Abstract
With the aim of in situ bioremediation of soil contaminated by hydrocarbons, anodes arranged with two different ways (horizontal or vertical) were compared in microbial fuel cells (MFCs). Charge outputs as high as 833 and 762C were achieved in reactors with anodes horizontally arranged (HA) and vertically arranged (VA). Up to 12.5 % of the total petroleum hydrocarbon (TPH) was removed in HA after 135 days, which was 50.6 % higher than that in VA (8.3 %) and 95.3 % higher than that in the disconnected control (6.4 %). Hydrocarbon fingerprint analysis showed that the degradation rates of both alkanes and polycyclic aromatic hydrocarbons (PAHs) in HA were higher than those in VA. Lower mass transport resistance in the HA than that of the VA seems to result in more power and more TPH degradation. Soil pH was increased from 8.26 to 9.12 in HA and from 8.26 to 8.64 in VA, whereas the conductivity was decreased from 1.99 to 1.54 mS/cm in HA and from 1.99 to 1.46 mS/cm in VA accompanied with the removal of TPH. Considering both enhanced biodegradation of hydrocarbon and generation of charge in HA, the MFC with anodes horizontally arranged is a promising configuration for future applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the essential energy sources, petroleum is an important material basis for the development of national economy. However, petroleum hydrocarbons have become one of the most important types of organic pollutants during the processes of exploration, extraction, refining, transporting, and marketing petroleum products (Margesin et al. 2003). Since petroleum commonly contains hazardous chemicals such as benzene, ethylbenzene, toluene, and xylenes (BETX), it threatens human health and ecosystem safety, inhibiting plant growth, destroying soil structures, and damaging groundwater quality (Guo and Zhou 2003; Meagher 2000; Sarkar et al. 2005; Ting et al. 1999; Zhou et al. 2005). Therefore, more and more attention has been paid to the remediation of petroleum hydrocarbons in soils.
Traditional strategies to treat petroleum contaminated soils include various physical and chemical technologies such as thermal treatment, soil washing, gaseous or liquid extraction, solidification, and stabilization or by using combination of the above (Kaimi et al. 2007). However, physical and chemical remediation is usually expensive and has high risk to incur additional air or aqueous pollution (Riser-Roberts 1998). Bioremediation is defined as a promising green technology to remove pollutants from contaminated soils. Bioremediation of hydrocarbon contaminants, such as biosparging, biostimulation, and bioaugmentation, has advantages of low cost, less interference with the soil structure and higher public acceptance than other approaches, which has been well utilized for the past decades (Das and Chandran 2010; Leahy and Colwell 1990; Prince and Bragg 1997; Tang et al. 2010a). However, the performance of bioremediation is affected by many factors such as the abundance of electron acceptor, bacterial competition, etc. (Morris et al. 2009; Tyagi et al. 2011).
Recently, a new device, soil microbial fuel cells (MFCs) that consist of an anode embedded in the anaerobic soil and a cathode directly faced to the air has attracted intensive attentions, because it can be used for organic pollutant degradation with simultaneous electricity generation and it does not need in situ addition of electron donor or electron acceptor into the subsurface. The exoelectrogens such as Geobacter spp. and Shewanella spp. in subsurface can utilize organic matter in soils, and these organics cannot be utilized for electricity generation without exoelectrogens (Anderson et al. 2003; Jung 2012). In the soil MFCs, electrons released from the substrate oxidation in the anodes are transferred via the external circuit to the cathode where the electrons are eventually consumed by the terminal electron acceptors (Lu et al. 2014). Electrodes offer an alternative electron acceptor for stimulating the anaerobic degradation of organic contaminants (Lovley 2006; Morris and Jin 2008; Zhang et al. 2010). The use of soil MFCs to enhance hydrocarbon remediation has a number of advantages. In particular, it stimulates and enhances hydrocarbon degradation accompanied with energy production without requiring any chemical addition or energy inputting. Wang et al. (Wang et al. 2012) demonstrated that a U-tube MFC increased total petroleum hydrocarbon (TPH) removal by 120 %, from 6.9 to 15.2 %, for water saturated saline soil close to the anode (<1 cm). Morris and Jin (Morris and Jin 2012) observed that the biodegradation rate of TPH in sediment increased by 12 times than that of background. While these studies demonstrated the possibility to use anodes as the electron acceptor for petroleum hydrocarbon removal, to our best knowledge, there has been no report to explore the impact of configuration of anodes on petroleum hydrocarbon degradation.
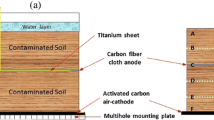
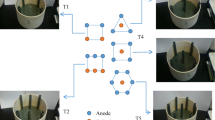
In this study, we constructed a new design of air-cathode MFCs with four layers of carbon mesh anodes to enhance biodegradation of petroleum hydrocarbons in soil. Two sets of reactors were designed through changing the configuration of the anodes, duplicate reactors with anodes horizontally arranged (HA) and duplicate reactors with anodes vertically arranged (VA) (Fig. 1). The main purpose of the study is to compare the biodegradation of petroleum hydrocarbons in the two configurations of the reactors, by measuring hydrocarbon concentration. We also monitored the production of electricity, as well as the change of pH and electrical conductivity of the soils in order to explore their impact on the biodegradation of petroleum hydrocarbons.
Materials and methods
Soil sample collection and characterization
Petroleum-contaminated soil was obtained from the Dagang Oil Field in Tianjin, China. The soil sample was taken from 5 to 10 cm below ground surface. The soil was sealed and transported to our laboratory. After being partially air-dried, soil samples were passed through a 2-mm sieve to remove plant roots and rocks. After the sieved soil was artificially homogenized, distilled water is added to a soil sample at a ratio of 1:5 (w/v). The mixture is shaken and centrifuged. pH and electrical conductivity of the supernatant are measured by using a pH meter (STARTER 3100, Ohaus Instruments Co., Ltd., Shanghai, China) and conductivity meter (DDS-11A, Shengci Instruments Co., Ltd., Shanghai, China) individually (Jung et al. 2014); the physical and chemical properties of the soil sample were measured as follows: pH 8.3, electrical conductivity 1.99 mS/cm, available nitrogen amount 46 mg/kg dry soil, available phosphate amount 198 mg/kg dry soil. Original concentration of TPH in soil sample was 25.7 g/kg.
MFC configuration and operation
The air-cathode soil MFC consisted of a rectangular organic glass storage container with the dimensions 6 × 6 × 9 cm (length × width × height) and was filled with the petroleum-contaminated soil. The cathode was placed in the bottom of the reactor and exposed to air; four layers of the anodes were embedded in the soil with 2 cm of distance between two adjacent anode layers as shown in Fig. 1a, b. The air cathode consisted of stainless steel mesh (8 × 8 cm, type 304 SS) with a catalyst layer (activated carbon, projected area of 36 cm2) on the soil side and a gas diffusion layer on the air side (Li et al. 2014). The anode was made of acetone-cleaned carbon meshes (3 k; Jilin Carbon Factory, Jilin, China) (Wang et al. 2009). Anodes were connected to the external circuit by a titanium plate. Anodes and cathodes were connected to 1,000 Ω external resistors (Domínguez-Garay et al. 2013; Morris and Jin 2012; Wang et al. 2012) according to the resistance used for exoelectrogenic bacteria acclimation in air-cathode MFCs with aqueous medium (Liu and Logan 2004).
MFCs were constructed in duplicate and filled with contaminated soils, the soil was immersed with a 2 cm of deionized water layer during regular experiments without adding buffer solution or additional hydrocarbons. The water lost via evaporation was routinely compensated with sterile deionized water to maintain a constant water level. All reactors were operated for 135 days under room temperature (23 ± 3 °C), and cell voltages were recorded every 30 min using a data acquisition system (PISO-813, ICP DAS Co., Ltd, Shanghai, China). Waterlogged soils under open circuit were used as the positive controls. At the end of the tests, soil samples in each reactor were collected between every two adjacent anodes and mixed before analyses and the soil samples of different layers were marked as S-1, S-2, S-3, and S-4 sequentially (Fig. 1a). The soil samples were analyzed for TPH, polycyclic aromatic hydrocarbons (PAHs), and n-alkanes.
Analytical methods
Determination of TPH content was conducted by Soxhlet apparatus for extraction according to the method reported earlier by Cai et al. (Cai et al. 2010). The freeze-dried soils were sieved through a 100 mesh, weighed, and extracted with dichloromethane (DCM) for 24 h using a Soxhlet apparatus. Then, the solution was transferred to an Erlenmeyer flask to evaporate volatile DCM. After evaporation of the solvent at 40 °C water bath in the hood, the amount of residual TPH was determined by weighing the flask before and after evaporation. After the gravimetric quantification, fractional analysis of petroleum hydrocarbons was carried out based on column chromatography introduced by Tang et al. (Tang et al. 2010b) with some modifications. About 12 cm of activated silica gel, 6 cm of anhydrous Al2O3 powder, and 1 cm of anhydrous Na2SO4 from the bottom to the top were filled into a glass column (Ф10 × 250 mm). The column was first washed with n-hexane and then was loaded and washed with 20 mL of n-hexane for saturated hydrocarbon extraction, and then, 75 mL n-hexane/DCM (1:1, v/v) was used to extract the PAHs. The measurements of n-alkanes and the 16 priority PAHs were performed on the same gas chromatograph (GC, Agilent 7890 GC, US) equipped with a model 5975 mass selective detector (MSD; SIM mode). Soil analytical results were reported on a dry weight basis. The pH and conductivity of soil were measured in a 1:5 (w/v) soil/deionized water mixture. Total charge output (Q, C) was obtained by \( Q={\displaystyle \underset{0}{\overset{T}{\int }}}\left(E/R\right) dt \), where T (s) is the cycle time, E (V) is the cell voltage, and R (Ω) is the external resistance.
Results
The performance of MFCs
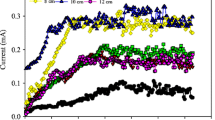
The voltages increased gradually after the connection of an external loading (1,000 Ω), as observed in all the reactors (Fig. 2a). Approximately, 50 h after filling soils into MFCs, the voltages reached their first peaks. The maximum voltages for HA and VA were 0.282 ± 0.015 and 0.285 ± 0.025 V, respectively. Over the tested period (135 days), the total charge accumulated slowly with time in all reactors (Fig. 2b). The total charge generated by HA was 833 ± 58C, which was 9.3 % higher than that of the VA (762 ± 34C).
Changes in characteristics of soils
After 135 days of operation, the pH in all the soil samples increased slightly, which could be due to the accumulation of oxygen reduction product of OH− at the cathode (Wang et al. 2014). In the soil remediation MFC system, the degradation of organic acids and the formation of bicarbonate salts might also increase the pH of the soil. In particular, the pH in the HA operated increased from 8.26 ± 0.12 at the beginning of the experiment to 9.12 ± 0.08 at the end of the study, which was consistent with the fact that the reactions took place more effectively and rapidly in the HA than those in VA (see below) (Jung et al. 2011). Opposite trend was observed for the electrical conductivity of soil samples, as shown in Table 1. The electrical conductivities of soil samples in HA, VA, and the controls were decreased compared with the consistent conductivity in the original soils (1.99 ± 0.09 mS/cm). The conductivity of the HA was 1.54 ± 0.09 mS/cm, which was slightly higher than that of VA (1.46 ± 0.02 mS/cm) and the controls (1.45 ± 0.06 mS/cm).
Degradation of petroleum hydrocarbons
The degradation of TPHs in soil under various different reactors was observed after 135 days of operation. The TPH degradation rates of the S-4 (Fig. 1a) were higher than other layers in all reactors, with the highest value of 15.3 ± 0.2 % observed in HA. As shown in Fig. 3, in the HA, approximately, 12.5 ± 0.6 % of the TPH was removed, which was 1.5 and 2.0 times higher than those obtained in VA (8.3 ± 0.1 %) and control (6.4 ± 0.2 %), showing that the horizontal arrangement of anodes substantially enhanced the biological degradation of TPH. Similar trend on TPH degradation were also observed in each layer in these reactors (HA > VA > control) (Fig. 3). In charge output, there were not much different among four reactors (Fig. 2). However, there is much greater difference in TPH degradation (Fig. 3). It indicates that petroleum hydrocarbons were degraded not only by exoelectrogenic bacteria but also by other microorganisms.
The concentrations of phenanthrene (PHE; C14), fluoranthene (FLU; C16), pyrene (PYR; C16), chrysene (CHR; C18), and benzo(b)fluoranthene (BbF; C20) were higher than 300 ng/g, accounting for 78 % of total PAHs (TPAHs) in the original soil. As shown in Fig. 4, after the experiment period, the concentrations of these five PAHs have the lowest values in the HA, corresponding to the highest degradation rates of 24.6 % (PHE), 4.6 % (FLU), 8.5 % (PYR), 10.4 % (CHR), and 9.4 % (BbF). For two-ring and three-ring PAHs, the reduction rate was 40 % for all the reactors. Groups of higher molecular weight compounds such as five-ring and six-ring PAHs showed a relatively low degradation rate (7.8 %).TPAHs calculated by adding the concentrations of 16 PAHs up also showed that the HA had the highest TPAHs degradation rate of 14 % than that of the control (7 %) and the VA (10 %).
Contents of PAHs in soil MFCs under different conditions. OS the original soil, NP, naphthalene, ACY acenaphthylenem, ACE acenaphthylene, FLN fluorene, PHE phenanthrene, ANT anthracene, FLU fluoranthene, PYR pyrene, BaA benzo(a)anthracene, CHR chrysenem, BbF benzo(b)fluoranthene, BkF benzo(k)fluoranthene, BaP benz-o(a)pyrene, IcdP indeno(1,2,3-cd)pyrene, DBah dibenzo(a,h)an-thracene, BghiP benzo(ghi)perylene
n-Alkanes were detected from C8 to C40 with main contents of C17–C40 (>20 ug/g for each content) in original soil (Table 2). The degradation of n-alkanes consistently showed a better performance in HA compared to that in VA. The maximum degradation rate up to 22 % from 1,120 to 877 ug/g soil on total n-alkanes (TNAs) was achieved in HA, whereas that in VA and control were only 9 and 7.8 %.
Discussion
All the voltages of connected soil MFCs exhibited a trend in which voltage rises, peaks, and decays after the waterlogged soils had been incubated, indicating that the soil MFCs with anodes arranged both in horizontal and in vertical can be started successfully. During 135 days of operation, the soil MFCs exhibited several peaks of voltages, which was presumably due to the step degradation of easily and slowly biodegradable organic matters in the soil (Hong et al. 2009). Besides, the HA generated relatively more Coulombs, showing that the arrangement of anodes had a solid effect on the electricity production in soil MFCs. The evaporation of water through the cathode brought more substrates for anodic bacteria by facilitating hydrocarbon diffusion from surrounding areas toward the anodes in the HA. However, the diffusion direction of water (from top to the bottom) and the direction of the anodes were placed in parallel in the VA, which would reduce the substrate availability of the anodes and had a negative effect on the electricity production. Besides, the vertical arrangement of anodes has a limited projected area on the cathode. The flux of electromotive intensity is proportional to the projected area, so the flux of electromotive intensity was reduced. This may also incur an additional resistance for ion transfer. It indicates that lower mass transport resistance in the HA than that of the VA resulted in more power and more TPH degradation.
The soil pH increased from 8.26 at the beginning of the experiment to 8.61–9.12 at the end of the study. Since no ion exchange membrane was employed in our study, the possible reason might be due to the poor permeability of proton/hydroxyl in the waterlogged soil, resulting in an accumulation of hydroxyl close to the cathode. The increase in soil pH close to the anode might be due to the depletion of existing organic acids and the formation of bicarbonate salts. After 135 days, the electrical conductivity of soils in all reactors obviously decreased compared to that of the original soil (1.99 ± 0.09 mS/cm). A possible reason for this may be the formation of metal (such as Ca2+ and Mg2+) bicarbonate precipitation at a high pH, leading to the reduction of ion concentration and thus reduce the conductivity of the soil. This could be further investigated in terms of carbon capture and saline soil reclaiming in the future.
The removal of TPH was stimulated by the application of MFCs in waterlogged soil. The carbon mesh anodes provided a low-cost, low-maintenance pathway for anaerobic oxidation of TPHs by co-localizing of the contaminants, electron acceptor (the anode) and degradative microorganisms on the same surface. Therefore, the presence of the carbon mesh anodes increased the microbial activity in the soil and accelerated the degradation of contaminations. This has important implications for potential uses for in situ bioremediation of soils or sediments contaminated with organic pollutants in conjunction with electricity generation.
The highest TPH degradation rate of 15.3 % was achieved in S-4, which was higher than those of other three layers, probably due to the smallest distance between the anode and the cathode. The hydroxyl transfer rate might be limited by the poor permeability of soil, which resulted in a resistance in the hydroxyl transfer to the anode (ohmic loss) and the accumulation of hydroxyl in the cathode chamber (concentration overpotential). However, since the cathode was shared by four anodes, hydroxyl produced by the distant anodes will accumulate in this layer and result in an increase in pH.
The removal of both n-alkanes and PAHs in soil MFCs were accelerated compared with open circuit and non-MFC conditions, which was consistent with our previous results (Wang et al. 2012). However, the removal rate of PAHs (14 %) was lower than that of n-alkanes (22 %), especially in the HA, implying that PAHs was not as readily used by the microorganisms in waterlogged soils as n-alkanes (Table 2). It is probably because the lighter saturated hydrocarbons were less toxic to microbes in the soil and can serve as a carbon source involved in the microbial metabolisms. Thus, they were more likely to be decomposed by microbes. On the other hand, heavier molecular weight compounds such as PAHs were more resistant to biodegradation as they were hydrophobic, with poor water solubility and bioavailability.
Conclusions
The air-cathode MFCs with anodes horizontally arranged (HA) and vertically arranged (VA) were developed for in situ soil bioremediation coupled to electrical energy production. The HA demonstrated a superior performance compared with VA for removal of the TPH. In particular, the highest TPH degradation rate was observed in S-4 of the HA (15.3 ± 0.2 %). Moreover, the n-alkanes were more easily used as substrate for the soil MFCs than the PAHs, although both of them can be degraded in MFCs. MFCs with anodes horizontally arranged were demonstrated as an effective design over vertically arranged systems to improve remediation efficiency of TPHs.
References
Anderson RT, Vrionis HA, Ortiz-Bernad I, Resch CT, Long PE, Dayvault R, Karp K, Marutzky S, Metzler DR, Peacock A (2003) Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl Environ Microbiol 69:5884–5891
Cai Z, Zhou Q, Peng S, Li K (2010) Promoted biodegradation and microbiological effects of petroleum hydrocarbons by Impatiens balsamina L. with strong endurance. J Hazard Mater 183:731–737
Das N, Chandran P (2010) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int
Domínguez-Garay A, Berná A, Ortiz-Bernad I, Esteve-Núñez A (2013) Silica colloid formation enhances performance of sediment microbial fuel cells in a low conductivity soil. Environ Sci Technol 47:2117–2122
Guo G, Zhou Q (2003) Advances of research on combined pollution in soil-plant systems. J Appl Ecol 14:823–828
Hong SW, Chang IS, Choi YS, Kim BH, Chung TH (2009) Responses from freshwater sediment during electricity generation using microbial fuel cells. Bioprocess Biosyst Eng 32:389–395
Jung S (2012) Impedance Analysis of Geobacter sulfurreducens PCA, Shewanella oneidensis MR-1, and their Coculture in Bioeletrochemical Systems. Int J Electrochem Sci 7:11091–11100
Jung S, Mench MM, Regan JM (2011) Impedance characteristics and polarization behavior of a microbial fuel cell in response to short-term changes in medium pH. Environ Sci Technol 45:9069–9074
Jung S, Kim Y, Kang H (2014) Denitrification rates and their controlling factors in streams of the Han River Basin with different land-use patterns. Pedosphere 24:516–528
Kaimi E, Mukaidani T, Tamaki M (2007) Screening of twelve plant species for phytoremediation of petroleum hydrocarbon-contaminated soil. Plant Prod Sci 10:211–218
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–315
Li X, Wang X, Zhang Y, Ding N, Zhou Q (2014) Opening size optimization of metal matrix in rolling-pressed activated carbon air-cathode for microbial fuel cells. Appl Energy 123:13–18
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046
Lovley DR (2006) Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol 4:497–508
Lu L, Huggins T, Jin S, Zuo Y, Ren ZJ (2014) Microbial metabolism and community structure in response to bioelectrochemically enhanced remediation of petroleum hydrocarbon-contaminated soil. Environ Sci Technol 48:4021–4029
Margesin R, Labbe D, Schinner F, Greer C, Whyte L (2003) Characterization of hydrocarbon-degrading microbial populations in contaminated and pristine alpine soils. Appl Environ Microbiol 69:3085–3092
Meagher RB (2000) Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 3:153–162
Morris JM, Jin S (2008) Feasibility of using microbial fuel cell technology for bioremediation of hydrocarbons in groundwater. J Environ Sci Health Part A Toxic/Hazard Subst Environ Eng 43:18–23
Morris JM, Jin S (2012) Enhanced biodegradation of hydrocarbon-contaminated sediments using microbial fuel cells. J Hazard Mater 213:474–477
Morris JM, Jin S, Crimi B, Pruden A (2009) Microbial fuel cell in enhancing anaerobic biodegradation of diesel. Chem Eng J 146:161–167
Prince RC, Bragg JR (1997) Shoreline bioremediation following the Exxon Valdez oil spill in Alaska. Biorem J 1:97–104
Riser-Roberts E (1998) Remediation of petroleum contaminated soils: biological, physical, and chemical processes. CRC Press, Boca Raton
Sarkar D, Ferguson M, Datta R, Birnbaum S (2005) Bioremediation of petroleum hydrocarbons in contaminated soils: comparison of biosolids addition, carbon supplementation, and monitored natural attenuation. Environ Pollut 136:187–195
Tang J, Wang R, Niu X, Wang M, Chu H, Zhou Q (2010a) Characterisation of the rhizoremediation of petroleum-contaminated soil: effect of different influencing factors. Biogeosciences 7:3961–3969
Tang J, Wang R, Niu X, Zhou Q (2010b) Enhancement of soil petroleum remediation by using a combination of ryegrass (Lolium perenne) and different microorganisms. Soil Tillage Res 110:87–93
Ting Y, Hu H, Tan H (1999) Bioremediation of petroleum hydrocarbons in soil microcosms. Resour Environ Biotechnol 2:197–218
Tyagi M, da Fonseca MMR, de Carvalho CC (2011) Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22:231–241
Wang X, Cheng S, Feng Y, Merrill MD, Saito T, Logan BE (2009) Use of carbon mesh anodes and the effect of different pretreatment methods on power production in microbial fuel cells. Environ Sci Technol 43:6870–6874
Wang X, Cai Z, Zhou Q, Zhang Z, Chen C (2012) Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U-tube microbial fuel cells. Biotechnol Bioeng 109:426–433
Wang X, Feng C, Ding N, Zhang Q, Li N, Li X, Zhang Y, Zhou Q (2014) Accelerated OH-transport in activated carbon air-cathode by modification of quaternary ammonium for microbial fuel cells. Environ Sci Technol 48:4191–4198
Zhang T, Gannon SM, Nevin KP, Franks AE, Lovley DR (2010) Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environ Microbiol 12:1011–1020
Zhou Q, Sun F, Liu R (2005) Joint chemical flushing of soils contaminated with petroleum hydrocarbons. Environ Int 31:835–839
Acknowledgments
This research was supported by the Ministry of Science and Technology as an 863 project (2012AA101403-2), the MOE Innovative Research Team in University (IRT13024), the National Natural Science Foundation of China(Nos. 21107053 and 21037002), the Ministry of Science and Technology as an 863 major project (2013AA06A205), and the Tianjin Research Program of Application Foundation and Advanced Technology (13JCQNJC08000).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, X., Li, X. et al. Horizontal arrangement of anodes of microbial fuel cells enhances remediation of petroleum hydrocarbon-contaminated soil. Environ Sci Pollut Res 22, 2335–2341 (2015). https://doi.org/10.1007/s11356-014-3539-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3539-7