Abstract
The aim of the present study was to investigate the persistence of endocrine effects by prochloraz, a fungicide known to have multiple effects on the endocrine system of vertebrates. Since discontinuous exposure is particularly relevant in aquatic ecosystems, an exposure scenario with an exposure phase and a subsequent recovery period was chosen to assess the potential for reversibility of effects by prochloraz on the sexual development of zebrafish (Danio rerio). Zebrafish were exposed to different concentrations of prochloraz (10–300 μg/L) until 60 days post hatch (dph), which includes the period of sexual differentiation. For the subsequent 40 days, fish were either held in clean water for depuration or under further continuous exposure. Histological investigations of the gonads revealed persistent effects on sexual differentiation. The sex ratio was skewed towards males and significantly more intersex individuals were found after exposure to prochloraz at 60 dph. No intersex fish, but masculinized sex ratios were still present after the depuration period, documenting that prochloraz irreversibly affects the sexual development of zebrafish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exposure of wildlife and humans to endocrine-disrupting chemicals (EDCs) represents a serious hazard for reproductive capacities of individuals and, thus, population fitness. Agriculture continuously and significantly contributes to the pollution of the environment via the use of fertilizers and pesticides. Especially aquatic organisms are massively affected, either by periodic peak exposures or chronic low-dose exposures. Prochloraz is a popular imidazole fungicide inhibiting the biosynthesis of ergosterol, which is part of the cell membrane in many fungi (Johnston et al. 1996). Imidazole derivatives are, therefore, widely used as antifungal agents not only in agriculture, but also in textile- and paint-producing industries. Through these applications, they can reach the environment and accumulate in aquatic organisms and their predators (reviewed by Mnif et al. 2011). Data on environmental concentrations suggest that environmentally realistic prochloraz concentrations in European surface waters are generally below 1 μg/L (Weltje 2013). A recent study reports average concentrations of 76.04 ng/L at different sample sites of the Jucar River, Spain (Belenguer et al. 2014). Other studies indicate a causal relation between human exposure to pesticides and poor sperm quality (Swan et al. 2003) or increased incidence of cryptorchidism in sons of female gardeners (Weidner et al. 1998). Although several studies reported significant effects of prochloraz on fish and other vertebrates, the underlying mechanisms are diverse and not yet fully understood. Prochloraz antagonizes the androgen and the estrogen receptors agonizes the Ah receptor and inhibits aromatase activity (reviewed by Vinggaard et al. 2006). Moreover, oocyte maturation can be triggered by prochloraz by the induction of luteinizing hormone (LH)-related genes (Rime et al. 2010). There are also indications that prochloraz has adverse effects on the thyroid system of vertebrates (Brande-Lavridsen et al. 2010; Liu et al. 2011). In zebrafish (Danio rerio), masculinization and inhibited vitellogenin (VTG) production have been reported, as well as increased numbers of intersex individuals and inhibited gonad maturation (Baumann et al. 2008, 2013; Holbech et al. 2012; Kinnberg et al. 2007; Thorpe et al. 2011). In an 8-day exposure experiment with adult fathead minnow (Pimephales promelas), Ankley et al. (2009) found multiple alterations such as inhibition of estradiol and testosterone production. These effects were compensated within a short recovery period of 8 days, indicating that the effects of prochloraz on the hormonal system are not persistent in adult fish. In contrast, in juvenile (developing) fish, the reversibility of prochloraz-induced effects has not been investigated. Therefore, the aim of the present study was to examine the persistence of adverse effects of prochloraz on zebrafish gonad development by means of an exposure scenario including prolonged exposure followed by a recovery period.
Materials and methods
Test substance
Prochloraz (CAS-No.: 67747-09-5) was obtained from Sigma-Aldrich (Deisenhofen, Germany). The following test concentrations were used for the exposure of zebrafish: 10, 30, 100, and 300 μg/L. Stock solutions were produced by dissolving prochloraz in warm deionized water (40 °C), stirred over night without solvent. Fresh stock solutions were produced every second day before use in light-isolated glass reservoirs, from which they were added to the exposure tanks via peristaltic pumps (MINIPULS 3, Gilson; Limburg, Germany).
Exposure and sampling
The exposure of zebrafish (D. rerio, Westaquarium strain) to prochloraz started, at latest, 1-h post fertilization (hpf) and ended at 100 days post hatch (dph). Fish were held in aerated 12-L flow-through glass tanks at 27 ± 1 °C and a dark–light cycle of 10/14 h. Temperature and flow-through rate (complete water exchange every 8 h) were controlled twice daily. Further parameters like hardness (200–280 mg/L), conductivity (600–750 μS), pH (8.0–8.2), and oxygen saturation (90–95 %) were controlled at least once weekly. Feces and food leftovers were removed daily. From days 4 to 14, larvae were fed with powdered dry food (Sera Micron; Sera, Ludwigshafen, Germany), and afterwards with granular flake food (TetraminTM; Tetra, Melle, Germany) and newly hatched nauplii of Artemia species (Great Salt Lake Artemia Cysts, Sanders Brine Shrimp Company).
The exposure to prochloraz was carried out until 60 days post hatch, followed by a recovery period in clear water of 40 days. Additionally, control groups with and without any continuous exposure over the entire time of the experiment (100 days) were analyzed. Each treatment was run in two replicates. One hundred fertilized eggs per replicate were used at the beginning. After 30 and 60 days, 30 individuals from each tank were randomly removed for analyses. The remaining 40 fish were sampled after 100 days. Results of the 30-days sampling are not presented. In case of slight differences in the number of individuals per tank, some additional fish were removed to avoid density-dependent effects. Fish euthanized with a saturated solution of tricaine (ethyl-m-aminobenzoate, Sigma-Aldrich). Trunks of the fish were placed in embedding cassettes (Histosette, Neolab; Heidelberg, Germany) and fixed in modified Davidson’s fixative (Romeis and Böck 2001) for subsequent histological analyses.
Histology
Samples were incubated in modified Davidsons’s fixative (Romeis and Böck 2001) at 4 °C for at least 24 h before embedding into paraffin by a tissue processor (TP 1020, Leica Microsystems; Nussloch, Germany). Embedding into blocks was performed with a heated paraffin embedding module (EG 1140 H, Leica Microsystems) with trunks orientated ventrally to the cutting surface. Sections of the gonads with a thickness of 4–5 μm were cut with a HN 40 microtome (Reichert-Jung; Nussloch, Germany), mounted on glass slides (Langenbrinck; Emmendingen, Germany) and stained with hematoxylin-eosin (Romeis and Böck 2001) the next day. Light microscopical evaluation of the tissue sections was performed according to the Organisation for Economic Co-operation and Development (OECD) Histopathology Guidance Document (OECD 2010). Each fish was identified either as female, male, or intersex or recorded as undifferentiated.
Statistics
For statistical evaluation, data were analyzed with SigmaStat 12.0 (Statsoft-Jandel Scientific; Erkrath, Germany). Chi-square analysis was used to identify differences in sex ratio between controls and each treatment group.
Results
No chemical analyzes were conducted, as previous experiments with the same substance in the same facility revealed high stability and small deviations from nominal concentrations as determined by chemical analyzes (Baumann 2008).
At 60 dph, 30 individuals were randomly picked out of an exposure group of 70 zebrafish. The ratio (%) of females/males/intersex in the control groups was 45:45:10 (Table 1). The relative amount of females decreased dose-dependently, resulting in 0 % females at 300 μg/L prochloraz. Interestingly, while at 10 μg/L prochloraz, significantly more females had developed; the number of male zebrafish did not change markedly, except in the lowest concentration of 10 μg/L prochloraz. The most striking effect was the increased occurrence of intersex individuals after exposure to 100 and 300 μg/L prochloraz. Females from the exposure groups tended to have less mature ovaries (Fig. 1).
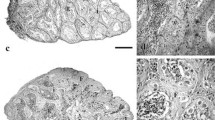
Gonad histology of zebrafish at 60 dph: a Female ovary from control group, maturity stage 2. b Female ovary from 100 μg/L group, maturity stage 1. c Male testis from control group, maturity stage 2. d Male testis from 300 μg/L group, maturity stage 1. e Testis ova from control group, arrow indicates perinucleolar oocyte. f Testis ova from 300 μg/L group, arrows indicate perinucleolar oocytes
At 100 dph, the remaining 40 fish were sampled and no intersex individuals were found. The sex ratio (females/males) was 39:61 in the controls. A sex ratio of 50:50 was consistently seen at 10 μg/L prochloraz. A masculinizing effect was observable from 30 μg/L prochloraz and higher, without significant differences between continuous and discontinuous exposure. No effects on gonad maturation were evident (Fig. 2).
Gonad histology of zebrafish at 100 dph: a Female ovary from control group, maturity stage 4. b Male testis from control group, maturity stage 3. c Female ovary from 100 μg/L exposure group, maturity stage 4. d Male testis from 300 μg/L exposure group, maturity stage 3. e Female ovary from 300 μg/L recovery group, maturity stage 4. f Male testis from 100 μg/L recovery group, maturity stage 3
Discussion
The present study shows that the fungicide prochloraz has strong and irreversible masculinizing effects on sexually differentiating zebrafish. Regarding the environmental relevance of fungicide exposure, it can be concluded that prochloraz might represent a serious hazard for aquatic vertebrates and their population fitness. Nevertheless, average measured concentrations of prochloraz in European surface waters do not exceed 1 μg/L (Weltje 2013). The concentrations used in the present study are comparably high, but may reflect periodic or peak exposure events, where concentrations can increase much higher than usual (Probst et al. 2005). Especially during sexual differentiation, this can cause severe and irreversible adverse effects on gonad development of aquatic animals.
After 60-day exposure to prochloraz, a dose-dependent increase of intersex individuals was striking. This agrees with the findings of Kinnberg et al. (2007) and Thorpe et al. (2011), who also observed high occurrence of intersex gonads after prochloraz exposure during sexual differentiation of zebrafish. Moreover, they also reported masculinization of sex ratios and inhibited gonad maturation of females, which was also observed in the present study.
Zebrafish is a protogynic fish species, i.e., all fish first develop immature female gonads, approximately 50 % of which will later transform into testes in genetic males (Takahashi 1977). This transformation is a process simultaneous to degeneration of immature oocytes and development and maturation of testicular tissue. Consequently, intersex gonads are part of the normal sexual differentiation of zebrafish males, known as “juvenile hermaphroditism” (Maack and Segner 2003). Therefore, the high percentage of intersex gonads after 60 days of exposure to prochloraz can be interpreted as retardation in the development of genetic males, possibly due to the anti-androgenic effects of prochloraz. Nevertheless, it is known that prochloraz elicits multiple modes of action that may cover each other (Vinggaard et al. 2006; Rime et al. 2010). In fact, at the highest concentration of prochloraz, no single female individual was found, which probably indicates a surplus of testosterone resulting from the aromatase-inhibiting effects of prochloraz. Since molecular mechanisms of sexual differentiation in zebrafish are not yet fully understood (Jørgensen et al. 2008; Orban et al. 2009; Tokarz et al. 2013), this interpretation remains to be certified.
So far, environmental factors seem to be the key regulators of zebrafish sex determination (Dranow et al. 2013). Recently, a communication has been published about a putative sex chromosome in zebrafish (Anderson et al. 2012), but further research is needed to confirm this hypothesis, as differences among strains were found. Another hint for masculinization, however, is that no intersex individuals were found at 100 dph. This indicates that development of intersex gonads due to prochloraz exposure at 60 dph was not permanent and that these individuals probably further developed into functional males. This is mirrored in the sex ratio at 100 dph; a concentration-related effect could be determined after exposure to 100 and 300 μg/L prochloraz, with a male dominance of 77–94 %. This is in line with results from previous studies (Holbech et al. 2012; Kinnberg et al. 2007) and proved to be not reversible through the recovery period, showing that masculinization of developing zebrafish is an irreversible process. The same effect was found in a previous study with the androgen 17β-trenbolone (Baumann et al. 2014a), where the same exposure scenario was studied. Following exposure to this strong androgen, zebrafish were not able to recover from the masculinizing effects, which resulted in all-male populations in the highest exposure groups with or without recovery period. As a conclusion, treatment with androgenic or aromatase-inhibiting EDCs during sexual differentiation of zebrafish leads to irreversible masculinization of gonad development. Similar effects have already been observed in other studies with androgens (Larsen and Baatrup 2010; Morthorst et al. 2010) and aromatase inhibitors (Fenske and Segner 2004).
In contrast, exposure to estrogens such as 17β-ethinylestradiol is mostly reversible (Baumann et al. 2014b; Fenske et al. 2005; Larsen et al. 2009; Nash et al. 2004; Schäfers et al. 2007). The underlying mechanism for the difference between the reversibility of feminization and masculinization by EDCs could be the loss of primordial germ cells (PGCs) during the development of testis tissue in zebrafish. This hypothesis was raised by Morthorst et al. (2010) and has not been refuted yet, even though inconsistencies in literature exist. PGCs are progenitors of gametes and differentiate either into eggs or sperms during sexual development (Saito et al. 2008). If PGCs are absent or ablated, only testis development is possible, since ovaries cannot develop without the presence of PGCs (Dranow et al. 2013; Slanchev et al. 2005; Uchida et al. 2002). Slanchev et al. (2005) could show that PGCs are also important for general gonad survival, which would indicate that testis tissue would not be able to persist for a longer period, e.g., until 100 dph. In contrast, Siegfried and Nüsslein-Volhard (2008) were able to show that germ line-deficient zebrafish are able to develop and maintain testis tissue for longer periods. Germ line-deficient zebrafish even display male coloration and male behavior, indicating that steroid hormones produced by the testis are sufficient to support masculinization of the whole organism (Siegfried and Nüsslein-Volhard 2008). Therefore, we assume that the exposure to androgens or aromatase inhibitors causes persistent testis development in genetic females due to PGC loss. As a consequence, later development of ovaries after depuration is impossible. In contrast, cessation of estrogenic exposure allows normal development of testis tissue in genetic males that were feminized (Baumann et al. 2014b). Consequently, masculinization of genetic zebrafish females represents a one-way development, whereas feminization of genetic zebrafish males can be reconverted. Regarding the extensive use of zebrafish in risk assessment of potential EDCs (Scholz and Klüver 2009; Segner 2009), much more research concerning its sexual development is still required to understand the basic mechanisms underlying this phenomenon.
References
Anderson JL, Rodriguez Mari A, Braasch I, Amores A, Hohenlohe P, Batzel P, Postlethwait JH (2012) Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One 7:e40701
Ankley GT, Bencic DC, Cavallin JE, Jensen KM, Kahl MD, Makynen EA, Martinovic D, Mueller ND, Wehmas LC, Villeneuve DL (2009) Dynamic nature of alterations in the endocrine system of fathead minnows exposed to the fungicide prochloraz. Toxicol Sci 112:344–353
Baumann L (2008) Effects of endocrine disruptors in zebrafish (Danio rerio) as revealed with the fish sexual development test. MSc thesis, Department of Zoology, University of Heidelberg, 102 pp.; http://archiv.ub.uni-heidelberg.de/volltextserver/13746/
Baumann L, Holbech H, Keiter S, Kinnberg KL, Knörr S, Nagel T, Braunbeck T (2013) The maturity index as a tool to facilitate the interpretation of changes in vitellogenin production and sex ratio in the fish sexual development test. Aquat Toxicol 128–129:34–42
Baumann L, Knörr S, Keiter S, Nagel T, Rehberger K, Volz S, Oberrauch S, Schiller V, Fenske M, Holbech H, Segner H, Braunbeck T (2014a) Persistence of endocrine disruption in zebrafish (Danio rerio) after periodic exposure to the androgen 17β-trenbolone. Environ Toxicol Chem. doi:10.1002/etc.2698
Baumann L, Knörr S, Keiter S, Rehberger K, Volz S, Schiller V, Fenske M, Holbech H, Segner H, Braunbeck T (2014b) Reversibility of endocrine disruption in zebrafish (Danio rerio) after periodic exposure to the estrogen 17α-ethinylestradiol. Toxicol Appl Pharmacol 278:230–237
Belenguer V, Martinez-Capel F, Masia A, Pico Y (2014) Patterns of presence and concentration of pesticides in fish and waters of the Júcar River (Eastern Spain). J Hazard Mat 265:271–279
Brande-Lavridsen N, Christensen-Dalsgaard J, Korsgaard B (2010) Effects of ethinylestradiol and the fungicide prochloraz on metamorphosis and thyroid gland morphology in Rana temporia. Open Zool J 3:7–16
Dranow D, Tucker R, Draper B (2013) Germ cells are required to maintain a stable sexual phenotype in adult zebrafish. Dev Biol 376:43–50
Fenske M, Segner H (2004) Aromatase modulation alters gonadal differentiation in developing zebrafish (Danio rerio). Aquat Toxicol 67:105–126
Fenske M, Maack G, Schäfers C, Segner H (2005) An environmentally relevant concentration of estrogen induces arrest of male gonad development in zebrafish Danio rerio. Environ Toxicol Chem 24:1088–1098
Holbech H, Kinnberg KL, Brande-Lavridsen N, Bjerregaard P, Petersen GI, Norrgren L, Orn S, Braunbeck T, Baumann L, Bomke C, Dorgerloh M, Bruns E, Ruehl-Fehlert C, Green JW, Springer TA, Gourmelon A (2012) Comparison of zebrafish (Danio rerio) and fathead minnow (Pimephales promelas) as test species in the fish sexual development test (FSDT). Comp Biochem Physiol Toxicol Pharmacol CBP 155:407–415
Johnston G, Dawson A, Walker CH (1996) Effects of prochloraz and malathion on the red-legged partridge: a semi-natural field study. Environ Poll 91:217–225
Jørgensen A, Morthorst JE, Andersen O, Rasmussen LJ, Bjerregaard P (2008) Expression profiles for six zebrafish genes during gonadal sex differentiation. Reprod Biol Endocrinol 6:25
Kinnberg K, Holbech H, Petersen GI, Bjerregaard P (2007) Effects of the fungicide prochloraz on the sexual development of zebrafish (Danio rerio). Comp Biochem Physiol Toxicol Pharmacol CBP 145:165–170
Larsen MG, Baatrup E (2010) Functional behavior and reproduction in androgenic sex reversed zebrafish (Danio rerio). Environ Toxicol Chem 29:1828–1833
Larsen MG, Bilberg K, Baatrup E (2009) Reversibility of estrogenic sex changes in zebrafish (Danio rerio). Environ Toxicol Chem 28:1783–1785
Liu C, Zhang X, Deng J, Hecker M, Al-Khedhairy A, Giesy JP, Zhou B (2011) Effects of prochloraz or propylthiouracil on the cross-talk between the HPG, HPA, and HPT axes in zebrafish. Environ Sci Technol 45:769–775
Maack G, Segner H (2003) Morphological development of the gonads in zebrafish. J Fish Biol 62:895–906
Mnif W, Hassine AI, Bouaziz A, Bartegi A, Thomas O, Roig B (2011) Effect of endocrine disruptor pesticides: a review. Int J Environ Res Publ Health 8:2265–2303
Morthorst JE, Holbech H, Bjerregaard P (2010) Trenbolone causes irreversible masculinization of zebrafish at environmentally relevant concentrations. Aquat Toxicol 98:336–343
Nash JP, Kime DE, Van der Ven LTM, Wester PW, Brion F, Maack G, Stahlschmidt-Allner P, Tyler CR (2004) Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ Health Persp 112:1725–1733
OECD (2010) Guidance document on the diagnosis of endocrine-related histopathology in fish gonads. OECD Series on Testing and Assessment No. 123. Paris, France: Organization for Economic Cooperation and Development
Orban L, Sreenivasan R, Olsson PE (2009) Long and winding roads: testis differentiation in zebrafish. Mol Cell Endocrinol 312:35–41
Probst M, Berenzen N, Lentzen-Godding A, Schulz R (2005) Scenario-based simulation of runoff-related pesticide entries into small streams on a landscape level. Ecotoxicol Environ Saf 62:145–159
Rime H, Nguyen T, Bobe J, Fostier A, Monod G (2010) Prochloraz-induced oocyte maturation in rainbow trout (Oncorhynchus mykiss), a molecular and functional analysis. Toxicol Sci 118:61–70
Romeis B, Böck P (2001) Mikroskopische Technik. Urban und Schwarzenberg, Munich, 697 pp
Saito, T., Goto-Kazeto, R., Arai, K. and Yamaha, E. (2008) Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biol Reprod 78:159–166
Schäfers C, Teigeler M, Wenzel A, Maack G, Fenske M, Segner H (2007) Concentration- and time-dependent effects of the synthetic estrogen, 17α-ethinylestradiol, on reproductive capabilities of the zebrafish, Danio rerio. Toxicol Environ Health A 70:768–779
Scholz S, Klüver N (2009) Effects of endocrine disrupters on sexual, gonadal development in fish. Sex Dev 3:136–151
Segner H (2009) Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp Biochem Physiol Part C 149:187–195
Siegfried KR, Nüsslein-Volhard C (2008) Germ line control of female sex determination in zebrafish. Dev Biol 324:277–287
Slanchev, K., Stebler, J., de la Cueva-Mendez, G. and Raz, E. (2005) Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proce Nat Ac Sci USA 102:4074–4079
Swan SH, Kruse RL, Liu F, Barr DB, Drobnis EZ, Redmon JB, Wang C, Brazil C, Overstreet JW (2003) Semen quality in relation to biomarkers of pesticide exposure. Environ Health Persp 111:1478–1484
Takahashi H (1977) Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bull Fac Fish Hokkaido Univ 28(2):57–65
Thorpe KL, Pereira ML, Schiffer H, Burkhardt-Holm P, Weber K, Wheeler JR (2011) Mode of sexual differentiation and its influence on the relative sensitivity of the fathead minnow and zebrafish in the fish sexual development test. Aquat Toxicol 105:412–420
Tokarz J, Möller G, Hrabe de Angelis M, Adamski J (2013) Zebrafish and steroids: what do we know and what do we need to know? J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2013.01.003
Uchida, D., Yamashita, M., Kitano, T. and Iguchi, T. (2002) Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. Exp Biol 205:711–718
Vinggaard AM, Hass U, Dalgaard M, Andersen HR, Bonefeld-Jorgensen E, Christiansen S, Laier P, Poulsen ME (2006) Prochloraz: an imidazole fungicide with multiple mechanisms of action. Int J Androl 29:186–192
Weidner IS, Moller H, Jensen TK, Skakkebaek NE (1998) Cryptorchidism and hypospadias in sons of gardeners and farmers. Environ Health Persp 106:793–796
Weltje L (2013) No proof of synergy at environmentally realistic concentrations of prochloraz and esfenvalerate—a reaction on “Synergy in microcosms with environmentally realistic concentrations of prochloraz and esfenvalerate” by Bjergager et al. Aquat Toxicol 101:412–422, Aquat. Toxicol. 140–141: 466–468
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Baumann, L., Knörr, S., Keiter, S. et al. Prochloraz causes irreversible masculinization of zebrafish (Danio rerio). Environ Sci Pollut Res 22, 16417–16422 (2015). https://doi.org/10.1007/s11356-014-3486-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3486-3