Abstract
A number of compounds found in particulate matter with an aerodynamic diameter <2.5 (PM2.5) can interact with DNA either directly or after enzymatic transformation to induce DNA modifications. These particulate matter (PM)-induced alterations in DNA may be associated with increased frequencies of pollution-associated diseases, such as lung cancer. In the present study, we applied different methods to assess the mutagenicity and genotoxicity of monthly PM2.5 organic extracts collected over a full year. We used the Salmonella assay, exposed cultured human embryonic lung fibroblasts and applied extracellular lactate dehydrogenase (LDH) and 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxyanilide inner salt (XTT) assays to assess the cytotoxicity of PM2.5 on the cells. We assessed both the expression levels of a number of DNA repair genes (using qRT-qPCR) and the genetic profile of the treated cells compared to the control. The expression levels of XRCC1 and APE1, which are involved in the first steps of base excision repair, as well as ERCC1, XPA and XPF, which encode nucleotide excision repair subunits, were analysed. The monthly mean of the PM2.5 collected was 35.16 ± 22.06 μg/m3. The mutagenicity of PM2.5 to TA98 was 46 ± 50 net revertants/m3, while the mutagenicity to TA98 + S9 was 17 ± 19 net revertants/m3. The mean IC50 values were 2.741 ± 1.414 and 3.219 ± 2.764 m3 of equivalent air in the XTT and LDH assays, respectively. A marked and significant increase in APE1 expression levels was observed in the exposed cells. This effect was also significantly correlated with mutagenicity (p < 0.01). No induced AFLP fragment profile alterations were detected. The proposed approach seems to be useful for integrated evaluation and for highlighting the mechanisms inducing DNA damage.

Mutagenic, cytotoxic and genotoxic effects that were recorded in vitro over a full year of investigation into PM2.5 organic extracts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban areas, particularly megacities, are the most polluted (Jain and Khare 2008; Nagdeve 2006; Zhang et al. 2008), along with areas with significant emission points and/or close valleys in which there is a lack of pollution dispersion (Nemery et al. 2001; Pope 1996; Ta et al. 2004; Voigt et al. 1998). Worldwide, several epidemiological studies have revealed morbidity and mortality increases following particle exposure. Fine particles are particularly dangerous (Eftim et al. 2008; Kappos et al. 2004; Krewski et al. 2005; Pope 2000). Particulate matter (PM) pollution is often measured using indicators, such as the concentration of dispersed particulate matter with an aerodynamic diameter <10 μm (PM10) and particulate matter with an aerodynamic diameter <2.5 (PM2.5) (Krzyzanowski 2008). The aerodynamic diameter of PM is inversely correlated with its ability to enter into the respiratory tree and to arrive at the alveolar level, at which blood-air exchange occurs (Sorensen et al. 2003a, b). The adverse health effects of PM include the exacerbation of asthma (Viera et al. 2009; Zhang et al. 2009), chronic obstructive pulmonary disease (COPD) (Lindgren et al. 2009) and death from cardiovascular disease (Mills et al. 2009; Nogueira 2009) and lung cancer (Beelen et al. 2008; Grant 2009; Vineis et al. 2007). The mechanisms driving these effects are not completely understood, but they are thought to involve the direct effects of organic toxins and oxidative stress, followed by inflammation (Moller et al. 2008).
The correlation between air pollution and cancer has been widely discussed in epidemiological terms (Valavanidis et al. 2008). Cohort studies have shown that populations exposed to high levels of air pollution have a 10–30 % greater risk of developing lung cancer than populations exposed to low levels of air pollution (Kam et al. 2013; Vinikoor-Imler et al. 2011); however, the contributions of confounding factors, particularly in studies of European populations, are debatable (Gallus et al. 2008).
In October 2013, the IARC declared that outdoor air pollution was the leading environmental cause of cancer death and classified this agent as a human carcinogen. Moreover, particulate matter, as a major component of outdoor pollution, was also classified as a group 1 agent (Loomis et al. 2013). Molecular studies have demonstrated that DNA damage was specifically correlated with fine PM pollution (Alfaro-Moreno et al. 2002; de Kok et al. 2005; Mehta et al. 2008). The effects of PM pollution on DNA can range from the formation of bulky DNA adducts or point mutations to single- or double-strand breaks that result in genetic and chromosomal instability. Early effects are normally corrected by repair mechanisms, but these repair mechanisms may fail during aging or disease processes. Some genotoxic agents not only damage the integrity of the genome but also directly or indirectly affect gene expression. These genotoxic effects may increase the incidence of different types of cancer and, in the long term, may result in decreased genetic integrity in exposed populations (Beelen et al. 2008; Brunekreef et al. 2009).
In recent years, molecular genetics studies have demonstrated both structural changes, such as genetic instability, and regulatory changes, including epigenetic phenomena (Ji and Hershey 2012). A variety of polymerase chain reaction (PCR)-based techniques are currently available to evaluate the frequency of induced DNA alterations in fragment profiles, expressed as number of lost and introduced fragments for each exposure in comparison to the control, all of which involve the random or semi-random amplification of genomic DNA (Atienzar and Jha 2006; Baurand et al. 2013; Ben Salem et al. 2014; Srut et al. 2013; Vardar et al. 2014). Induced variations in the nucleotide sequence are the result of a complex equilibrium between the genetic response to environmental stressors, DNA repair systems and DNA damage resulting from the stressors. DNA repair systems include the base excision repair (BER) and nucleotide excision repair (NER) systems (Wood et al. 2005). These systems can be affected by urban particulate exposure (Libalova et al. 2012; Mukherjee et al. 2013). Fine PM is a complex mixture of compounds that have both antagonistic and synergistic effects, making the application of in vitro assays relevant when evaluating a global effect on a biologic model (Pfuhler et al. 2013).
In the present study, we assessed the results of parallel in vitro methods applied to the analysis of cells exposed to urban PM2.5 organic extracts collected in Turin, one of the most polluted cities in Europe (Traversi et al. 2011). These methods included the following assays: Salmonella test to assess the mutagenic potential of PM2.5, lactate dehydrogenase (LDH) and 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxyanilide inner salt (XTT) to assess the cytotoxic potential, real-time quantitative polymerase chain reaction (RT-qPCR) expression to assess the modulation of DNA repair proteins and amplified fragment length polymorphism (AFLP) to measure the induced DNA alterations in fragment profiles.
Materials and methods
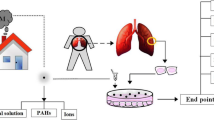
The complete study design, from the sampling of the environmental PM2.5 to the early effect tests, is illustrated in Fig. 1. The assays include the Salmonella test and cytotoxicity, AFLP and gene expression assays in human embryonic lung fibroblasts (HELFs). The aim of such test choice was to use a classic mutagenic test as Salmonella test and to place side by side, using the same samples, various tests on human cells to observe the following: cytotoxicity, gene expression modulations and DNA alterations in fragment profiles.
An overview of the study design from the PM2.5 sampling to the expected biological responses. After PM2.5 was collected on filters, the mass was evaluated. Organic extraction was then performed. The extracts were subdivided and used for mutagenicity evaluations in bacteria and for cytotoxicity and DNA alteration evaluations in HELF cells. The cytotoxicity assays included the MTT and LDH assays, while the genotoxic assays included both gene expression analyses and AFLP. The last step shows the appearance or disappearance of a band on the polyacrylamide gel
PM2.5 sampling and organic extraction
PM2.5 was collected from January to December 2008 in a meteorological-chemical station located in a background urban area in southern Turin using a sampler (Analitica Strumenti, Pesaro, Italy), according to directive UNI-EN14907. PM2.5 (PM passing through a size-selective inlet with 50 % efficiency cutoff at an aerodynamic diameter of 2.5 μM) was collected on glass micro-fibre filters (Type A/E, 8″ × 10″, PALL Corporation, 25 Harbor Park Drive, Port Washington, NY 11050, USA) at a flow rate of approximately 500 l/min. The samples were collected over a 24-h period, and sample duration was controlled by a timer that was accurate to ±15 min. The exact flow was calculated daily and corrected for variations in atmospheric pressure and actual differential pressure across the filter. The filters were conditioned for 48 h and weighed using an analytical balance (±10 μg) before and after sampling to calculate the concentration of PM in the air sampled. The procedures were conducted according to the European Committee for Standardisation (CEN 1998). The sampling station was located in an urban area that was not directly exposed to any relevant emission source; therefore, the PM concentration was representative of the exposure level of the resident population, without accounting for specific traffic-related or industrial exposure.
Each day, the collected filters were pooled to obtain a unique month-long organic sample. Extractions of each pooled sample were performed using at least 80 cycles of a Soxhlet apparatus (BUCHI B-811, Savatec, Torino, Italy) with acetone (product number 439126 Sigma-Aldrich), followed by evaporation induced by a Rotavapor (Savatec, Torino, Italy) instrument and resuspension of the sample with dimethyl-3-sulfoxide (DMSO, product number 13409023 Sigma-Aldrich) to obtain an equivalent concentration of 0.2 m3/μl.
We chose the organic extraction because in the literature, it showed most of the genotoxicity (Claxton et al. 2004; Claxton and Woodall 2007). The doses for the following assays were defined as equivalent PM2.5 organic extract corresponding to a cubic metre of inhalable air to describe real human exposure conditions during a year of exposure to urban air pollution (Maruyama et al. 2006). Moreover, it was possible to evaluate the dose corresponding to PM2.5 mass (Supplementary Material 1) and the effect per mass unit of PM2.5, as shown in Fig. 2.
Mutagenic and cytotoxic effects observed by month. Seasonal analysis of the data collected is subdivided into winter, autumn, spring and summer means and standard deviations. The seasonal ANOVA for this data series is significant (p < 0.01). a Mutagenicity data resulting from a Salmonella assay that was conducted with Salmonella typhimurium TA98 in the presence and absence of S9 mix. The data are expressed on the primary ordinate axis as net revertants/cubic meter of equivalent air and as net revertants/microgram of PM2.5 on the secondary ordinate axis. b Cytotoxicity data resulting from the LDH and XTT assays. The data are expressed on the ordinate axis as the IC50 as cubic meter of equivalent air and as the IC50 as microgram of PM2.5 on the secondary ordinate axis
Salmonella reversion test
Mutagenicity assays were performed for each of the monthly DMSO extracts, according to the classic procedure. Increasing doses of the PM2.5 organic extract (5, 10, 20 and 50 μl of the 0.2 m3/μl DMSO suspension) were tested. All experiments were performed in triplicate. The slope of the dose–response curve (revertants/m3) was calculated by the least squares linear regression from the first linear portion of the dose–response curve (Gilli et al. 2007). The results are displayed as net revertants per cubic metre (rev/m3), but the net revertants per microgram of PM2.5 are also presented. The mutagenic activity of airborne particulate extracts was studied using Salmonella typhimurium strain TA98, both in the presence and absence of Aroclor-induced rat-liver homogenate activation (S9). The inclusion of a mammalian liver homogenate ensured that some of the potential carcinogens, such as polycyclic aromatic hydrocarbons (PAHs), were converted into their active forms by liver enzyme systems (Claxton et al. 2004). The number of spontaneous revertants obtained during the bioassay sessions were 18 ± 2 and 23 ± 6 for S. typhimurium strain TA98 in the absence and presence of S9 mix, respectively.
Cell exposures
Low-passage human embryonic lung fibroblast (HELF) cells were grown as a monolayer in minimum essential medium (MEM) (M2279 Sigma-Aldrich) containing 10 % foetal bovine serum (FBS) (N4762 Sigma-Aldrich), 4 mM l-glutamine, 2 mM sodium pyruvate and 100 μg/ml of penicillin/streptomycin at 37 °C in a humidified atmosphere of 5 % CO2. Cytotoxicity was evaluated using both an extracellular lactate dehydrogenase (LDH) assay and an assay measuring mitochondrial dehydrogenase activity in living cells by cleaving formazan to a tetrazolium salt (XTT). The experiments were performed according to the instructions provided in the Cytotoxicity Kit in terms of number of technical repeats (at least 3), plate size (96-well plates), cell number (20,000 cells/well), positive and negative controls, duration of the exposition and acceptability of variation (In CytoTox, PAN I, Xenometrix) using different concentrations of the PM2.5 organic extracts. Doses ranging from 0.25 % (0.5 m3 of equivalent air) to 3 % (6 m3 of equivalent air) were tested and corresponded to the equivalent doses shown in Supplementary Material 1. The results for the two assays are expressed as the equivalent air (in m3 per ml) required to observe 50 % of the effect (IC50). Moreover, the results for the PM2.5 mass unit are also shown.
RNA and DNA extractions
HELF cells were cultured at densities of 5 × 106 cells/plate in 90-mm cell culture plates. Six plates were exposed to each sample, and six plates were used as controls. Two plates were exposed to each of the three concentrations of PM2.5 organic extracts (1, 2 and 3 %), and two plates were exposed only to the organic solvent DMSO and served as controls (1, 2 and 3 %). We performed two replicates. After 72 h of incubation with the PM2.5 organic extracts, we isolated pure genomic DNA (GenElute Mammalian Genomic DNA Miniprep Kit, Sigma-Aldrich, GN70) from the three different exposure doses for each sample and control. In addition, we extracted RNA (GenElute Mammalian total RNA Miniprep kit, Sigma-Aldrich, RTN70) from the other plates for the three different exposure doses for each sample and control. The concentration and quality of the nucleic acid extracts was tested using spectrophotometric analyses, as outlined in the extraction kits. The DNA extracts were stored at −20 °C prior to the AFLP analyses, and the RNA extracts were stored at −80 °C prior to the retrotranscription reactions.
Gene expression analysis
Reverse transcription of total RNA for all monthly samples was achieved using the High Capacity cDNA Archive Kit (iScript cDNA Synthesis Kit, Bio-Rad). APE1, XRCC1, XPA, ERCC1 and XPF gene expression levels were evaluated using a PCR master mix (SsoFast EvaGreen Supermix, Bio-Rad) and the Chomo 4 Real-time PCR system (Bio-Rad) with Opticon Monitor 3.0 software. The thermal protocol consisted of enzymatic activation at 98 °C for 30 s, followed by 40 denaturation cycles at 95 °C for 10 s, annealing/extension at 60 °C for 30 s and a plate read. After all cycles had been completed, a melt curve was generated at 65–95 °C (in 0.5 °C increments). The primers for each expression target are shown in Table 1 (Arase et al. 2002; Chang et al. 1999; Silva et al. 2008). The relative changes in gene expression were calculated using the ΔΔCt method (Livak and Schmittgen 2001), taking into account both the control expression levels and normalisation against the endogenous housekeeping genes, HPRTI and GAPDH, as suggested in the literature (Vandesompele et al. 2002). The results are expressed as the fold increase in the expression following exposure to each dose (1, 2 and 3 %, which corresponded to 2, 4 and 6 m3/ml, respectively).
AFLP analyses
AFLP is a DNA-fingerprinting technique (Vos et al. 1995). In this technique, a set of distinct DNA fragments is generated from a single DNA sample using PCR after DNA digestion with restriction enzymes and adapter ligation. These fragments are then separated on a high-resolution denaturing polyacrylamide gel, producing a complex electrophoretic profile. AFLP was conducted using a commercial kit (Invitrogen AFLP Analysis System I and Starter Primer Kit), according to the manufacturer’s specifications and a previously described protocol (Vos et al. 1995). We used this technique to detect induced DNA alterations in AFLP fragment profiles in the DNA extracts of the cells that had been exposed to 3 % doses compared to the controls exposed only for the winter months. We used the amplification reaction profile that was described in the kit manual.
This cell line has greater genomic stability than transformed cells, as do other embryonic cells (Bylund et al. 2004). This high genomic stability is critical for experiments assessing induced DNA alterations in AFLP fragment profiles and was verified by the extraction and amplification of DNA from two different vials of cells that were frozen from the same cell culture aliquot, stored in liquid nitrogen and thawed on different days.
The DNA amplification products were analysed by separating an aliquot of each reaction on 5 % denaturing polyacrylamide gels in TBE electrophoresis buffer for 3 h at 100 W. Bands were visualised using silver staining (Bassam and Gresshoff 2007). The polymorphic bands were scored by visual inspection of the stained gels. The frequency of observed DNA alterations in AFLP fragment profiles was calculated as the ratio of the number of polymorphic bands to the total number of recorded bands and is reported as a percentage.
Statistics
The local Environmental Protection Agency (ARPA Piemonte) produced an air pollution data set (ARPA Piemonte PdT 2008) that examined PM10 and PM2.5 (collected in the sampling station), including benzo(a)pyrene and total PAHs, with the addition of 1, 2A and 2B PAH, according to the IARC assessment (Belpomme et al. 2007). These data were included in the experimental database. Statistical analyses were performed using the IBM SPSS Package, version 21.0, for Windows. Analysis of variance (ANOVA) was used to compare the means of the variables using Tukey’s post hoc test, and Spearman’s rank-order correlation coefficient was used to assess the relationships between the variables. The difference between two means was considered to be significant at p < 0.05. Probit linear regression was used to calculate the IC50 values for the cytotoxicity tests. Seasonal analyses were performed. December, January and February were considered to be the winter months; March, April and May were considered to be the spring months; June, July and August were considered to be the summer months; and September, October and November were considered to be the autumn months
Results and discussion
Environmental levels and mutagenicity of the PM2.5
The PM2.5 concentration ranged from 15.41 to 83.71 μg/m3, as described in Table 2. The annual mean was well above the reference levels for the protection of human health, which were established to be 20 μg/m3 by the EU and 10 μg/m3 by the WHO (EEA 2012). The concentrations of PM2.5 displayed a seasonal trend, with higher concentrations in the winter and autumn. All seasonal mean comparisons were significant: winter, 62.32 ± 16.42; spring, 18.72 ± 3.32; summer, 16.57 ± 1.10; and autumn, 43.01 15.96 (ANOVA, F = 31.645, p < 0.001). The annual ratio of PM2.5 to PM10 was 0.69, revealing the prevalence of fine particles that is typical of urban locations.
The mutagenicity incidence was sevenfold that of spontaneous revertants, but generally, it was not high, particularly in the presence of S9 enzyme-mediated activation (Table 2). The mutagenicity observed in the absence of metabolic activation was greater, highlighting the fact that direct mutagens play major roles in mutagenic processes. Generally, the contribution of S9 mix to mutagen activation is quite relevant (Claxton et al. 2004). However, this contribution, which is imputable mainly to the polycyclic aromatic hydrocarbons (PAHs), can vary consistently based on the sampling site (Claxton and Woodall 2007). The annual mean of the PM2.5 benzo(a)pyrene and PAHs were 0.6 and 3.1 ng/m3, respectively (ARPA Piemonte PdT 2008). The correlation between PAHs and mutagenicity was statistically significant (Spearman’s rho 0.870, p < 0.01). Such compounds may play roles in the mutagenic potential of PM2.5. Moreover, Turin and southern Europe are characterised by the presence of directly mutagenic nitro compounds, such as the nitro-PAHs (Traversi et al. 2011), which are more often mutagenic and carcinogenic than PAH derivatives (Albinet et al. 2008).
As for the gravimetric data, the mutagenicity observed with the Salmonella assay differed in a seasonal manner (F = 25.144, p < 0.001 for the TA98 strain and F = 34.888, p < 0.001 for the TA98 strain with S9 mix), with the exception of summer versus spring (Fig. 2a). When comparing the PM2.5 level and mutagenicity in the absence and presence of S9 mix, the calculated Spearman’s rho was 0.846 (p = 0.001) and 0.825 (p = 0.001), respectively. The mutagenic responses with and without metabolic activation were correlated (Spearman’s rho 0.993, p < 0.001). Moreover, when mutagenicity was expressed as net revertants per microgram of PM2.5, valuable responses were observed, particularly during the months of January, February, November and December (Fig. 2a). This evidence confirms not only that PM2.5 concentration is greater in winter, but also the mutagenicity per mass unit is also higher, likely because of alterations in the chemical composition of the mixture of particles, as previously discussed (Traversi et al. 2011). In contrast, other types of genotoxic effects, such as oxidative stress, may be higher during the spring or other seasons (Bastonini et al. 2011). The observed results are comparable to previous studies in which data regarding the mutagenic properties of polluted urban areas in Europe in general and Southern Europe in particular were collected (Cassoni et al. 2004; Claxton et al. 2004; Claxton and Woodall 2007).
The year 2013 has been designated by the WHO as the year of air, in reference to the serious economic problems caused by air pollution and the impact of aero-dispersed pollution on human health (WHO-Europe 2013). Some estimates indicate that the incidence of lung cancer increases 8 % for every 10 μg/m3 increase in PM2.5 levels in the air above the background level. It is assumed that the threshold below which no harmful effects are observed is equal to approximately 5 μg of PM2.5/m3 (WHO 2005). The annual mean level of PM2.5 is above this reference concentration in all southern European regions, particularly in urban areas. This finding was confirmed in Turin during our sampling period, despite the fact that the annual mean PM2.5 levels have markedly decreased over the past few years (ARPA Piemonte SP, CSI Piemonte 2014; WHO 2005).
Cytotoxicity
Cytotoxic effects were observed in HELF cells. A dose-dependent response was observed, and the mean monthly IC50 was equal to 2.741 ± 1.414 and 3.219 ± 2.764 m3 of equivalent air/millilitre for the XTT and LDH assays, respectively, which corresponds to approximately 96 and 113 μg, respectively, of aero-dispersed PM2.5. The maximum effect on mitochondrial activity of the samples reached 80 % of total inhibition for every sample but was particularly high during spring and the first few months of summer. The cytotoxic effects were also affected by different seasonal environmental mixtures (ANOVA, F = 92.044, p < 0.001 for LDH, where a significantly higher IC50 was observed during autumn than during the other seasons; F = 27.937, p < 0.001 for XTT, where the IC50 values during autumn and winter differed significantly from those during summer and spring) (Fig. 2b). The two IC50 cytotoxicity evaluations were significantly correlated (Spearman’s rho, 0.622; p < 0.001). In this case, the observed effect on the cells was more relevant because of both the higher quantity and the higher toxicity of the PM2.5 composition, as evidenced by the data analysis per unit mass of exposure (Fig. 2b, secondary x–y axis).
Plasma membrane damage, as assessed by measuring LDH release, is comparable to the effect recorded in other studies, in which the LDH increase was approximately two times the effect of the control at a dose of 200 μg/ml of PM10 and in which a major effect was observed for the finer PM size fractions (Happo et al. 2010). The mitochondrial respiratory chain effects, as measured using XTT reduction, in the current study were similar to those reported in the literature when lung cells were used as a biological model of exposure to various particles (Breznan et al. 2013). The cytotoxic effects were relevant, and the results were sub-lethal for the cells at levels greater than 2 m3 of equivalent air/millilitre, corresponding to 70 μg of PM2.5 mass.
Gene expression
Gene expression analysis revealed only mild modulation (modulation of APE1 expression was only evident at the lowest exposure dose). Dose-dependent effects on levels of expression were not observed. However, we examined sub-lethal exposure doses, as revealed by the previously described cytotoxicity tests. At the higher doses (2 and 3 %), the cellular gene expression machinery may become completely disordered.
APE1 and XRCC1 are genes involved in the first steps of BER after the recognition of damaged bases by specific DNA glycosylases, incision of the affected DNA strand by an apurinic/apyrimidinic (AP) endonuclease and subsequent repair involving the XRCC1 protein. APE1 was clearly upregulated (Table 2), particularly following exposure to non-lethal doses (i.e. lower than the IC50 values identified in the cytotoxic tests); however, only modest and non-significant XRCC1 modulation was observed after the first dose was applied (Table 3). Recently, other authors observed that nanoparticles in particular were leading to defective XRCC1 DNA repair, proliferation arrest and inflammatory responses. The reduced changes with respect to the expected effects seemed to be caused by the capability of the particle to adsorb cytosolic proteins on its surface (Asharani et al. 2012).
After recognition of the damaged base by the appropriate DNA glycosylase, the glycosylase creates an apurinic or apyrimidinic site (AP site). The DNA backbone is then cleaved by a DNA AP endonuclease (APE1). Its activity creates a single-stranded DNA nick 5′ to the AP site, as opposed to the nick being created 3′ to the AP site as a result of DNA AP lyase activity. APE1 was constitutively upregulated, and the ANOVA analysis of APE1 expression was significant when the first dose was considered in respect to the other doses (F = 4.983, p = 0.013), which were either lethal or displayed cytotoxic effects that were too high to reveal evidence of gene expression modulation.
NER is the main DNA repair pathway involved in the repair of UV- and carcinogen-induced DNA damage in mammalian cells and is highly conserved in nature. DNA damage is first recognised by XPA, but our results revealed that modulation of this target was not an effective marker, because the mean observed expression was lower than control levels (Table 2). When the first dose was considered with regard to the others, the expression level differences were significant (ANOVA, F = 3.364, p = 0.048). Other authors recently demonstrated that XPA expression was not clearly increased when studying its modulation following benzo(a)pyrene exposure in human bronchial epithelial cells (Yang et al. 2013). The regulation of ERCC1 and XPF expression is quite similar. During the DNA repair process, ERCC1 and XPF form a complex and are involved in the incision of the 3′ side of the damage, resulting in the removal of a piece of DNA that is approximately 29 nucleotides in length. Our data indicated that ERCC1 and XPF are significantly correlated based on the expression data obtained following exposure to the first dose. Constant and moderate increases in their expression were observed, equating to approximately 20–30 % (Table 3). Similarly, the induction of relevant genes involved in DNA repair and apoptosis, and ERCC1 induction specifically, has been reported after exposure to PM10 (Bastonini et al. 2011).
To correlate the gene expression results with the Salmonella assay data and PM2.5 levels (Table 4), we used only the data obtained from the two cytotoxicity tests following exposure to the lowest dose (the dose that was below the IC50 value), which were expressed as cubic metre of equivalent air per square centimetr of cell culture.
Interestingly, we observed a significant and proportional correlation between APE1 overexpression and the mutagenicity observed in the Salmonella assay that was induced by monthly samples (Table 4). A proportional effect was observed both in base insertions into a bacterial cell in the S. typhimurium TA98 strain and in the necessity of removing AP sites in human lung fibroblasts.
AFLP
Our AFLP analyses were performed on the DNA extracts that were derived from cell cultures that had been exposed to the highest dose from the winter months. No lost or introduced fragments were observed. Recently, a study of the effects of exposure to benzo(a)pyrene and ethyl methanesulfonate revealed that the percentage of lost or introduced fragments (compared to the control) was 12 % (Srut et al. 2013). Furthermore, it may be inferred that an effect that results from exposure to high concentrations of a single chemical compound is more repeatable than an effect observed after exposure to environmental mixtures, such as the PM2.5 urban organic extracts.
It has been suggested that 100 % reproducibility of AFLP fingerprints may be achieved by excluding bands with intensities <1 % of the combined intensity of all bands and by excluding the largest and the smallest bands, which account for 10 % of the total recorded bands (Bagley et al. 2001). However, this approach can be improved in terms of resolution and repeatability using an automatic method for the detection of the AFLP fragments with respect to the visual inspection of the stained gels.
Conclusions
The process of scientific investigation can be used to determine the level of exposure to mutagenic and genotoxic contaminants and to define an appropriate protection factor for human health. A threshold effect should also be evaluated, considering individual susceptibility and also the susceptibility specific to some population groups, such as children, elderly patients, patients with predisposing polymorphisms and patients with previous pathologies.
The screening methods that are used for genotoxic assessments are generally based on consolidated protocols, such as the Salmonella assay in bacteria and the Comet assay in human cells. These methods provide a useful model for assessing the ability of these environmental mixtures to induce genetic and chromosomal damage in the exposed cells (Eastmond et al. 2009). After this damage has occurred, however, it is not possible to define the nature and chromosomal location of the induced damage. PCR-based methods may be a tool to address this problem. Although the PCR-based techniques are promising, many problems remain, including low reproducibility and the lack of standardisation (Bustin et al. 2009). Moreover, other kinds of methods able to analyse a great number of genes are now available such as microarray.
In the present study, mutagenicity, cytotoxicity and gene expression assays were applied to a panel of the same samples. Overall, the results revealed the capacity of PM2.5 to produce DNA alterations and support genotoxic processes. Interesting correlations between damage processes that were similar from a biologic point of view were observed, such as point mutations in Salmonella and increases in AP site removal necessity in HELF cells. On the other hand, the dose of exposure is a crucial point, and careful consideration must be given to distinguish a dose that is able to produce a detectable effect from a lethal dose. An extension of the endpoints investigated, including epigenetic targets, may help to clarify the complex carcinogenic pathways activated by exposure to PM2.5.
Abbreviations
- AFLP:
-
Amplified fragment length polymorphism
- BER:
-
Base excision repair
- LDH:
-
Extracellular lactate dehydrogenase
- NER:
-
Nucleotide excision repair
- PAHs:
-
Polycyclic aromatic hydrocarbons
- PCR:
-
Polymerase chain reaction
- PM:
-
Particulate matter
- PM10:
-
Particulate matter with an aerodynamic diameter <10 μm
- PM2.5:
-
Particulate matter with an aerodynamic diameter <2.5 μm
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- XTT:
-
2,3-Bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxyanilide inner salt
References
Albinet A, Leoz-Garziandia E, Budzinski H, Villenave E, Jaffrezo JL (2008) Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleys—part 1: concentrations, sources and gas/particle partitioning. Atmos Environ 42:43–54
Alfaro-Moreno E, Martinez L, Garcia-Cuellar C, Bonner JC, Murray JC, Rosas I, Rosales SP, Osornio-Vargas AR (2002) Biologic effects induced in vitro by PM10 from three different zones of Mexico City. Environ Health Perspect 110:715–720
Arase Y, Nomura J, Sugaya S, Sugita K, Kita K, Suzuki N (2002) Effects of 3-D clino-rotation on gene expression in human fibroblast cells. Cell Biol Int 26:225–233
ARPA Piemonte PdT (2008) Uno sguardo all’aria 2008. Provincia di Torino, Torino
ARPA Piemonte SP, CSI Piemonte (2014) ARIAWEB–Sistema Regionale di Rilevamento della Qualità dell’Aria della Regione Piemonte
Asharani P, Sethu S, Lim HK, Balaji G, Valiyaveettil S, Hande MP (2012) Differential regulation of intracellular factors mediating cell cycle, DNA repair and inflammation following exposure to silver nanoparticles in human cells. Genome Integr 3:2
Atienzar FA, Jha AN (2006) The random amplified polymorphic DNA (RAPD) assay and related techniques applied to genotoxicity and carcinogenesis studies: a critical review. Mutat Res 613:76–102
Bagley MJ, Anderson SL, May B (2001) Choice of methodology for assessing genetic impacts of environmental stressors: polymorphism and reproducibility of RAPD and AFLP fingerprints. Ecotoxicology 10:239–244
Bassam BJ, Gresshoff PM (2007) Silver staining DNA in polyacrylamide gels. Nat Protoc 2:2649–2654
Bastonini E, Verdone L, Morrone S, Santoni A, Settimo G, Marsili G, La Fortezza M, Di Mauro E, Caserta M (2011) Transcriptional modulation of a human monocytic cell line exposed to PM(10) from an urban area. Environ Res 111:765–774
Baurand PE, de Vaufleury A, Scheifler R, Capelli N (2013) Coupling of random amplified polymorphic DNA profiles analysis and high resolution capillary electrophoresis system for the assessment of chemical genotoxicity. Environ Sci Technol 47:9505–9513
Beelen R, Hoek G, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, Armstrong B, Brunekreef B (2008) Long-term exposure to traffic-related air pollution and lung cancer risk. Epidemiology 19:702–710
Belpomme D, Irigaray P, Hardell L, Clapp R, Montagnier L, Epstein S, Sasco AJ (2007) The multitude and diversity of environmental carcinogens. Environ Res 105:414–429
Ben Salem Z, Capelli N, Grisey E, Baurand PE, Ayadi H, Aleya L (2014) First evidence of fish genotoxicity induced by heavy metals from landfill leachates: the advantage of using the RAPD-PCR technique. Ecotoxicol Environ Saf 101:90–96
Breznan D, Goegan P, Chauhan V, Karthikeyan S, Kumarathasan P, Cakmak S, Nadeau D, Brook JR, Vincent R (2013) Respiratory burst in alveolar macrophages exposed to urban particles is not a predictor of cytotoxicity. Toxicol in Vitro 27:1287–1297
Brunekreef B, Beelen R, Hoek G, Schouten L, Bausch-Goldbohm S, Fischer P, Armstrong B, Hughes E, Jerrett M, van den Brandt P (2009) Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: the NLCS-AIR study. Res Rep Health Eff Inst 5–71; discussion 73–89
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Bylund L, Kytola S, Lui WO, Larsson C, Weber G (2004) Analysis of the cytogenetic stability of the human embryonal kidney cell line 293 by cytogenetic and STR profiling approaches. Cytogenet Genome Res 106:28–32
Cassoni F, Bocchi C, Martino A, Pinto G, Fontana F, Buschini A (2004) The Salmonella mutagenicity of urban airborne particulate matter (PM2.5) from eight sites of the Emilia-Romagna regional monitoring network (Italy). Sci Total Environ 324:79–90
CEN (1998) Air quality—determination of the PM10 fraction of suspended particulate matter—reference method and field test procedure to demonstrate reference equivalence of measurement methods. In: Standardization ECf (Hrsg.), European Standard EN 12341 Bruxelles
Chang LC, Sheu HM, Huang YS, Tsai TR, Kuo KW (1999) A novel function of emodin—enhancement of the nucleotide excision repair of UV- and cisplatin-induced DNA damage in human cells. Biochem Pharmacol 58:49–57
Claxton LD, Woodall GM Jr (2007) A review of the mutagenicity and rodent carcinogenicity of ambient air. Mutat Res 636:36–94
Claxton LD, Matthews PP, Warren SH (2004) The genotoxicity of ambient outdoor air, a review: Salmonella mutagenicity. Mutat Res 567:347–399
de Kok TM, Hogervorst JG, Briede JJ, van Herwijnen MH, Maas LM, Moonen EJ, Driece HA, Kleinjans JC (2005) Genotoxicity and physicochemical characteristics of traffic-related ambient particulate matter. Environ Mol Mutagen 46:71–80
Eastmond DA, Hartwig A, Anderson D, Anwar WA, Cimino MC, Dobrev I, Douglas GR, Nohmi T, Phillips DH, Vickers C (2009) Mutagenicity testing for chemical risk assessment: update of the WHO/IPCS Harmonized Scheme. Mutagenesis 24:341–349
EEA (2012) European Environmental Agency—air quality in Europe—2012 report Office for Official Publications of the European Union, Copenhagen
Eftim SE, Samet JM, Janes H, McDermott A, Dominici F (2008) Fine particulate matter and mortality: a comparison of the six cities and American Cancer Society cohorts with a medicare cohort. Epidemiology 19:209–216
Gallus S, Negri E, Boffetta P, McLaughlin JK, Bosetti C, La Vecchia C (2008) European studies on long-term exposure to ambient particulate matter and lung cancer. Eur J Cancer Prev 17:191–194
Gilli G, Pignata C, Schiliro T, Bono R, La Rosa A, Traversi D (2007) The mutagenic hazards of environmental PM2.5 in Turin. Environ Res 103:168–175
Grant WB (2009) Air pollution in relation to U.S. cancer mortality rates: an ecological study; likely role of carbonaceous aerosols and polycyclic aromatic hydrocarbons. Anticancer Res 29:3537–3545
Happo MS, Salonen RO, Halinen AI, Jalava PI, Pennanen AS, Dormans JAMA, Gerlofs-Nijland ME, Cassee FR, Kosma VM, Sillanpaa M, Hillamo R, Hirvonen MR (2010) Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six European cities. Inhal Toxicol 22:402–416
Jain S, Khare M (2008) Urban air quality in mega cities: a case study of Delhi City using vulnerability analysis. Environ Monit Assess 136:257–265
Ji H, Hershey GKK (2012) Genetic and epigenetic influence on the response to environmental particulate matter. J Allergy Clin Immunol 129:33–41
Kam W, Delfino RJ, Schauer JJ, Sioutas C (2013) A comparative assessment of PM2.5 exposures in light-rail, subway, freeway, and surface street environments in Los Angeles and estimated lung cancer risk. Environ Sci Process Impacts 15:234–243
Kappos AD, Bruckmann P, Eikmann T, Englert N, Heinrich U, Hoppe P, Koch E, Krause GH, Kreyling WG, Rauchfuss K, Rombout P, Schulz-Klemp V, Thiel WR, Wichmann HE (2004) Health effects of particles in ambient air. Int J Hyg Environ Health 207:399–407
Krewski D, Burnett R, Jerrett M, Pope CA, Rainham D, Calle E, Thurston G, Thun M (2005) Mortality and long-term exposure to ambient air pollution: ongoing analyses based on the American Cancer Society cohort. J Toxicol Environ Health A 68:1093–1109
Krzyzanowski M (2008) WHO air quality guidelines for Europe. J Toxicol Environ Health A 71:47–50
Libalova H, Uhlirova K, Klema J, Machala M, Sram RJ, Ciganek M, Topinka J (2012) Global gene expression changes in human embryonic lung fibroblasts induced by organic extracts from respirable air particles. Part Fibre Toxicol 9
Lindgren A, Stroh E, Montnemery P, Nihlen U, Jakobsson K, Axmon A (2009) Traffic-related air pollution associated with prevalence of asthma and COPD/chronic bronchitis. A cross-sectional study in Southern Sweden. Int J Health Geogr 8:2
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25:402–408
Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K, IARC (2013) The carcinogenicity of outdoor air pollution. Lancet Oncol 14:1262–1263
Maruyama W, Hirano S, Kobayashi T, Aoki Y (2006) Quantitative risk analysis of particulate matter in the air: interspecies extrapolation with bioassay and mathematical models. Inhal Toxicol 18:1013–1023
Mehta M, Chen LC, Gordon T, Rom W, Tang MS (2008) Particulate matter inhibits DNA repair and enhances mutagenesis. Mutat Res 657:116–121
Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, Sandstrom T, Blomberg A, Newby DE (2009) Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med 6:36–44
Moller P, Folkmann JK, Forchhammer L, Brauner EV, Danielsen PH, Risom L, Loft S (2008) Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett 266:84–97
Mukherjee B, Dutta A, Roychoudhury S, Ray MR (2013) Chronic inhalation of biomass smoke is associated with DNA damage in airway cells: involvement of particulate pollutants and benzene. J Appl Toxicol 33:281–289
Nagdeve D (2006) Urban air pollution and its influence on human health in mega cities of India. Epidemiology 17:S261–S262
Nemery B, Hoet PH, Nemmar A (2001) The Meuse Valley fog of 1930: an air pollution disaster. Lancet 357:704–708
Nogueira JB (2009) Air pollution and cardiovascular disease. Rev Port Cardiol 28:715–733
Pfuhler S, Elespuru R, Aardema MJ, Doak SH, Donner EM, Honma M, Kirsch-Volders M, Landsiedel R, Manjanatha M, Singer T, Kim JH (2013) Genotoxicity of nanomaterials: refining strategies and tests for hazard identification. Environ Mol Mutagen 54:229–239
Pope CA 3rd (1996) Particulate pollution and health: a review of the Utah valley experience. J Expo Anal Environ Epidemiol 6:23–34
Pope CA 3rd (2000) Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who’s at risk? Environ Health Perspect 108(Suppl 4):713–723
Silva JP, Gomes AC, Coutinho OP (2008) Oxidative DNA damage protection and repair by polyphenolic compounds in PC12 cells. Eur J Pharmacol 601:50–60
Sorensen M, Autrup H, Hertel O, Wallin H, Knudsen LE, Loft S (2003a) Personal exposure to PM2.5 and biomarkers of DNA damage. Cancer Epidemiol Biomarkers Prev 12:191–196
Sorensen M, Autrup H, Moller P, Hertel O, Jensen SS, Vinzents P, Knudsen LE, Loft S (2003b) Linking exposure to environmental pollutants with biological effects. Mutat Res 544:255–271
Srut M, Stambuk A, Klobucar GI (2013) What is comet assay not telling us: AFLP reveals wider aspects of genotoxicity. Toxicol in Vitro 27:1226–1232
Ta W, Wang T, Xiao H, Zhu X, Xiao Z (2004) Gaseous and particulate air pollution in the Lanzhou Valley, China. Sci Total Environ 320:163–176
Traversi D, Schiliro T, Degan R, Pignata C, Alessandria L, Gilli G (2011) Involvement of nitro-compounds in the mutagenicity of urban Pm2.5 and Pm10 in Turin. Mutat Res Genet Toxicol Environ Mutagen 726:54–59
Valavanidis A, Fiotakis K, Vlachogianni T (2008) Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 26:339–362
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3
Vardar C, Basaran E, Cansaran-Duman D, Aras S (2014) Air-quality biomonitoring: assessment of genotoxicity of air pollution in the Province of Kayseri (Central Anatolia) by use of the lichen Pseudevernia furfuracea (L.) Zopf and amplified fragment-length polymorphism markers. Mutat Res Genet Toxicol Environ Mutagen 759:43–50
Viera L, Chen K, Nel A, Lloret MG (2009) The impact of air pollutants as an adjuvant for allergic sensitization and asthma. Curr Allergy Asthma Rep 9:327–333
Vineis P et al (2007) Lung cancers attributable to environmental tobacco smoke and air pollution in non-smokers in different European countries: a prospective study. Environ Health 6:7
Vinikoor-Imler LC, Davis JA, Luben TJ (2011) An ecologic analysis of county-level PM2.5 concentrations and lung cancer incidence and mortality. Int J Environ Res Public Health 8:1865–1871
Voigt T, Bailey M, Abramson M (1998) Air pollution in the Latrobe Valley and its impact upon respiratory morbidity. Aust N Z J Public Health 22:556–561
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
WHO (2005) Air quality guidelines—global update 2005
WHO-Europe (2013) Review of evidence on health aspects of air pollution—Technical Report—REVIHAAP Project
Wood RD, Mitchell M, Lindahl T (2005) Human DNA repair genes, 2005. Mutat Res Fundam Mol Mech Mutagen 577:275–283
Yang J, Wang Z, Chen W, Yin J (2013) Role of ubiquitin protein ligase Ring2 in DNA damage of human bronchial epithelial cells exposed to benzo[a]pyrene. J Biochem Mol Toxicol 27:357–363
Zhang M, Song Y, Cai X, Zhou J (2008) Economic assessment of the health effects related to particulate matter pollution in 111 Chinese cities by using economic burden of disease analysis. J Environ Manag 88:947–954
Zhang JJ, McCreanor JE, Cullinan P, Chung KF, Ohman-Strickland P, Han IK, Jarup L, Nieuwenhuijsen MJ (2009) Health effects of real-world exposure to diesel exhaust in persons with asthma. Res Rep Health Eff Inst 5–109; discussion 111–23
Acknowledgments
This study was funded by the University of Turin as a local project (ex. 60 %, 2012). The authors thank their colleagues (Prof. Giorgio Gribaudo and Dr. Anna Luganini) for supplying the HELF cells and the Environmental Protection Agency of Piedmont (particularly Dr. F. Lollobrigida) for collecting the PM2.5 samples. Moreover, the authors thank Dr. Roberto Arbore for his collaboration during his training period.
Authors’ contributions
Dr. Traversi designed the study and performed the PM2.5 sample extractions, Salmonella, cytotoxicity and gene expression assays. Dr. Cervella performed the AFLP assay. Dr. Gilli provided the PM2.5 samples and contacted the local Environmental Protection Agency. Dr. Gilli and Dr. Traversi analysed the data. Dr. Traversi prepared the manuscript. All authors approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
Fine particles are crucial urban pollution indicators.
PM2.5 produces marked mutagenic and cytotoxic effects in vitro.
APE1 gene overexpression provided an effect evidence, but no AFLP profile alterations were induced.
These effects displayed a significant seasonal trend.
Multi-assay approach displayed an advantage in conducting genotoxic assessments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Materials 1
(GIF 204 kb)
High resolution
(TIFF 2902 kb)
Rights and permissions
About this article
Cite this article
Traversi, D., Cervella, P. & Gilli, G. Evaluating the genotoxicity of urban PM2.5 using PCR-based methods in human lung cells and the Salmonella TA98 reverse test. Environ Sci Pollut Res 22, 1279–1289 (2015). https://doi.org/10.1007/s11356-014-3435-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3435-1