Abstract
In this work, we studied the influence of air pollution on the morpho-structural, biochemical and chemical composition of Eucalyptus camaldulensis leaves. Analyses were carried out on 22 samples collected in Palermo (Italy) area. Considering the mean concentrations (in unwashed leaves) of investigated metals, nutrient elements as Fe (214 mg kg−1 dry weight (d.w.)), Mn (160 mg kg−1 d.w.) and Zn (39 mg kg−1 d.w.) were the most abundant, whereas Pb (5.6 mg kg−1 d.w.) and Cd (0.072 mg kg−1 d.w.) showed the lowest concentrations. The values of metal pollution index (MPI) ranged from 6.0 (station no. 15) to 25 (station no. 8) and from 4.0 (station no. 16) to 17 (stations no. 7 and no. 15) for unwashed and washed leaves, respectively. The station no. 8, located in an area interested by traffic mostly caused by the activities of university, showed the highest value of MPI. The station no. 15 (Industrial area) showed the lowest MPI value, which is similar to those determined in the reference stations. Considering that the station considered is located in a large and open area interested only by commercial activities and there are no production activities, this data is not surprising. In this study, the washing of the leaves with distilled water has caused a little reduction of metal concentrations. Microphotography reveals a correlation between zones of necrosis, modified cuticles and accumulations of acid phosphatases in the leaves collected in polluted areas. The diaphanized leaves from the more polluted areas show irregular areolas, several idioblasts both on the ribs and scattered in the mesophyll. With polarized light, we observe many crystal deposits near the ribs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial development and urbanization processes have gradually caused a general increase in the number and concentrations of organic and inorganic toxic substances released to the environment (Orecchio and Amorello 2011) increasing risks both to human health and to environmental matrices and cultural heritages (Orecchio and Amorello 2010).
Most of trace pollutants are present in the air in the form of particles, whilst only few are found in the form of both gas and particles. In urban areas, some contaminants (polycyclic aromatic hydrocarbons (PAHs), Cd, Cu, Fe, Pb, Zn, Pt, Pd, Rh, etc.) are mainly derived from coal and petroleum combustion, oil burning, incineration of waste and other activities such as motor vehicles (Orecchio and Amorello 2010, 2011).
Metals are removed from the atmosphere by wet and dry deposition, diffusion and retention on solid surfaces. Since metals may be also dispersed in silt, soil and water, a rapid accumulation in the environment was reported. As an example, in Germany, between 1999 and 2005, a distinct increase of metal concentrations by a factor of 2.1 to 8.9 in soils nearly alongside heavy traffic roads was observed (Gomez et al. 1998). Organic and inorganic pollutants are often deposited along roadways (Orecchio and Amorello 2011), on adjacent vegetation (Calamari et al. 1991; Lombardo et al. 2001; Dongarra et al. 2003; Gianguzza et al. 2008; Orecchio and Amorello 2010, 2010) and in water bodies (Gianguzza et al. 2006). Vegetation intercepts aerosol and particulate, consequentially the concentrations of some pollutants are high in the samples of plants located in urban and industrial areas (Aksoy and Ozturk 1997; Culotta et al. 2005) and their deposition on plants depend on pollutant air concentrations (Market 1993; Ugolini et al. 2013). The high surface/volume ratio and the waxy or resinous (Dongarra et al. 2003; Lehndorff and Schwark 2004) coating on the leaves of several plants make possible the accumulation on their structure of pollutants, which are present in the air in the form of particulate matter.
In some cases, bio monitoring uses, as living instrument, organisms capable of exhibiting the toxic effects of the pollutants whilst resistant organisms may be used by bio accumulators that do not show, to the naked eye, damages (Market 1993; Lombardo et al. 2001). The use of plants as bio accumulator, compared to animals, is more appropriate because they are immobile. So, leaves represent a suitable biological tool for monitoring atmospheric pollutants (Calamari et al. 1991; Lombardo et al. 2001; Dongarra et al. 2003; Lehndorff and Schwark 2004; Orecchio and Amorello 2010).
The determination of metal concentrations in the leaves is useful to evaluate the air quality over a longer period compared to when using the traditional methods, because the pollutants accumulate over the whole lifetime of the leaves (Market 1993). Usually, concentrations of various air-dispersed pollutants are checked with monitoring systems consisting of fixed or mobile automatic survey installations fitted out with equipment that constantly measures pollutant concentrations. In general, these survey systems have some limitations, for example:
-
Provide data which do not allow to establish risk thresholds unlike bio monitoring methods which follow the variations in organism populations, without losing sight of the fact that biotic diversity does not depend on level of pollution alone;
-
Require specialized personnel;
-
Are expensive.
Pollutant injury symptoms in leaves are extremely significant for bio monitoring purposes; in particular, analyses of the leaves are particularly helpful in the assessment of air quality (Aksoy and Ozturk 1997; Lombardo et al. 2001). The cell diagnosis of plants is complicated because the plants may respond in a similar way to a number of anthropogenic or natural stress inducers (Lombardo et al. 2001). It is therefore useful to gather several parameters for a comparative analysis. This type of diagnosis is an important indicator of environmental pollution when correlated with the morphological and biochemical analyses (Lombardo et al. 2001).
In the ecosystem, living organisms undergo modifications due to environmental alterations, and in some cases, these alterations may generate extinction. However, the disappearance of a population of organisms is the last step of response to stress. Some organisms constitute primary systems of information on the alterations underway. These organisms are known as biological indicators (Market 1993; Ugolini et al. 2013). All organisms are potential bio indicators of environmental quality in the sense that the definition itself of living means that they are sensitive to environmental solicitations, though not all of them can provide clear information on the modifications underway. When one or more atmospheric pollutants are presents in the environment, in some cases, they can interfere with the normal growth and reproduction processes of the plants (Zhang et al. 2007). Several metals can affect pollen germination and the lengthening of the pollen tube, as well as seed germination and the production of flowers and strobili. Macroscopic evidence of injury caused by pollutants is preceded by so-called invisible injuries, a sum of biochemical and physiological alterations that can only be highlighted with instrumental methods (Lombardo et al. 2001).
Literature has demonstrated a correlation between level of pollution and the increase of some protein content in plant tissues, such as metallothioneins or enzymes such as acid phosphatases and secondary metabolites (Lombardo et al. 2001). Toxic concentrations of heavy metal affect a variety of processes in vegetal organisms (Siedlecka and Krupa 2002; Zhang et al. 2007). One of the major consequences is the enhanced production of reactive oxygen species (ROS), which damage cell membranes, nucleic acids and chloroplast pigments (Chaoui et al. 1997). In uncontaminated environment, concentration of oxygen radicals in plants is generally low because the efficient activity of anti oxidative enzymes. Accumulation of reactive oxygen species may be the consequence of disruption of the balance between their production and the anti oxidative system activity, composed of enzymic antioxidants such as catalase, peroxidases and superoxide dismutases and nonenzymic scavengers. The activities of previous enzymes are induced in plants species by heavy metals (Li et al. 2006; Zhang et al. 2007).
Acid phosphatase is an indicator of the plant’s response to atmospheric pollutants since these enzymes are connected to different modifications of the tissues; an abnormal presence of these leads us to suppose that there have been proliferative cell reactions. Red-brown accumulations of phosphatases in the transversal sections of the leaves can be highlighted with histochemical staining (Lombardo et al. 2001).
The aim of this study was to identify metal pollution levels in Eucalyptus collected in Palermo area and examine biological modifications induced in leaves and pollen under stress conditions. Leaves and pollen samples of Eucalyptus genus were chosen for this research because Eucalyptus is one of the most common evergreen plants in Palermo and in Europe. Of the many metals released into the atmosphere, we chose, based on the risk which some of them cause to health and for their potential bioaccumulation, Cd, Cu, Fe, Pb, Mn and Zn. Considering that essential elements for organisms as iron and manganese are not considered to be of great interest for toxicology and environmental points, they were used to assess natural background.
Experimental
Eucalyptus
We studied the morpho-structural, biochemical and chemical characteristics of Eucalyptus camaldulensis (genus Eucalyptus), a fast-growing evergreen plant, which is often used as a windbreak in coastal areas or for reforestation. Eucalyptus grows in gardens and is popular as an urban ornamental plant all through south and insular Europe but is native to Australia, where it is widespread, especially beside inland watercourses.
The leaves of Eucalyptus are stalked, with the broad at the base, tapering to the tip; colour is a dull blue-green. The leaves also are an important source of essential oils, produced by glands located in the un-veined areas, with a wide range of biological activities such as antibacterial, antifungal, analgesic and anti-inflammatory properties and used against the effects of colds, influenza, respiratory infections, rhinitis and sinusitis. Eucalyptus leaves contain several secondary metabolites that vary qualitatively and quantitatively between species. Also, their concentrations vary with leaf age. Eucalyptus secondary metabolites control many ecological interactions. For example, formylated phloroglucinol compounds are significant antifeedants against several herbivore animals, essential oil concentration affects plant flammability and phenolic compounds influence leaf litter decomposition (McKiernan et al. 2012).
The green parts of higher plants as Eucalyptus are covered by a hydrophobic epicuticular wax that will sorb compounds, such as environmental contaminants, from the surrounding air (Marco and Kishimba 2006). Eucalyptus, as for all plant species, can take up heavy metals from the air directly via the stomata or via deposition on the foliage surfaces. Previous studies have demonstrated that Eucalyptus is a good bio monitor of air quality being able to accumulate metals and PAHs in leaf tissues (Rodriguez et al. 2012).
Site and sampling
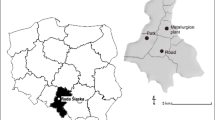
Palermo is a populated town-city (about 850,000 residents) with a heavy load of vehicular traffic, and major activities are selected within the urban area. It is characterized by conspicuous air pollution (Orecchio and Amorello 2010, 2011). In order to have a complete picture on air quality, 22 stations are located in different areas affected by various types of emissions. For reference purposes, we chose two sites far from human sources of metals. The stations chosen for the analysis of the leaves are shown in Fig. 1 and described in Table 1.
Previous leaves analyses conducted in the same area during different periods of the year have demonstrated that the contamination trend among stations is independent by seasonality. So, the sampling was carried out at different times of year, randomly at about 1–2.5 m above the ground surface, from the whole perimeter of the plant, discarding badly damaged, diseased or parasite-infested leaves.
In order to maximize the reproducibility and representativeness of the analyzed samples, leaves of different ages were taken from different trees of the same station. The reproducibility of the sampling was preliminarily checked by analyzing for copper six different samples of leaves collected at different points of the same plant. The standard deviation on sampling, with respect to that of the analytical process, was similar (about 5 %). For the successive analysis, from each station, we collected about 200–300 g of leaves. Samples were collected using rubber gloves (without talc), immediately refrigerated (4 °C) and stored, then rapidly carried to the laboratory and frozen before analysis.
To investigate the leaf capacity to hold heavy metals, two sample treatments were used. Samples from each station were divided into two batches; one batch was washed with Milli-Q water to remove any substances on the leaf surface. To quantify heavy metals, both the washed and unwashed leaves from each station were put in ovens at a temperature of 80 °C for one night. After being dried this way, the samples were finely ground in a porcelain mortar.
Chemical and cytological analyses on the Eucalyptus leaves were performed immediately after the sampling.
Chemicals
Standard solution containing 26 elements was purchased from Ultra Scientific, USA, (catalogue number: 191 IQC-026, Lot no. J00022). The diluted standard solutions were prepared daily. The reagents used throughout were analytical grade (Carlo Erba, Milano, Italy) and all solutions were prepared in Milli-Q water. Concentrated HNO3 (Suprapur Carlo Erba, Milano, Italy) was used to mineralize the samples and to acidify the standard solutions.
Quality assurance
The procedural blanks were routinely analyzed in every five samples. The analytical procedure was checked for accuracy by analyzing enriched samples prepared by us. The average recoveries of added analytes ranged from 92 to 104 %. The relative standard deviations on the metal measurements of recovery are less than 5 %. All the vessels and flasks were cleaned before use by rinsing several times with HNO3 (2 %) and three times with Milli-Q water. All the samples were analyzed in triplicate.
Chemical analyses
Digestion procedure
About 0.5 g of dried sample material was digested in 6 mL HNO3 + 1 mL H2O2 in a Milestone model MLS-1200 Mega (Milestone Laboratory Systems, Italy) microwave oven (1000 W maximum power) equipped with six high-pressure (up to 100 bar) Teflon containers. The instrumental parameters for the microwave digestion are reported in Table 2. After digestion was completed, the clear solution was transferred into a volumetric flask and brought to volume with water purified by Milli-Q system. For every mineralization cycle (5 samples), a blank was prepared.
ICP-OES analysis
Elemental analyses were carried out on the solutions obtained from the microwave treatment of the samples using a Perkin Elmer Optima 2100 series ICP Optical Emission Spectrometer. The instrument is equipped with a Perkin S10 model auto sampler. Data acquisition and processing were performed using the Win Lab 32 software (Perkin Elmer). Six elements (Cd, Cu, Fe, Mn, Pb and Zn) were determined in each sample, chosen on the basis of their significance in the study of environmental matrices. The inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of trace elements was performed in axial viewing mode.
The quantitative analysis was carried out at two different spectral lines for each element (Table 3). The data of the metals reported in this paper have been calculated considering the average of the three concentrations obtained at the two wavelengths that, for all analytes, differed by less than 5 %. The repeatability of the whole method calculated as relative standard deviation (RSD %) of three independent analysis of the same sample was less than 10 %.
The metal concentrations are expressed in milligrammes of element per kilogramme of dry weight of the plant (mg kg−1 d.w.).
Calibration curves
The detection limit (LOD) and the quantification limit (LOQ) were estimated as reported in previous papers (Mannino and Orecchio 2011; Orecchio 2013) and respectively ranged from 3.0 to 10 μg L−1 and from 10 to 33 μg L−1. Calibration standard solutions were prepared by dilution with HNO3 2 % of the multielement calibration standard solution. The range of concentration of the calibration curves was between 10 and 50,000 μg L−1. For calibration, a solution of HNO3 2 % as blank was used. The analyses of the five standard solutions were replicated in every 2 days. Correlation coefficients of the calibration curves were in the range of 0.9980–0.9999. To eliminate memory effects related to the previous sample analysis, between two subsequent samples, a 25s washing was settled. A blank was run-up every five samples. All reported data were blank corrected.
Cytological analyses
In addition to chemical analyses, which allowed to know the concentrations of heavy metals in the leaves, histological observations were carried out in order to correlate the presence of the metals with cell injury. During the samplings, the botanical characteristics of the plant were evaluated, often observing some anomalies in the leaf (galls, browning, chlorosis). The cytochemical analyses were completed with cytological and morphological analyses (necrosis, chlorosis), extended also to the pollen of the plant. Cryosections of the apical and basal portions of the Eucalyptus leaves were prepared, choosing stations no. 1 and 2 for reference, stations no. 11 and 13 as areas with average traffic flow and stations no. 8 and 12 as an area with heavy traffic flow.
The sections, once prepared, were treated with a test solution to observe the presence of phosphatases. The test solution was prepared as follows (Ashrafi and Fisk 1961): 10 mg of substrate (naphthol AS-BI phosphate for acid phosphatase and naphthol AS-MX phosphate for alkaline phosphatase) were dissolved in 0.50 ml N,N-dimethylformamide followed by 50 mL of water and 50 mL of buffer (0.2 M acetate of pH 5.2 for acid phosphatase, and 0.2 M trishydroxymethylaminomethane buffer of pH 8.3 for alkaline phosphatase). After that, 60 mg of Red Violet salt for acid phosphatase was added. This mixture was shaken and filtered. 0.2 mL of 10% MnCl2 were added to the incubation mixture prepared for acid phosphatase localization. As a result of phosphatase activity during incubation, AS-naphthol was liberated from naphthol AS-BI phosphate and naphthol AS-MX phosphate. The liberated AS-naphthol couples instantly with diazonium Red Violet LB salt, producing a red pigment.
The slides were incubated both at a temperature of 37 °C for 30 min (Fuchs 1963; Gahan 1984) and at room temperature, in order to compare results on the formation of coloured granules (phosphatases in particulate form within the cells) and a characteristic diffuse staining, due to the presence, also, of secondary metabolites.
The study was also extended to leaf architecture using diaphanization. The literature reports various procedures for diaphanization; the one we used is a modification of Fuchs method (Fuchs 1963). At the end of these operations, the material was observed with a polarized light optical microscope (Ortoplan Leica) to highlight the colourless ribs.
Pollen vitality was tested with a fluorescein diacetate solution (0.1 %) in acetone diluted with phosphate buffer 0.05 M at pH 5.8. The pollen on the slides, treated with a few drops of the abovementioned solution, was observed and photographed with fluorescence microscope (Ortoplan Leica).
Results
Figure 2 shows the total heavy metal in milligrammes of metal per kilogramme of dry weight, before and after washing with Milli-Q water.
Considering the mean concentrations of all the stations in unwashed leaves, Fe (214 mg kg−1 d.w.), Mn (160 mg kg−1 d.w.) and Zn (39 mg kg−1 d.w.), being nutrient elements, were the most abundant metals, whereas Cd (0.072 mg kg−1 d.w.) showed the lowest concentrations. To compare the total metal content at different sampling sites, the metal pollution index (MPI) was used, obtained with the equation:
where C men = concentration of the single metal in the sample. The values of MPI (Fig. 3) ranged from 6.0 (station no. 15) to 25 (station no. 8) and from 4 (station no. 16) to 17 (stations no. 7 and no. 15) for unwashed and washed leaves respectively. The station no. 8 (University) showed the highest values of MPI and the highest concentrations of the considered pollutants. This is not surprising, considering that the station is located in an area interested by traffic mostly caused by the activities of university, etc. The station no. 15 (industrial area) showed the lowest MPI value and the lowest concentrations of metals, which are similar to those determined in the reference stations (no. 1 and no. 2). This is not surprising, considering that the station is located in a large and open area interested only by commercial activities, and there are no production activities.
The obtained results are in agreement with other published data (Orecchio and Amorello 2011) regarding the same area. In detail, considering the spatial distribution of platinum in soil, the greater concentrations are measured in the proximity of the Palermo historical town centre. This area is characterized by narrow streets frequented by heavy traffic consisting of cars and buses. Also, high Pt levels in soil have been measured in a station located in a large area interested by traffic mostly caused by the activities of university, hospitals, cemetery, etc. In another paper (Orecchio and Amorello 2010), we have previously reported that the higher amounts of Pt and Rh in Nerium oleander were detected in leaves from the urban area of Palermo compared to the reference. The differences of Pt and Rh concentrations in leaves between green areas and road and anthropized areas are in agreement with differences in PAH contents measured by us in the same area using oleander and other bio accumulators (Culotta et al. 2005).
As reported in literature (Ugolini et al. 2013), sample washing is necessary if the purpose is to distinguish between pollutants deposited on the surface of leaves and those accumulated within the internal tissues. In Fig. 4 are shown the lead concentrations in the Eucalyptus leaves collected in various stations. For stations no. 1, 2, 3, 4, 14, 15 and 18, concentrations are fairly low, as we had expected. In particular, lead concentration in the leaves of station no. 16 (Lincoln) is comparable with the fore-mentioned stations, even though it is a main road with average traffic flow. This can be attributed to the fact that this road is highly ventilated and near to the coast. In most stations (no. 5, 10, 19, 20, 21, 22) with average traffic flow, lead concentrations in unwashed leaves vary from 3.4 to 6 mg kg−1 d.w. Higher concentrations were found in the stations with heavier traffic flow (no. 6, 7, 8, 9, 11, 12, 13, 17, 22). In many of these stations, semaphores do not allow at the vehicles to flow continuously, and cars, buses and other vehicles are forced to frequently stop and go with the result that pollutants emitted by vehicles enter the atmosphere and are gradually deposited on the leaf surfaces.
Concentrations of lead are in the range from 1.8 to 14 and 0.79 to 11 mg kg−1 d.w. in unwashed and washed samples, respectively. This trend is in good agreement to recent literature (De Nicola et al. 2008) that report a decrease of lead concentration in washed leaves.
The sampling station no. 17 (Piemonte road) showed the highest concentrations. This station, despite being located outside of the historic centre, is in the Palermo residential and commercial area, route frequented by heavy and slow traffic.
Lead concentrations were higher in the thresholds (10 mg kg−1 d.w.) for plant biology (Ugolini et al. 2013) only in three stations (no. 11, no. 12, no. 17). The lead (nonessential metal) is accumulated passively in leaves by a detoxification process (Ugolini et al. 2013). This would explain the high concentration in most washed samples.
Considering all the stations, the average concentration of lead in unwashed leaves is 5.6 mg kg−1 d.w., whilst in the washed samples is 4.2 mg kg−1 d.w. Therefore, we can affirm that, generally, most of the lead is contained within the leaf structure with the exception of some stations (no. 2, 3, 8, 16, 17, 22) in which the metal present on the surface is not negligible (on average 50 % compared to total lead).
According to several authors (Celik et al. 2005), Cu, Zn and Pb are directly associated to the traffic density, and the concentrations found in some sampling stations would confirm this hypothesis.
The higher amounts of Pb detected in leaves of Eucalyptus from the urban Palermo area, compared to the control sites, are diagnostic of conspicuous air contamination. These results are in good agreement to Dongarra et al. (2003) that affirms a geochemical anomaly of origin of lead in needles of Pinus pinea L. in Palermo city: the highest concentrations of Pb were measured in trees growing within the city area. The excess of Pb, compared to natural background, in the analyzed pine needles was attributed, on the basis of the Pb isotope composition, to the anthropogenic emissions, mainly gasoline combustion.
Concentrations of zinc are in the range from 19 to 64 and 7 to 58 mg kg−1 d.w. in unwashed and washed samples, respectively. The sampling station no. 8 (University) showed the highest concentrations. The found concentrations are considered normal for plants (Ugolini et al. 2013). In the washed samples, the zinc concentrations are similar to ones total (Fig. 5). The obtained data confirm that the zinc is present within the structure of the leaves, and this result is in agreement with the fact that this element is a macronutrient. To establish the origin of zinc (and later of the other metals also) in the leaves, given that the lead derives mainly from anthropic activities, we constructed a graph (not reported), placing lead concentrations on the abscissa and zinc concentrations on the ordinate. The concentrations of Pb and Zn are not correlated, excluding their common origin. This is because the amount of natural zinc in the Eucalyptus leaves is much higher than the quantity that might derive from human sources, and therefore, the latter does not modify the natural values. The intercept value of the best straight line passing through the points, on the ordinate axis, allows us to determine natural base concentrations (about 40 mg kg−1 d.w.), which diverge greatly from those reported in the literature but which are valid for any type of plant (Kabata Pendias and Pendias 1992). This anomaly can be justified by considering that Sicilian soils show high zinc concentrations (Giacalone et al. 2005). This element is rather uniformly distributed among the carbonate, Fe–Mn oxides and organic phases, whilst lower contents are associated with the exchangeable fraction. It has been documented that Fe–Mn oxides may adsorb Zn on their surface (Catts and Langmuir 1986) with high stability constants.
Copper concentrations in the unwashed leaves ranged from 9 to 20 mg kg−1 d.w. (Fig. 6). As for zinc, the concentrations of copper on washed and unwashed samples, excluding few cases, have a more or less constant trend; above-average (15 mg kg−1 d.w.) concentrations were identified for stations with a heavy traffic flow which coincide with those already identified for lead. In all the stations, excluding the stations no. 6 (Highway 113), no. 7 (Tukory road), no. 8 (University) and no. 16 (Lincoln road) which have the lowest value of the metal, copper is present within the structure of the leaves; in fact, the concentrations of the samples washed are similar to the total (Fig. 6). As for zinc, also in this case, the obtained data confirm that the copper is present within the structure of the leaves, and this result is in good agreement with the fact that this element is an essential macronutrient. Figure 7 shows a significant positive correlation between concentrations of Cu and Pb (correlation coefficient = 0.60, P = 0.05). The intercept value on the ordinate axis allows us to determine the natural base concentrations (Gonzales et al. 1996). The found concentration (12 mg kg−1 d.w.) is in agreement with the natural concentrations of copper determined in the leaves by various researchers (5–20 mg kg−1 d.w.) (Kabata Pendias and Pendias 1992; Gonzales et al. 1996). Unlike zinc, the positive correlation suggest that in all the stations, most of the copper derives from natural sources whilst a slight part derives from human sources, which can only be traffic since there were no other possible sources of emissions in the areas examined.
Figure 8 shows average concentrations of cadmium in the leaves from the various stations. The concentration of Cd in the unwashed leaves ranged from 0.01 to 0.62 mg kg−1 d.w. The mean value was 0.072 mg kg−1 d.w. In heavy anthropized areas (stations no. 6, 8), the concentrations were higher (0.62 mg kg−1 d.w.). However, the concentrations found in all the stations, excluding few cases (stations no. 7, 8, 10, 11 and 22) were of the same order of magnitude as those natural (0.05 mg kg−1 d.w.) and far from the phytotoxic level (5 mg kg−1 d.w.) (Kabata Pendias and Pendias 1992). In Eucalyptus leaves, considering experimental uncertainties, the sample washing did not cause significant differences for Cd concentration, suggesting a stock capacity, with the exception of stations no. 6, no. 8 and no. 20 which showed significant lower concentrations in washed leaves. The concentrations of Pb and Cd are not correlated, excluding their common origin. This is because the concentrations of Cd in the Eucalyptus leaves were very low and similar to the natural values.
Iron concentrations ranged from 131 to 410 mg kg−1 d.w. and from 75 to 378 mg kg−1 d.w. in unwashed and washed leaves, respectively. Unwashed leaves showed significantly higher Fe concentration than washed ones, except some stations (no. 5, no. 7, no. 18, no. 20). Maximum concentration was determined in station no. 17 (the same stations where lead concentrations were higher than average).
Figure 9 shows a positive correlation (r = 0.82, p = 0.05) coefficient between concentrations of Fe and Pb. The intercept value on the ordinate axis allows to determine the natural concentrations (about 100 mg kg−1 d.w.), which correspond with those reported in the literature by some authors (Kabata Pendias and Pendias 1992; Gonzales et al. 1996).
Manganese concentrations ranged from 34 to 520 mg kg−1 d.w. and from 32 to 355 mg kg−1 d.w. in unwashed and washed samples, respectively. As reported for other elements, the Mn concentrations on washed and unwashed samples, excluding few cases, have a more or less constant trend; above-average (160 mg kg−1 d.w.) concentrations were identified in few stations with a heavy traffic flow (no. 8 which coincide with those already identified for lead, no. 6, etc.). Manganese concentration in both sample treatments was into the range considered as normal in plant tissues, 17–600 mg kg−1 d.w. (Bowen 1979).
As already stated, the analyses were carried out on washed and unwashed samples. Total metal concentrations include the fraction deposited on the leaf surface, which is easily washed away by the rain, and an internal fraction, mainly accumulated in the leaf mesophyll cells. In general, in this study, washing with distilled water had low considerable (meanly 16 %) result on the reduction of contaminant concentration for all the metals.
The heavy metal concentrations in the Eucalyptus leaves collected at Palermo were similar to those reported in other studies carried out in industrial and urban areas (Rodriguez et al. 2012).
Analyzing, with an optical microscope (Fig. 10), the transversal cryosections of the apical and basal portions of the Eucalyptus leaves collected in urban areas with heaviest traffic, such as stations no. 6 (Highway SS113) and no. 8 (University), we observed characteristic accumulations of secondary metabolites. These, according to Maffei (1998), are produced from the plant in order to defend itself from the abiotic stresses, protecting and detoxifying the plant from the dangerous chemicals (Figs. 10 and 11a–c). In urban areas with average traffic flow, such as station no. 5 (Avenue of the Mille), considered representative of the city of Palermo, the secondary metabolites (Fig. 12a–c) are found mainly in the mesophyll cells inside the vacuolar structure, whilst in the areas with heavier traffic, such as in station no. 8, the secondary metabolites also invade the epidermis. Microphotography reveals a correlation between presence of necrosis zones, modified cuticles and accumulations of acid phosphatases in the areas with heavy traffic. The cytological observations were also extended to the leaves with a characteristic tissue reaction, also visible to the naked eye (galls), mainly close to the median rib. In this case, the acid phosphatase was accumulated around the regular contours of the galls, more or less spherical, and in the more internal peripheral tissues (Fig. 12).
a Transversal cryosections of the apical portion of the eucalyptus leaves collected in urban areas with heaviest traffic (no. 7 Tukory). ×25. b Transversal cryosections of the basal portion of the eucalyptus leaves collected in urban areas with heaviest traffic (no. 7 Tukory). ×25. c Transversal cryosections of the basal portion of the eucalyptus leaves collected in urban areas with heaviest traffic (no. 7 Tukory). ×25
a Transversal cryosections of the apical portion of eucalyptus leaves collected in urban areas with heaviest traffic (no. 8 University). ×25. b Transversal cryosections of the basal portion of eucalyptus leaves collected in urban areas with heaviest traffic (no. 8 University). ×25. c Transversal cryosections of the basal portion of eucalyptus leaves collected in urban areas with heaviest traffic (no. 8 University). ×10
a Transversal cryosections of the eucalyptus leaves collected in urban areas with heaviest traffic (no. 5 Avenue of the Mille). 5×. b Transversal cryosections of the eucalyptus leaves collected in urban areas with heaviest traffic (no. 5 Avenue of the Mille). 10×. c Transversal cryosections of the eucalyptus leaves collected in urban areas with heaviest traffic (no. 5 Avenue of the Mille). 50×
The photographs (Fig. 13a–c) of the diaphanized leaves from the more polluted areas (stations no. 6, 8, 22) show irregular areolas, several idioblasts (coloured brown) both on the ribs and scattered in the mesophyll. With polarized light, we can see, on the transparent blade of the Eucalyptus leaf, the outline of the areolas, the thickness of the ribs and many crystal deposits near to the ribs. The same investigations were carried out on the samples from station no. 2 (Botanic Garden), chosen as a control (Fig. 14a–c). These leaves are integral with leaning mesophyll cells, without necrosis and dysplasia. The cytochemical staining reveals low qualitative levels of phosphatases. Botanic Garden leaves (Fig. 14a, b) show a cytological harmonic organization, without coarctation or cell collapse, in Fig. 14c are visible few areas of necrosis, whilst in the leaves of station University no. 8 (Fig. 11), between the mesophyll cells and the cells that surround the central rib are visible deposits of acid phosphatase. Also, in the secretor cavities are accumulations of secondary metabolites. The leaves of the station Tukory no. 6 (Fig. 10) show several areas of necrosis.
a Transversal cryosections of the apical portion of the eucalyptus leaves collected in urban area far from traffic (no. 7 Botanical Garden). 10×. b Transversal cryosections of the apical portion of the eucalyptus leaves collected in urban area far from traffic (no. 7 Botanical Garden). 25×. c Transversal cryosections of the apical portion of the eucalyptus leaves collected in urban area far from traffic (no. 7 Botanical Garden). 10×
The studies were also extended to the pollen of Eucalyptus. By means of an optical microscope, it was possible to show that the pollen from the more polluted stations is particularly viscous; therefore, the granules tend to aggregate and many granules are coarctated (Fig. 15). The pollen grains from the control station, on the other hand, are well spaced out on the slide and show no irregular morphology.
The chemical and cell tests demonstrate that the Eucalyptus is a most favourable bio indicator and bio accumulator particularly sensitive to urban pollutants. In our case, the results relating to the different stations vary considerably, regarding both the concentrations of the pollutants (Pb, Cd, Cu, Fe and Zn) and phosphatase accumulations.
Conclusions
This study demonstrates that atmospheric pollutants can lead to the disturbance of the normal functioning of plants. The analysis of metals in leaves showed a relationship between the highest values in the samples and proximity to the emission source. In addition, Eucalyptus leaves with high metal accumulation showed structural and functional modification as compared to the less contaminated ones. In detail, areas with average traffic flow, the secondary metabolites are found mainly in the mesophyll cells inside the vacuolar structure, whilst in the areas with heavier traffic, the secondary metabolites occupy the epidermis. In the areas with heavy traffic, the microphotographs show zones of necrosis, modified cuticles and accumulations of acid phosphatases.
Eucalyptus in urban areas plays an important role not only for green embellishment, shading and mitigation of microclimate but also for the capacity through leaf surfaces to adsorb atmospheric contaminants. Leaves of Eucalyptus were efficient in intercepting heavy metals (Pb, Fe, Zn, Cd, Cu and Mn). Leaf washing is indispensable to distinguish between pollutants deposited on the surface of leaves and metals accumulated within the internal tissues.
Moreover, this study highlights that Eucalyptus is fairly resistant to heavy metals. This high resistance is due to some characteristic of the leaves, such as typical waxy scales secreted from the epidermis cells on the surface. Moreover, the mature leaves are leathery, which obstructs infiltration of pollutants in the mesophyll spaces. The Eucalyptus indicates environmental problems and, thanks to its morpho-structural characteristics, it is a bio indicator which suffers a modest degree of damage, such as browning leaves, initial blade loss (defoliation).
The results clearly indicate that the leaves of Eucalyptus offer the possibility to assess the quality of air in a geographic area with a modest economic burden, if compared to what is needed to set up and run automatic survey installations.
The results on air quality of the city of Palermo obtained in this research using Eucalyptus leaves are in good agreement with results of other studies that measured other metals (Pd, Rh) and other environmental contaminants (PAHs). It is likely that anthropic are the major causes for high concentrations of these contaminants in the Eucalyptus leaves collected in the Palermo area.
As a final point, we believe that this research should now be extended to other elements (Pt, Pd, Rh, etc.) which are used in the catalytic converters of vehicles, which run on lead-free petrol.
References
Aksoy A, Ozturk MA (1997) Nerium oleander L. as a bio monitor of lead and other heavy metals in Mediterranean environments. Sci Total Environ 205:145–150
Ashrafi SH, Fisk FW (1961) Histochemical localization of phosphatases in the stable fly, Stomoxys Calcitrans (L.), using naphthol As-phosphate. Ohio Journal of Science 61: 7–13 http://hdl.handle.net/1811/4746.
Bowen HJM (1979) Environmental chemistry of the elements. Academic, London, p 333
Calamari D, Bacci E, Focardi S, Gaggi C, Morosini M, Vighi M (1991) Role of plant biomass in the global environmental partitioning of chlorinated hydrocarbons. Environ Sci Technol 25:1489–1495
Catts JG, Langmuir D (1986) Adsorption of Cu, Pb and Zn by MnO2: applicability of the site binding-surface complexation model. Appl Geochem 1:255
Celik A, Kartal AA, Akdoganb A, Kaska Y (2005) Determining the heavy metal pollution in Denizli (Turkey) by using Robinio pseudo-acacia L. Environ Int 31:105–112
Chaoui A, Mazhoudi S, Ghorbal MN, Ferjani EE (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzymes activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Culotta L, Gianguzza A, Orecchio S (2005) Leaves of Nerium oleander L. as bioaccumulators of polycyclic aromatic hydrocarbons (PAH) in the air of Palermo (Italy). Extraction and GC–MS analysis, distribution and sources. Polycycl Aromat Compd 25:327–344
De Nicola F, Maisto G, Prati MV, Alfani A (2008) Leaf accumulation of trace elements and polycyclic aromatic hydrocarbons (PAHs) in Quercus ilex L. Environ Pollut 153:376–383
Dongarra G, Varrica D, Sabatino G (2003) Occurrence of platinum, palladium and gold in pine needles of Pinus pinea L. from the city of Palermo (Italy). Appl Geochem 18:109–116
Fuchs C (1963) Fuchsin staining with NaOH clearing for lignified elements of whole plants or plants organ. Stain Tecnol 38:141–144
Gahan B (1984) Plant histochemistry and cytochemistry. Academic, London, pp 218–242
Giacalone A, Gianguzza A, Orecchio S, Piazzese D, Dongarrà G, Sciarrino S, Varrica D (2005) Metal distribution in the organic and inorganic fractions of soil: a case-study on soils from Sicily. Chem Speciat Bioavailb 17:83–94
Gianguzza A, Culotta L, Mannino MR, Orecchio S (2006) Pelargonium leaves as bioaccumulator of polynuclear aromatic hydrocarbons: analytical method and evaluation of sources and air quality in Palermo area. Fresenius Environ Bull 15:928–935
Gianguzza A, Culotta L, Orecchio S (2008) Absorption of polycyclic aromatic hydrocarbons by pinus bark: analytical method and use for environmental pollution monitoring in the Palermo area (Sicily, Italy). Environ Res 107:371–379
Gomez B, Gomez M, Sanchez JL, Fernandez L, Palacios MA (1998) Platinum and rhodium distribution in airborne particulate matter and road dust. Sci Total Environ 59:215–222
Gonzales SE, Alonso RE, Lopez MP, Muniategui LS, Prada RD (1996) Determination of trace elements in tree leaves. J Anal and Environ Chem 86:181–191
Kabata Pendias A, Pendias H (1992) Trace element in soil and plant. CRC Press Inc.
Lehndorff E, Schwark L (2004) Biomonitoring of air quality in the Cologne conurbation using pine needles as a passive sampler – part II: polycyclic aromatic hydrocarbons. Atmos Environ 38:3793–3808
Li M, Hu CW, Zhu Q, Chen L, Kong ZM, Liu ZL (2006) Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalga Pavlova viridis (Prymnesiophyceae). Chemosphere 62:565–572
Lombardo M, Melati MR, Orecchio S (2001) Assessment of the quality of the air in the city of Palermo through chemical and cell analyses on Pinus needles. Atmos Environ 35:6435–6445
Maffei M (1998) Biochimica vegetale. Piccin, Padova
Mannino MR, Orecchio S (2011) Chemical characterization of ancient potteries from Himera and Pestavecchia necropolis (Sicily, Italy) by inductively coupled plasma–optical emission spectrometry (ICP–OES). Microchem J 97:165–172
Marco JAM, Kishimba MA (2006) Pesticides and metabolites in cassava, Eucalyptus, plum and cashew leaves and roots in relation to a point source in Kibaha, Tanzania. Chemosphere 64:542–548
Market B (1993) Plants as biomonitors. Weinhem-New York-Basel- Cambridge 2–19:57–59
McKiernan AB, O’Reilly-Wapstra JM, Price CJ, Davies NW, Potts BM, Hovenden MJ (2012) Stability of plant defensive traits among populations in two Eucalyptus species under elevated carbon dioxide. J Chem Ecol 38:204–212
Orecchio S (2013) Microanalytical characterization of decorations in handmade ancient floor tiles using inductively coupled plasma optical emission. Microchem J 108:137–150
Orecchio S, Amorello D (2010) Platinum and rhodium associated with the leaves of Nerium oleander L.; analytical method using voltammetry; assessment of air quality in the Palermo (Italy) area. J Hazard Mater 174:720–727
Orecchio S, Amorello D (2011) Platinum levels in urban soils from Palermo (Italy); analytical method using voltammetry. Microchem J 99:283–288
Rodriguez JH, Wannaz ED, Salazar MJ, Pignata ML, Fangmeier A, Franzaring J (2012) Accumulation of polycyclic aromatic hydrocarbons and heavy metals in the tree foliage of Eucalyptus rostrata, Pinus radiata and Populus hybridus in the vicinity of a large aluminium smelter in Argentina. Atmos Environ 55:35–42
Siedlecka A, Krupa Z (2002) Functions of enzymes in heavy metal treated plants. In: Prasad, M.N.V., Kazimierz, S. (Eds.), Physiology and biochemistry of metal toxicity and tolerance in plants. Kluwer, Netherlands, 314–3177
Ugolini F, Tognetti R, Raschia A, Baccia L (2013) Quercus ilex L. as bioaccumulator for heavy metals in urban areas: effectiveness of leaf washing with distilled water and considerations on the trees distance from traffic. Urban For Urban Green 12:576–584
Zhang F-Q, Wang Y-S, Lou Z-P, Dong J-D (2007) Effect of heavy metal stress on anti oxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Orecchio, S., Culotta, L. Assessment of quality of air in Palermo by chemical (ICP-OES) and cytological analyses on leaves of Eucalyptus camaldulensis . Environ Sci Pollut Res 22, 1891–1905 (2015). https://doi.org/10.1007/s11356-014-3570-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3570-8