice (Oryza sativa) in a typical area of the Yangtze River Delta. Environ Pollut 157:2542–2549
Jain C (2004) Metal fractionation study on bed sediments of River Yamuna, India. Water Res 38:569–578
Jha PK, Subramanian V, Sitasawad R, Van Grieken R (1990) Heavy metals in sediments of the Yamura River (a tributary of the Ganges). India Sci Total Environ 95:7–27
Jiao W, Lu SY, Li GD JXC, Yu H, Cai MM (2010) Heavy metal pollution of main inflow and outflow rivers around the Taihu Lake and assessment of its potential ecological risk. Chin J Appl Environ Biol
Karadede-Akin H, Ünlü E (2007) Heavy metal concentrations in water, sediment, fish and some benthic organisms from Tigris River, Turkey. Environ Monit Assess 131:323–337
Klavinš M, Briede A, Rodinov V, Kokorite I, Parele E, Klavina I (2000) Heavy metals in rivers of Latvia. Sci Total Environ 262:175–183
Lin AYC, Panchangam SC, Ciou PS (2010) High levels of perfluorochemicals in Taiwan’s wastewater treatment plants and downstream rivers pose great risk to local aquatic ecosystems. Chemosphere 80:1167–1174
Lindsay W, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Liu J, Li Y, Zhang B, Cao J, Cao Z, Domagalski J (2009) Ecological risk of heavy metals in sediments of the Luan River source water. Ecotoxicology 18:748–758
Long H, Zou J, Liu Y (2009) Differentiation of rural development driven by industrialization and urbanization in eastern coastal China. Habitat Int 33:454–462
Nakajima A, Sakaguchi T (1986) Selective accumulation of heavy metals by microorganisms. Appl Microbiol Biot 24:59–64
Nemati K, Bakar NKA, Abas MR, Sobhanzadeh E (2011) Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J Hazard Mater 192:402–410
Niu H, Deng W, Wu Q, Chen X (2009) Potential toxic risk of heavy metals from sediment of the Pearl River in South China. J Environ Sci-China 21:1053–1058
Ongley ED ZXL, Tao Y (2010) Current status of agricultural and rural non-point source pollution assessment in China. Environ Pollut 158:1159–1168
Pérez G, López-Mesas M, Valiente M (2008) Assessment of heavy metals remobilization by fractionation: comparison of leaching tests applied to roadside sediments. Environ Sci Technol 42:2309–2315
Passos EDA, Alves JC, Dos Santos IS, Alves JDPH, Garcia CAB, Spinola Costa AC (2010) Assessment of trace metals contamination in estuarine sediments using a sequential extraction technique and principal component analysis. Microchem J 96:50–57
Pempkowiak J, Sikora A, Biernacka E (1999) Speciation of heavy metals in marine sediments vs their bioaccumulation by mussels. Chemosphere 39:313–321
Rauret G, Lopez-Sanchez J, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Sahuquillo A, Lopez-Sanchez J, Rubio R, Rauret G, Thomas R, Davidson C, Ure A (1999) Use of a certified reference material for extractable trace metals to assess sources of uncertainty in the BCR three-stage sequential extraction procedure. Anal Chim Acta 382:317–327
Sheykhi V, Moore F (2013) Evaluation of potentially toxic metals pollution in the sediments of the Kor river, southwest Iran. Environ Monit Assess 185:3219–3232
Suthar S, Nema AK, Chabukdhara M, Gupta SK (2009) Assessment of metals in water and sediments of Hindon River, India: impact of industrial and urban discharges. J Hazard Mater 171:1088–1095
Ure A, Quevauviller P, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Environ An Ch 51:135–151
Varol M (2011) Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater 195:355–364
Wang M, Webber M, Finlayson B, Barnett J (2008) Rural industries and water pollution in China. J Environ Manage 86:648–659
Wei FS, Chen JS, Wu YY (1990) Background values of soil elements in China. China Environmental Science Press, Beijing (In Chinese)
Yang Z, Wang Y, Shen Z, Niu J, Tang Z (2009) Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J Hazard Mater 166:1186–1194
Yap CK, Ismail A, Tan SG, Omar H (2002) Correlations between speciation of Cd, Cu, Pb and Zn in sediment and their concentrations in total soft tissue of green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia. Environ Int 28:117–126
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585
Yu R, Hu G, Wang L (2010) Speciation and ecological risk of heavy metals in intertidal sediments of Quanzhou Bay, China. Environ Monit Assess 163:241–252
Yu T, Zhang Y, Hu XN, Meng W (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotox Environ Safe 81:55–64
Zhang G, Wang DJ, Chen XM (2007) Roles of buffer strips in reducing nutrient loss from paddy field in Taihu Lake region. Acta Pedol Sin 44:873–877 (In Chinese)
Zheng N, Wang Q, Zhang X, Zheng D, Zhang Z, Zhang S (2007) Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Sci Total Environ 387:96–104
Acknowledgments
This work was supported by National Key Technology R&D Program of the Ministry of Science and Technology (no. 2012BAJ24B06).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bo, L., Wang, D., Li, T. et al. Accumulation and risk assessment of heavy metals in water, sediments, and aquatic organisms in rural rivers in the Taihu Lake region, China. Environ Sci Pollut Res 22, 6721–6731 (2015). https://doi.org/10.1007/s11356-014-3798-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3798-3